Extraction and Modification of Cellulose Microfibers Derived from Biomass of the Amazon Ochroma pyramidale Fruit
Abstract
:1. Introduction
2. Experimental
2.1. Materials
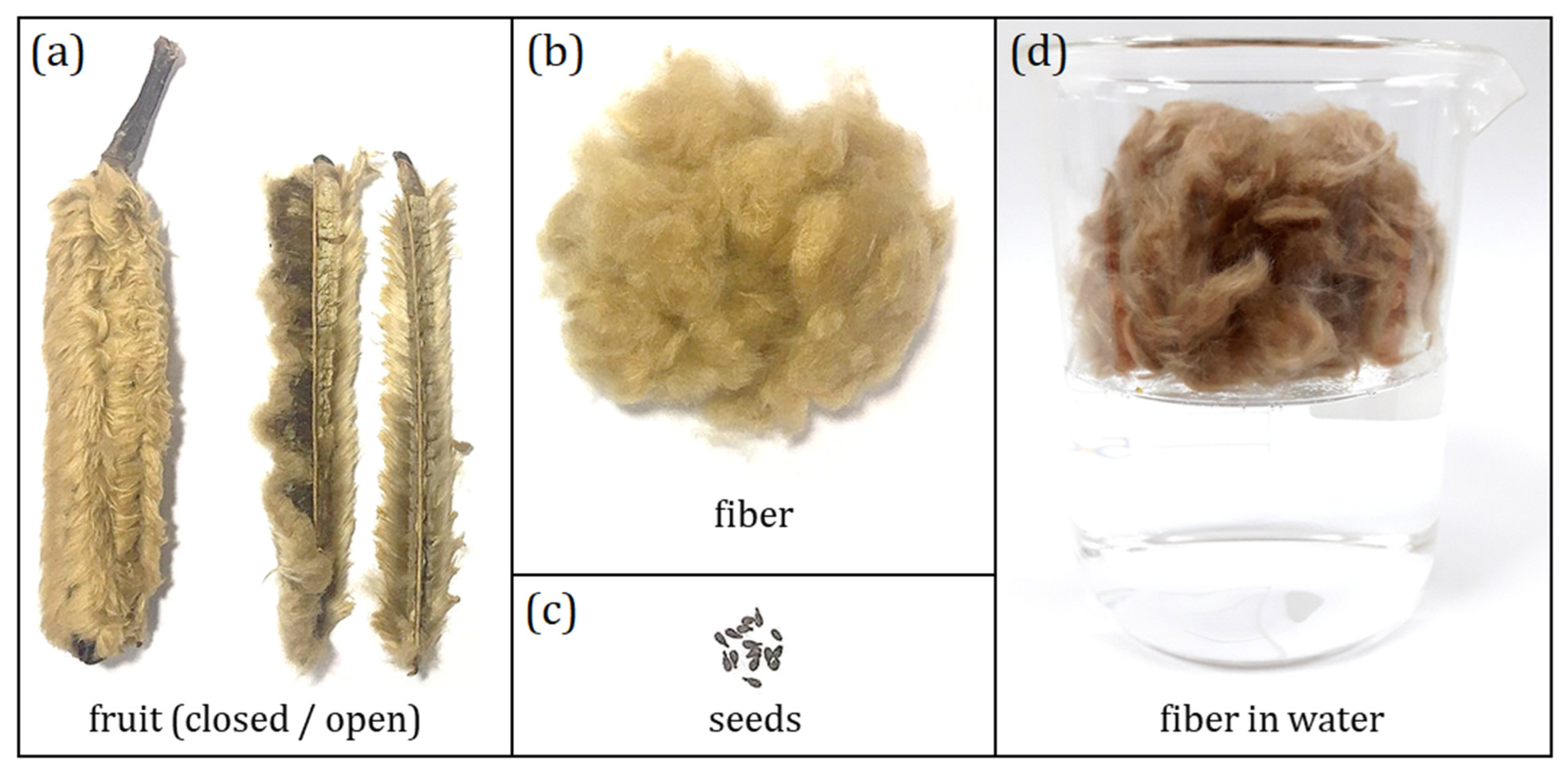
Plant Material
2.2. Methods
2.2.1. Physicochemical Composition
2.2.2. X-ray Diffraction (XRD) and Percentage of Crystallinity
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.5. Thermogravimetry/Derivative Thermogravimetry (TG/dTG) and Differential Scanning Calorimetry (DSC)
2.2.6. Thermal Conductivity
3. Results and Discussion
3.1. Physicochemical Characterization
| Specie | M% | E% | He% | C% | L% | A% | Reference |
|---|---|---|---|---|---|---|---|
| Balsa fruit fiber (Ochroma pyramidale) | 8.7 ± 0.2 | 3.7 ± 0.3 | 33 ± 1 | 35.8 ± 0.1 | 32 ± 3 | 2.04 ± 0.03 | (Present work) |
| Balsa fruit fiber (Ochroma pyramidale) | 11.45 | 2.29 | 37.35 | 44.62 | 16.6 | 0.94 | [16] |
| Kapok fruit fiber (Ceiba pentandra) | 11.23 | 2.34 | 45.64 | 38.09 | 14.1 | 1.05 | [16] |
| Kapok bark (Ceiba pentandra) | 7.46 | 0.38 | 17.53 | 60.9 | 23.5 | 1.05 | [51] |
| Kapok fruit fiber (Ceiba pentandra) | – | 23 | 23 | 64 | 19 | – | [17] |
| Coconut fiber (Cocos nucifera) | – | – | 8.51 | 35.62 | 37.59 | 0.97 | [23] |
| Jute stem (Corchorus capsularis) | – | 0.5 | 14–20 | 61–71 | 12–13 | – | [51] |
| Sisal leaf (Agave sisalana) | – | 2 | 12 | 65 | 9.9 | – | [51] |
| Kenaf stem (Hibiscus cannabinus) | – | 0.3 | 20.3 | 72 | 9 | – | [51] |
| Oil palm leaves (Elaeis guineensis) | – | – | 34 | 42.67 | 22.9 | – | [51] |
| Umbrella thorn bark (Acacia tortilis) | 6.47 | 17.43 | – | 61.89 | 21.26 | 4.33 | [1] |
| Buriti leaf fiber (Mauritia flexuosa) | 9 | 6 | 1 | 58 | 19 | 2 | [19] |
| Date palm (Phoenix dactylifera) | 5.4–15.6 | – | 9.75–26 | 35–44 | 11–29 | 3–12 | [45] |
| Sabai grass (Eulaliopsis binata) | – | – | 21.1 | 42.9 | 18.5 | 13.4 | [44] |
| Portia tree bark (Thepesia populnea) | 9.8–11.5 | 0.7–0.8 | 12–16 | 64–70 | 16–18 | 1.7–2.1 | [52] |
| Roselle stems (Hibiscus sabdariffa) | – | – | 16–20 | 58–64 | 6–10 | – | [46] |
3.2. XRD Analysis
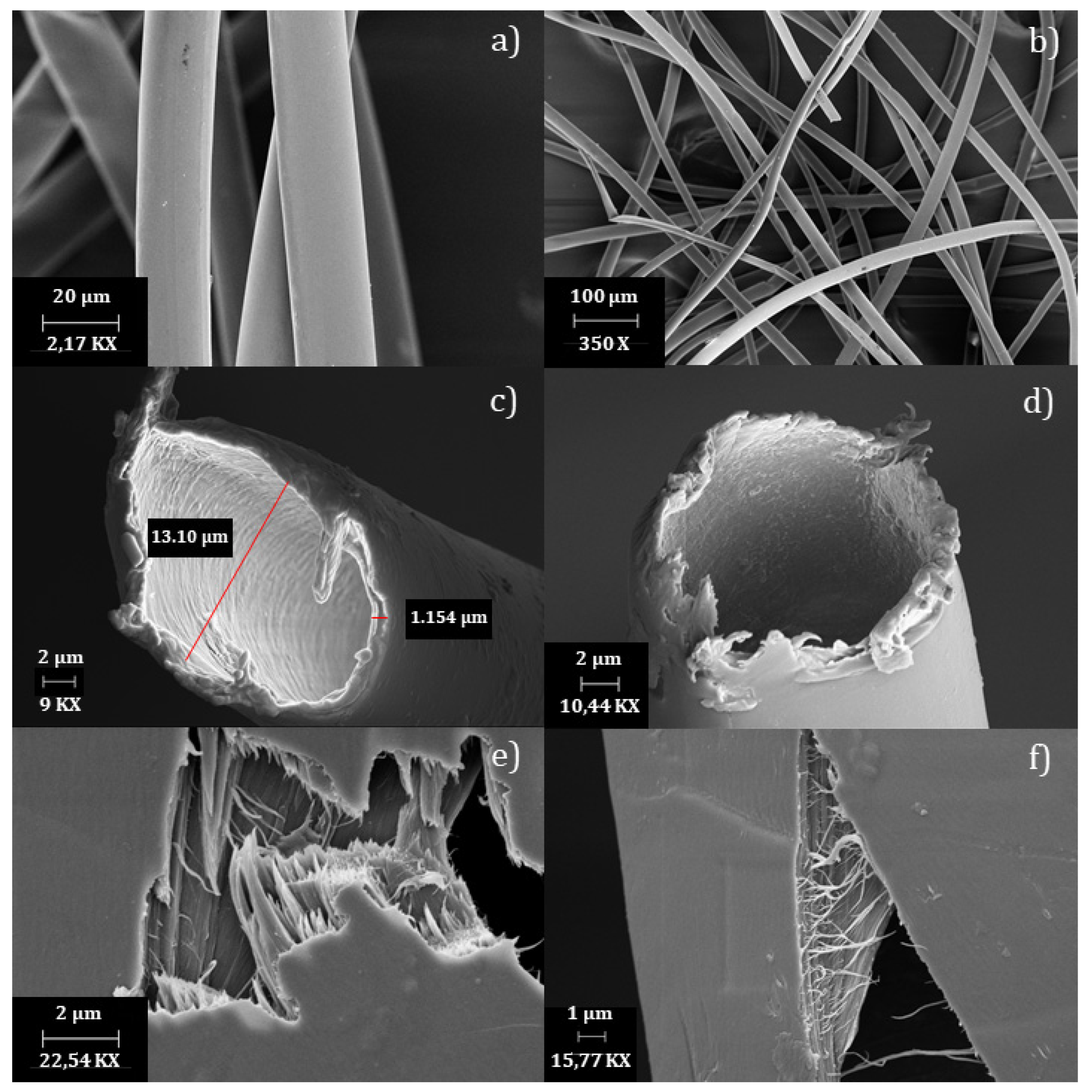
3.3. Morphological Analysis
3.4. FTIR Analysis
| Wavenumber (cm−1) | Vibrational Modes | References |
|---|---|---|
| 3350 | O–H groups of cellulose or moisture | [19,62,64] |
| 2913 | C–H stretching | [47,64] |
| 1739 | C=O stretching of lignin and hemicellulose fractions | [55,59,61] |
| 1644 | Adsorbed water | [23,46,55] |
| 1597/1503 | C=C stretching of aromatic ring of lignin | [46,55,60] |
| 1463 | CH3 deformation of lignin | [60,62] |
| 1429 | CH2 symmetrical bending of cellulose | [24,46,47,63] |
| 1375 | C–H bending | [46,61] |
| 1337/1327/1282 | CH2 wagging vibration and C–O aromatic ring of cellulose | [55,59,62,63] |
| 1246 | C–O stretching of lignin | [47,55,63] |
| 1202/1164/1113/ 1059/1034 | Multiple peaks of C–O–C pyranose ring | [30,61,62] |
| 897 | β-glycosidic linkages between glucose units of cellulose | [24,46,63] |
3.5. TG/dTG Analysis
3.6. DSC Analysis
3.7. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawit, J.B.; Regassa, Y.; Lemu, H.G. Property characterization of Acacia tortilis for natural fiber reinforced polymer composite. Results Mater. 2020, 5, 100054. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Guan, F.; Li, G.; Song, Z.; Yu, D.; Wang, H.; Liu, H. Using cellulose fibers to fabricate transparent paper by microfibrillation. Carbohydr. Polym. 2019, 214, 26–33. [Google Scholar] [CrossRef]
- Leão, N.; de Freitas, A.D.D. Informativos Técnico Rede de Sementes da Amazônia Pau-de-balsa. Rede Sementes Amazônia. 2008, 17–19. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/173038/1/19-Pau-de-balsa.pdf (accessed on 29 June 2023).
- Borrega, M.; Ahvenainen, P.; Serimaa, R.; Gibson, L. Composition and structure of balsa (Ochroma pyramidale) wood. Wood Sci. Technol. 2015, 49, 403–420. [Google Scholar] [CrossRef]
- Malek, S.; Gibson, L.J. Multi-scale modelling of elastic properties of balsa. Int. J. Solids Struct. 2017, 113, 118–131. [Google Scholar] [CrossRef]
- Masselter, T.; Kempe, A.; Caliaro, S.; Neinhuis, C.; Speck, T. Comparing structure and biomechanics of extant Carica papaya and Ochroma pyramidale stems allows re-evaluating the functional morphology of the fossil ‘seed fern’ Lyginopteris oldhamia. Rev. Palaeobot. Palynol. 2017, 246, 258–263. [Google Scholar] [CrossRef]
- Santos, D.G.D.J.; Deuner, C.; De Almeida, A.P.F.; Meneghello, G.E.; Xavier, F.D.M. Superação de dormência em sementes de pau de balsa (Ochroma pyramidale). Rev. Verde Agroecol. E Desenvolv. Sustentável 2016, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Rezende, G.M.; Vieira, D.L.M. Forest restoration in southern Amazonia: Soil preparation triggers natural regeneration. For. Ecol. Manag. 2019, 433, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic-oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Pooja, S.; Anbarasan, B.; Ponnusami, V.; Arumugam, A. Efficient production and optimization of biodiesel from kapok (Ceiba pentandra) oil by lipase transesterification process: Addressing positive environmental impact. Renew. Energy 2021, 165, 619–631. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Wan Abdullah, W.N.; Leh, C.P. Potential of Ceiba pentandra (L.) Gaertn. (kapok) fiber as a resource for second generation bioethanol: Parametric optimization and comparative study of various pretreatments prior enzymatic saccharification for sugar production. Bioresour. Technol. 2013, 140, 10–14. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Investigation of acetylated kapok fibers on the sorption of oil in water. J. Environ. Sci. 2013, 25, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Zhu, Y.; Wang, A. Research and application of kapok fiber as an absorbing material: A mini review. J. Environ. Sci. (China) 2015, 27, 21–32. [Google Scholar] [CrossRef]
- Purnawati, R.; Febrianto, F.; Wistara, I.N.J.; Nikmatin, S.; Hidayat, W.; Lee, S.H.; Kim, N.H. Physical and chemical properties of kapok (Ceiba pentandra) and balsa (ochroma pyramidale) fibers. J. Korean Wood Sci. Technol. 2018, 46, 393–401. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Chaiwatyothin, S.; Mueangta, S.; Hanchana, A. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Peraza-Ku, S.A.; Escobar-Morales, B.; Rodríguez-Fuentes, N.; Cervantes-Uc, J.M.; Uribe-Calderon, J.A. Ceiba pentandra cellulose crosslinked with citric acid for drug release systems. Carbohydr. Res. 2021, 504, 108334. [Google Scholar] [CrossRef] [PubMed]
- Demosthenes, L.C.D.C.; Nascimento, L.F.C.; Monteiro, S.N.; Costa, U.O.; Filho, F.D.C.G.; da Luz, F.S.; Oliveira, M.S.; Ramos, F.J.H.T.V.; Pereira, A.C.; Braga, F.O. Thermal and structural characterization of buriti fibers and their relevance in fabric reinforced composites. J. Mater. Res. Technol. 2020, 9, 115–123. [Google Scholar] [CrossRef]
- Mercedes, L.; Gil, L.; Bernat-Maso, E. Mechanical performance of vegetal fabric reinforced cementitious matrix (FRCM) composites. Constr. Build. Mater. 2018, 175, 161–173. [Google Scholar] [CrossRef]
- Olacia, E.; Pisello, A.L.; Chiodo, V.; Maisano, S.; Frazzica, A.; Cabeza, L.F. Sustainable adobe bricks with seagrass fibres. Mechanical and thermal properties characterization. Constr. Build. Mater. 2020, 239, 117669. [Google Scholar] [CrossRef]
- ben Hammouda, S.; Chen, Z.; An, C.; Lee, K. Recent advances in developing cellulosic sorbent materials for oil spill cleanup: A state-of-the-art review. J. Clean. Prod. 2021, 311, 127630. [Google Scholar] [CrossRef]
- Cardoso, C.K.M.; Mattedi, S.; Lobato, A.K.D.C.L.; Moreira, T.A. Remediation of petroleum contaminated saline water using value-added adsorbents derived from waste coconut fibres. Chemosphere 2021, 279, 130562. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Arteaga-Cardona, F.; de Anda Reyes, M.E.; Gervacio-Arciniega, J.J.; Salazar-Kuri, U. Magnetic bioplastics based on isolated cellulose from cotton and sugarcane bagasse. Mater. Chem. Phys. 2019, 238, 121921. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Rios-Rivera, A.C.; Von Bennevitz, M.R. Living biomass supported on a natural-fiber biofilter for lead removal. J. Environ. Manag. 2019, 231, 825–832. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Irvine JT, S.; Lawton, L.A.; Robertson PK, J. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.K.; Goyal, P.; Sehgal, S. Effect of cellulose fibre based insulation on thermal performance of buildings. Mater. Today Proc. 2021, 45, 5778–5781. [Google Scholar] [CrossRef]
- Piégay, C.; Glé, P.; Gourdon, E.; Gourlay, E.; Marceau, S. Acoustical model of vegetal wools including two types of fibers. Appl. Acoust. 2018, 129, 36–46. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Dacrory, S.; Hasanin, M.; Saber, E.; Kamel, S. Biocompatible hydrogel based on aldehyde-functionalized cellulose and chitosan for potential control drug release. Sustain. Chem. Pharm. 2021, 21, 100419. [Google Scholar] [CrossRef]
- Soliman, M.; Sadek, A.A.; Abdelhamid, H.N.; Hussein, K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res. Vet. Sci. 2021, 137, 262–273. [Google Scholar] [CrossRef]
- Morais, J.P.S.; Rosa, M.D.F.; Marconcini, J.M. Procedimentos Para Análise Lignocelulósica; EMBRAPA: Brasilia, Brazil, 2010. [Google Scholar]
- TAPPI. T 211 om-02 Ash in Wood Pulp Paper Paperboard: Combustion at 525 °C; Technical Association of the Pulp and Paper Industry: Tokyo, Japan, 2017; pp. 525–530. [Google Scholar] [CrossRef]
- TAPPI. T 207 cm-99 Water Solubility of Wood and Pulp; Technical Association of the Pulp and Paper Industry: Tokyo, Japan, 1999; pp. 6–8. [Google Scholar] [CrossRef]
- TAPPI. T 204 cm-97 Solvent Extractives of Wood and Pulp; Technical Association of the Pulp and Paper Industry: Tokyo, Japan, 2007; pp. 7–10. [Google Scholar]
- TAPPI. T 222 om-02 Lignin in Wood and Pulp; Technical Association of the Pulp and Paper Industry: Tokyo, Japan, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- TAPPI. T 203 cm-99 Alpha-, Beta- and Gamma-Cellulose in Pulp; Technical Association of the Pulp and Paper Industry: Tokyo, Japan, 1999; pp. 5–9. [Google Scholar]
- Mccusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Biondo, M.M.; de Oliveira, L.M.; Lima, S.X.; de Souza Carolino, A.; Rocha AL, F.; da Silva, J.P.; Sanches, E.A. Chemically synthesized poly(o-methoxyaniline): Influence of counterions on the structural and electrical properties. J. Mol. Struct. 2020, 1205, 127588. [Google Scholar] [CrossRef]
- Veras, T.N.; Carolino, A.S.; Lima, S.X.; Biondo, M.M.; Santos, N.A.; Campelo, P.H.; Ruiz, Y.L.; Frota, H.O.; Sanches, E.A. Characterization and DFT calculation of poly(m-anisidine) synthesized with different dopant acids. J. Mol. Struct. 2020, 1201, 127182. [Google Scholar] [CrossRef]
- Götz, A.; Senz, V.; Schmidt, W.; Huling, J.; Grabow, N.; Illner, S. General image fiber tool: A concept for automated evaluation of fiber diameters in SEM images. Meas. J. Int. Meas. Confed. 2021, 177, 109265. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH [rad] and ABTS [rad] assays: Estimation methods for EC 50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Guna, V.; Ilangovan, M.; Adithya, K.; Akshay, A.K.; Srinivas, C.V.; Yogesh, S.; Nagananda, G.S.; Venkatesh, K.; Reddy, N. Biofibers and biocomposites from sabai grass: A unique renewable resource. Carbohydr. Polym. 2019, 218, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.R.; Almutairi, Z.; Jawaid, M. Characterization of natural fiber obtained from different parts of date palm tree (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Isolation and characterization of cellulose fibers from Thespesia populnea barks: A study on physicochemical and structural properties. Int. J. Biol. Macromol. 2019, 129, 396–406. [Google Scholar] [CrossRef]
- Spinacé, M.A.S.; Lambert, C.S.; Fermoselli, K.K.G.; De Paoli, M.A. Characterization of lignocellulosic curauá fibres. Carbohydr. Polym. 2009, 77, 47–53. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chudina, A.I.; Kazachenko, A.S.; Fetisova, O.Y.; Borovkova, V.S.; Vorobyev, S.A.; Karacharov, A.A.; Gnidan, E.V.; Mazurova, E.V.; Skripnikov, A.M.; et al. Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin. Polymers 2023, 15, 2671. [Google Scholar] [CrossRef]
- Kumar, P.S.S.; Allamraju, K.V. A review of natural fiber composites [Jute, Sisal, Kenaf]. Mater. Today Proc. 2019, 18, 2556–2562. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Assessment of cellulose in bark fibers of Thespesia populnea: Influence of stem maturity on fiber characterization. Carbohydr. Polym. 2019, 212, 439–449. [Google Scholar] [CrossRef]
- Feitosa, B.d.A.; Rocha AL, F.; Lima, S.X.; de Oliveira, L.M.; Biondo, M.M.; Campelo, P.H.; Sanches, E.A. Nanocomposites based on the cellulose extracted from the Amazon Peperomia pellucida and polyaniline derivatives: Structural and thermal properties. Chem. Pap. 2020, 75, 1809–1821. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, M.; Zhang, D. Pyrolysis Characteristics of Cellulose Isolated from Selected Biomass Feedstocks using a Thermogravimetric Analyser. Energy Procedia 2017, 142, 636–641. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Faria LU, S.; Pacheco BJ, S.; Oliveira, G.C.; Silva, J.L. Production of cellulose nanocrystals from pineapple crown fibers through alkaline pretreatment and acid hydrolysis under different conditions. J. Mater. Res. Technol. 2020, 9, 12346–12353. [Google Scholar] [CrossRef]
- Bajpai, P. Carbon Fibre from Lignin; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Kumneadklang, S.; O-Thong, S.; Larpkiattaworn, S. Characterization of cellulose fiber isolated from oil palm frond biomass. Mater. Today Proc. 2019, 17, 1995–2001. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Y.; Yu, W. Effects of extraction methods on morphology, structure and properties of bamboo cellulose. Ind. Crops Prod. 2021, 169, 113640. [Google Scholar] [CrossRef]
- Caputo, D.; Fusco, C.; Nacci, A.; Palazzo, G.; Murgia, S.; D’Accolti, L.; Gentile, L. A selective cellulose/hemicellulose green solvents extraction from buckwheat chaff. Carbohydr. Polym. Technol. Appl. 2021, 2, 100094. [Google Scholar] [CrossRef]
- Manzato, L.; Rabelo LC, A.; de Souza, S.M.; da Silva, C.G.; Sanches, E.A.; Rabelo, D.; Mariuba LA, M.; Simonsen, J. New approach for extraction of cellulose from tucumã’s endocarp and its structural characterization. J. Mol. Struct. 2017, 1143, 229–234. [Google Scholar] [CrossRef]
- Devnani, G.L.; Sinha, S. Extraction, characterization and thermal degradation kinetics with activation energy of untreated and alkali treated Saccharum spontaneum (Kans grass) fiber. Compos. Part B Eng. 2019, 166, 436–445. [Google Scholar] [CrossRef]
- Leite AL, M.P.; Zanon, C.D.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr. Polym. 2017, 157, 962–970. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S. The structural and thermal characteristics of wheat straw hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Athayde, J.N.; Fernandes, B.L.; de Siqueira, C.J.M.; Nohama, P.; Fernandes, C.R. Device for in vitro wear analysis of biomaterials in the hinged prosthesis configuration. Facta Univ. Ser. Mech. Eng. 2018, 16, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, P.L.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Ijjada, N.; Nayaka, R.R. Review on properties of some thermal insulating materials providing more comfort in the building. Mater. Today Proc. 2022, 58, 1354–1359. [Google Scholar] [CrossRef]
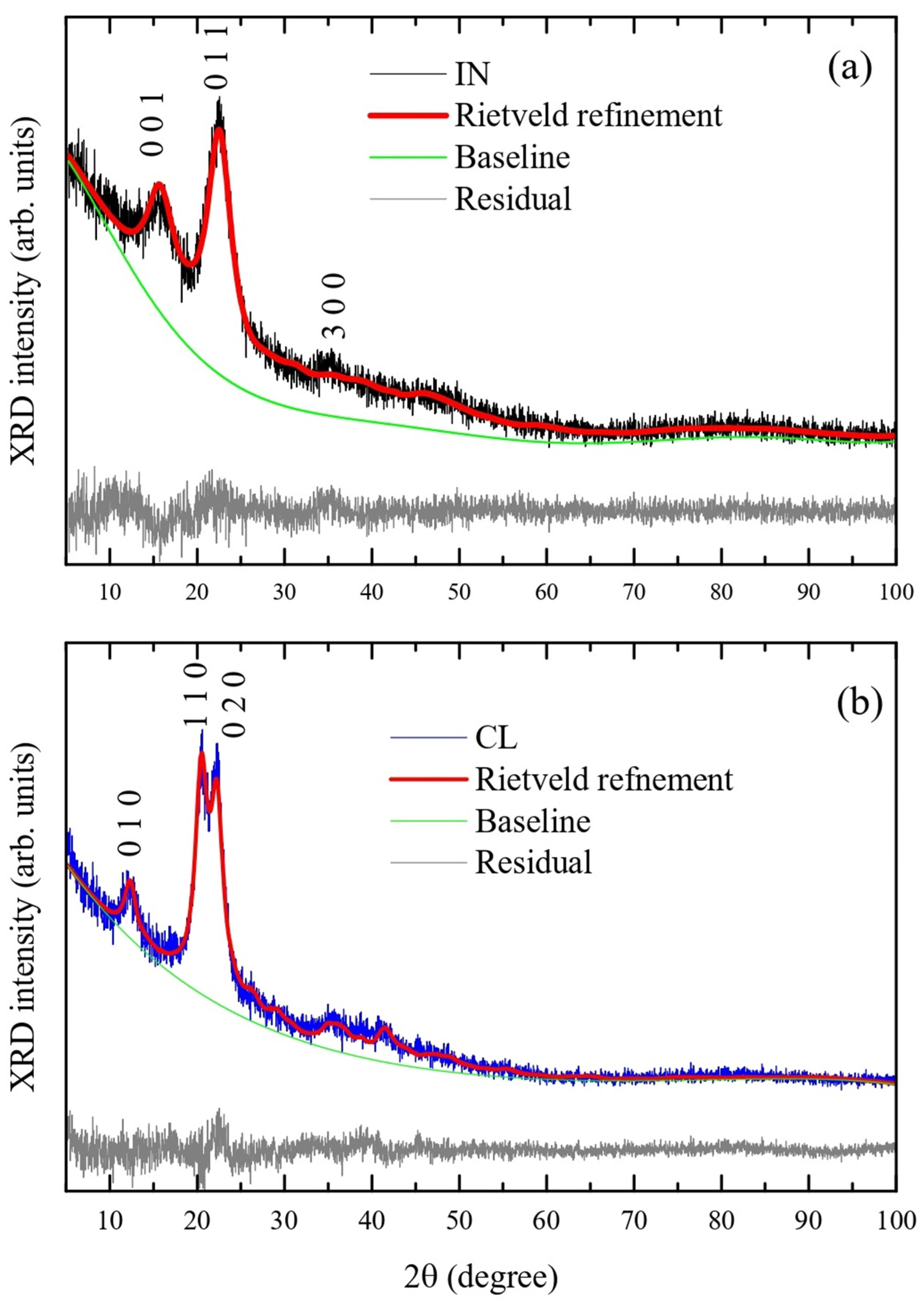



| IN Sample [38] | IN Sample | PU Sample [39] | PU Sample | |
|---|---|---|---|---|
| a (Å) | 10.4 | 10.49 ± 0.06 | 8.10 | 7.87 ± 0.01 |
| b (Å) | 6.717 | 6.74 ± 0.03 | 9.03 | 9.01 ± 0.01 |
| c (Å) | 5.962 | 6.26 ± 0.02 | 10.31 | 10.19 ± 0.02 |
| α (°) | 80.37 | 76.3 ± 0.3 | 90 | 90 |
| β (°) | 118.08 | 112.5 ± 0.4 | 90 | 90 |
| γ (°) | 114.8 | 127.4 ± 0.2 | 117.1 | 117.77 ± 0.07 |
| Volume (Å3) | - | 325 ± 2 | - | 641 ± 2 |
| χ2 | - | 1.104 | - | 1.205 |
| Rwp (%) | - | 0.0862 | - | 0.1091 |
| IN Sample | |||
| 1st Event | 2nd Event | 3rd Event | |
| Temperature range (°C) | 22–100 | 213–363 | 363–526 |
| Mass loss (%) | 9 | 54 | 35 |
| Tmax dTG (°C) | 47 | 314 | 490 |
| DSC effect/temperature (°C) | Endo/50 °C | Exo/343 °C | Exo/500 °C |
| Remaining sample mass = 2.0% | |||
| PU sample | |||
| 1st Event | 2nd Event | 3rd Event | |
| Temperature range (°C) | 20–100 | 235–364 | 364–600 |
| Mass loss (%) | 8.8 | 63.0 | 26.0 |
| Tmax dTG (°C) | 52 | 339 | 529 |
| DSC effect/temperature (°C) | Endo/62 °C | Exo/357 °C | Exo/391 °C |
| Remaining sample mass = 2.2% | |||
| Material | Thermal Conductivity (W/m·K) | References |
|---|---|---|
| O. pyramidale fibers | 0.036 | Current work |
| Cotton | 0.06 | Current work |
| EPS | 0.44 | Current work |
| Cork | 0.23–0.406 | [71] |
| Cellulose | 0.031 | |
| Bamboo | 0.077–0.088 | [51] |
| Corn | 0.101–0.139 | |
| Hemp | 0.039–0.123 | |
| Kenaf | 0.026–0.044 | |
| Sunflower | 0.038–0.05 | |
| Rice husk | 0.048–0.08 | |
| Cotton | 0.058–0.082 | |
| Pineapple | 0.035–0.057 | |
| Wood fiber | 0.038–0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, A.L.F.; Feitosa, B.d.A.; Carolino, A.d.S.; Nunes, R.Z.d.A.; Macalia, C.M.A.; da Silva, K.A.; Dias, C.O.; de Souza, S.M.; Campelo, P.H.; Bezerra, J.d.A.; et al. Extraction and Modification of Cellulose Microfibers Derived from Biomass of the Amazon Ochroma pyramidale Fruit. Micro 2023, 3, 653-670. https://doi.org/10.3390/micro3030046
Rocha ALF, Feitosa BdA, Carolino AdS, Nunes RZdA, Macalia CMA, da Silva KA, Dias CO, de Souza SM, Campelo PH, Bezerra JdA, et al. Extraction and Modification of Cellulose Microfibers Derived from Biomass of the Amazon Ochroma pyramidale Fruit. Micro. 2023; 3(3):653-670. https://doi.org/10.3390/micro3030046
Chicago/Turabian StyleRocha, Ana Luisa Farias, Bianca de Andrade Feitosa, Adriano de Souza Carolino, Ronald Zico de Aguiar Nunes, Célio Matias Airone Macalia, Kalil Araújo da Silva, Cleverton Oliveira Dias, Sérgio Michielon de Souza, Pedro Henrique Campelo, Jaqueline de Araújo Bezerra, and et al. 2023. "Extraction and Modification of Cellulose Microfibers Derived from Biomass of the Amazon Ochroma pyramidale Fruit" Micro 3, no. 3: 653-670. https://doi.org/10.3390/micro3030046
APA StyleRocha, A. L. F., Feitosa, B. d. A., Carolino, A. d. S., Nunes, R. Z. d. A., Macalia, C. M. A., da Silva, K. A., Dias, C. O., de Souza, S. M., Campelo, P. H., Bezerra, J. d. A., & Sanches, E. A. (2023). Extraction and Modification of Cellulose Microfibers Derived from Biomass of the Amazon Ochroma pyramidale Fruit. Micro, 3(3), 653-670. https://doi.org/10.3390/micro3030046







