Aligning Liquid Crystal Materials through Nanoparticles: A Review of Recent Progress
Abstract
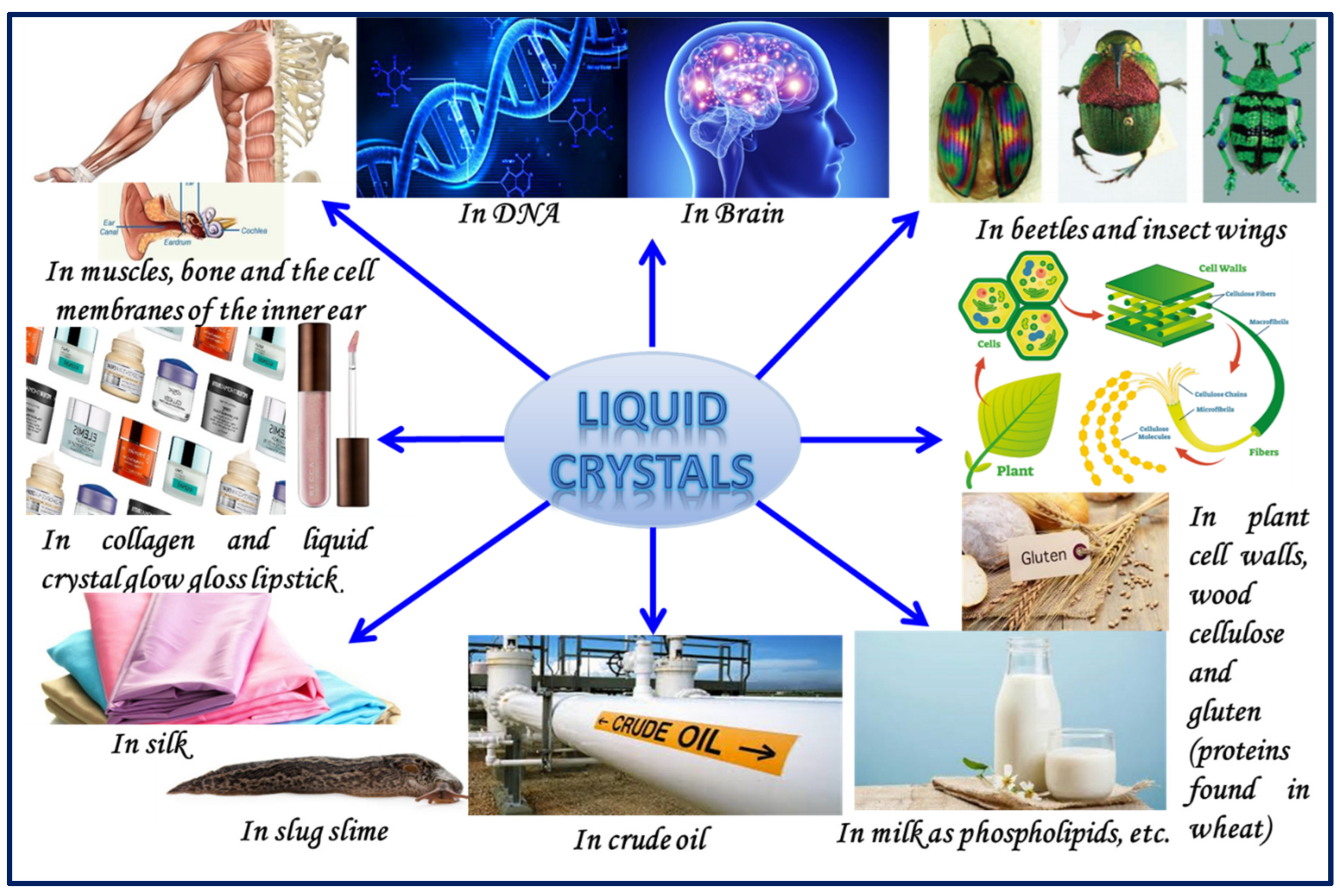
:1. Introduction
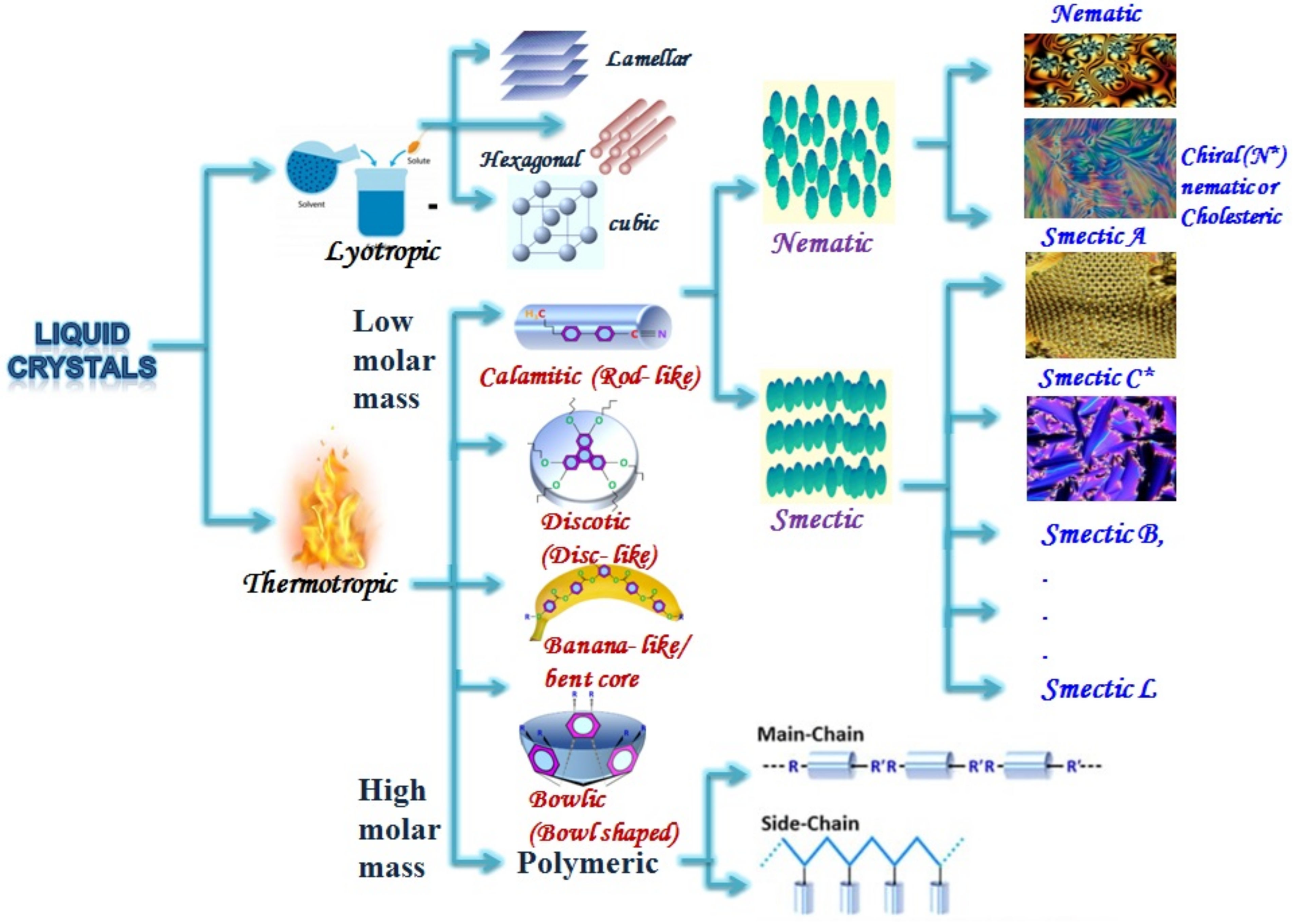
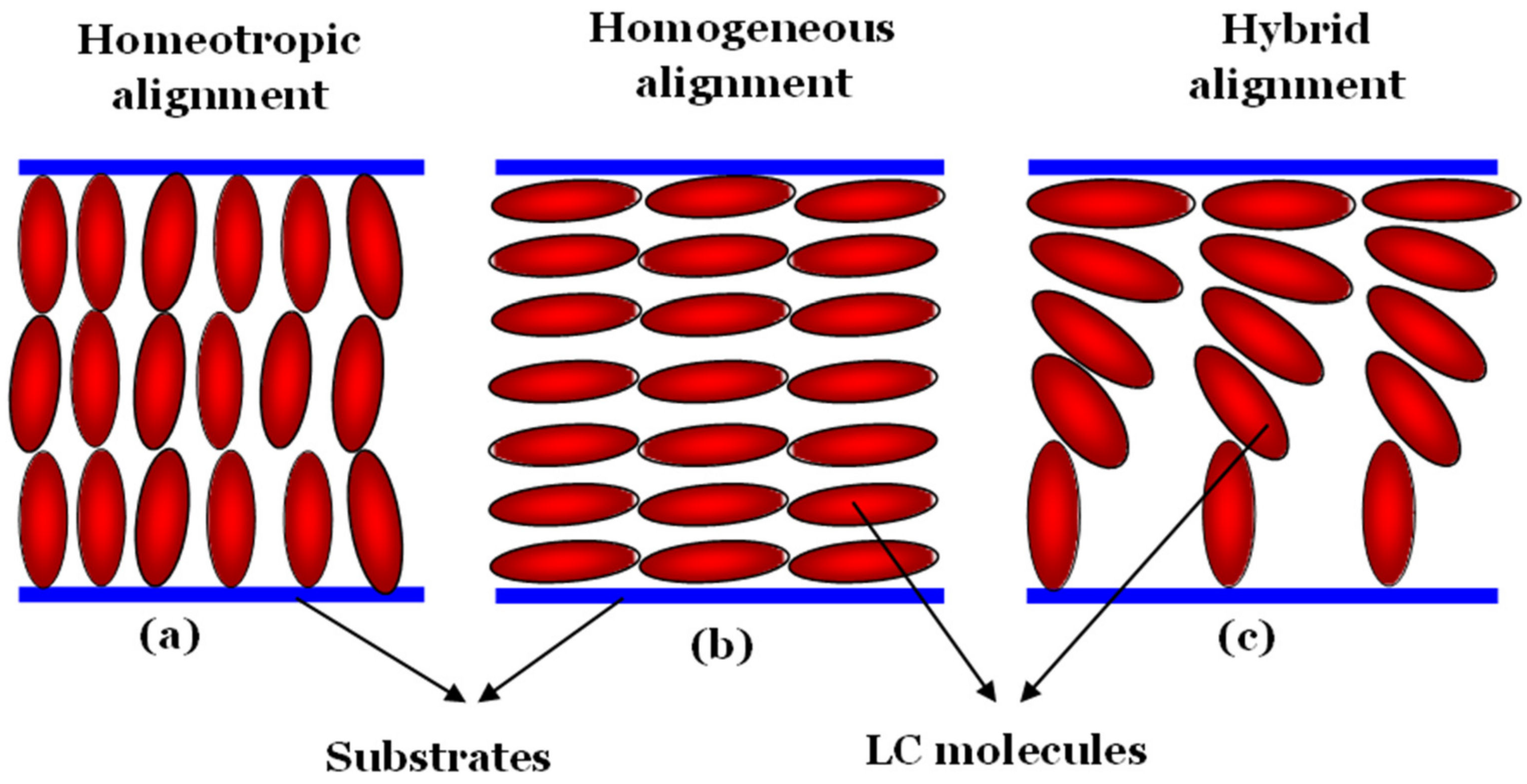
2. Ways to Align Liquid Crystal Materials
3. Nanoparticle-Induced Alignment
4. Development Status
5. Applications of Nanoparticles-Induced Alignment
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prakash, J.; Chandran, A.; Biradar, A.M. Scientific developments of liquid crystal-based optical memory: A review. Rep. Prog. Phys. 2016, 80, 016601. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dunmur, P.D.A. Liquid Crystals; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2002; pp. 1–531. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Prakash, J.; Khan, S.; Chauhan, S.; Biradar, A. Metal oxide-nanoparticles and liquid crystal composites: A review of recent progress. J. Mol. Liq. 2020, 297, 112052. [Google Scholar] [CrossRef]
- Andrienko, D. Introduction to liquid crystals. J. Mol. Liq. 2018, 267, 520–541. [Google Scholar] [CrossRef]
- Kamei, T.; Utsumi, Y.; Moritake, H.; Toda, K.; Suzuki, H. Measurements of the dielectric properties of nematic liquid crystals at 10 kHz to 40 GHz and application to a variable delay line. Electron. Commun. Jpn. 2003, 86, 49–60. [Google Scholar] [CrossRef]
- Naemura, S. Liquid-crystal-material technologies for advanced display applications. J. Soc. Inf. Disp. 2000, 8, 5–9. [Google Scholar] [CrossRef]
- Miniewicz, A.; Gniewek, A.; Parka, J. Liquid crystals for photonic applications. Opt. Mater. 2002, 21, 605–610. [Google Scholar] [CrossRef]
- Wang, L.; Urbas, A.M.; Li, Q. Nature-Inspired Emerging Chiral Liquid Crystal Nanostructures: From Molecular Self-Assembly to DNA Mesophase and Nanocolloids. Adv. Mater. 2018, 32, e1801335. [Google Scholar] [CrossRef]
- Brach, K.; Hatakeyama, A.; Nogues, C.; Olesiak-Banska, J.; Buckle, M.; Matczyszyn, K. Photochemical analysis of structural transitions in DNA liquid crystals reveals differences in spatial structure of DNA molecules organized in liquid crystalline form. Sci. Rep. 2018, 8, 4528. [Google Scholar] [CrossRef]
- Kowerdziej, R.; Parka, J.; Krupka, J.; Olifierczuk, M.; Nowinowski-Kruszelnicki, E.; Jaroszewicz, L.; Chojnowska, O. Dielectric properties of highly anisotropic nematic liquid crystals for tunable microwave components. Appl. Phys. Lett. 2013, 103, 172902. [Google Scholar] [CrossRef]
- Canli, N.Y.; Özdemir, Z.G.; Okutan, M.; Güzeller, D.; Ocak, H.; Eran, B.B. Dielectric Properties of 4-Cyano-4′-pentylbiphenyl (5CB): 4-[4-(S)-2-Methylbutoxybenzoyloxy]benzoic Acid (BAC) Composite. Mol. Cryst. Liq. Cryst. 2015, 623, 17–30. [Google Scholar] [CrossRef]
- Kumar, A.; Varshney, D.; Prakash, J. Role of ionic contribution in dielectric behaviour of a nematic liquid crystal with variable cell thickness. J. Mol. Liq. 2020, 303, 112520. [Google Scholar] [CrossRef]
- Popov, N.; Honaker, L.W.; Popova, M.; Usol’Tseva, N.; Mann, E.K.; Jákli, A.; Popov, P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials 2017, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darla, M.R.; Varghese, S. Synthesis and characterisation of azomethine class thermotropic liquid crystals and their application in nonlinear optics. Liq. Cryst. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Kovshev, E.I.; Blinov, L.M.; Titov, V.V. Thermotropic Liquid Crystals and Their Applications. Russ. Chem. Rev. 1977, 46, 395–419. [Google Scholar] [CrossRef]
- Bunjes, H.; Rades, T. Thermotropic liquid crystalline drugs. J. Pharm. Pharmacol. 2005, 57, 807–816. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jahn, A.; Cho, S.-J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2014, 45, 1–11. [Google Scholar] [CrossRef]
- Garti, N.; Libster, D.; Aserin, A. Lipid polymorphism in lyotropic liquid crystals for triggered release of bioactives. Food Funct. 2012, 3, 700–713. [Google Scholar] [CrossRef]
- Forrest, B.J.; Reeves, L.W. New lyotropic liquid crystals composed of finite nonspherical micelles. Chem. Rev. 1981, 81, 1–14. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: A new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef] [PubMed]
- Boden, N.; Bushby, R.J.; Clements, J.; Movaghar, B.; Donovan, K.J.; Kreouzis, T. Mechanism of charge transport in discotic liquid crystals. Phys. Rev. B 1995, 52, 13274–13280. [Google Scholar] [CrossRef] [PubMed]
- Bushby, R.J.; Kawata, K. Liquid crystals that affected the world: Discotic liquid crystals. Liq. Cryst. 2011, 38, 1415–1426. [Google Scholar] [CrossRef]
- Ros, M.B.; Serrano, J.L.; de la Fuente, M.R.; Folcia, C.L. Banana-shaped liquid crystals: A new field to explore. J. Mater. Chem. 2005, 15, 5093–5098. [Google Scholar] [CrossRef]
- De Almeida, R.R.; Zhang, C.; Parri, O.; Sprunt, S.; Jakli, A. Nanostructure and dielectric properties of a twist-bend nematic liquid crystal mixture. Liq. Cryst. 2014, 41, 1661–1667. [Google Scholar] [CrossRef]
- Kim, Y.B.; Hur, I.K. High speed response time of nematic liquid crystal mixtures for LCD monitor and TV applications. J. Inf. Disp. 2001, 2, 32–38. [Google Scholar] [CrossRef]
- Yazdanpanahi, M.; Bulja, S.; Mirshekar-Syahkal, D.; James, R.; Day, S.E.; Fernandez, F.A. Measurement of Dielectric Constants of Nematic Liquid Crystals at mm-Wave Frequencies Using Patch Resonator. IEEE Trans. Instrum. Meas. 2010, 59, 3079–3085. [Google Scholar] [CrossRef]
- Varshney, D.; Kumar, A.; Prakash, J.; Meena, R.; Asokan, K. Gamma irradiation induced dielectric modulation and dynamic memory in nematic liquid crystal materials. J. Mol. Liq. 2020, 320, 114374. [Google Scholar] [CrossRef]
- Dierking, I.; Mitov, M.; Osipov, M.A. Smectic layer instabilities in liquid crystals. Soft Matter 2014, 11, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Bohley, C.; Stannarius, R. Inclusions in free standing smectic liquid crystal films. Soft Matter 2008, 4, 683–702. [Google Scholar] [CrossRef]
- Langer, S.A.; Sethna, J.P. Textures in a chiral smectic liquid-crystal film. Phys. Rev. A 1986, 34, 5035–5046. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Bishnoi, D.D.; Soni, S. Ramswroop liquid crystals and applications of chlosteric liquid crystal in laser. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 736–740. [Google Scholar] [CrossRef]
- Kim, Y.; Wada, M.; Tamaoki, N. Dicholesteryl icosanedioate as a glass-forming cholesteric liquid crystal: Properties, additive effects and application in color recording. J. Mater. Chem. C 2014, 2, 1921–1926. [Google Scholar] [CrossRef]
- Ryabchun, A.; Bobrovsky, A. Cholesteric Liquid Crystal Materials for Tunable Diffractive Optics. Adv. Opt. Mater. 2018, 6, 1–20. [Google Scholar] [CrossRef]
- Coates, D. Development and applications of cholesteric liquid crystals. Liq. Cryst. 2015, 42, 1–13. [Google Scholar] [CrossRef]
- Salamończyk, M.; Vaupotič, N.; Pociecha, D.; Walker, R.; Storey, J.; Imrie, C.T.; Wang, C.; Zhu, C.; Gorecka, E. Multi-level chirality in liquid crystals formed by achiral molecules. Nat. Commun. 2019, 10, 1922. [Google Scholar] [CrossRef]
- Tournilhac, F.; Blinov, L.M.; Simon, J.; Yablonsky, S.V. Ferroelectric liquid crystals from achiral molecules. Nature 1992, 359, 621–623. [Google Scholar] [CrossRef]
- Skarp, K.; Handschy, M. Ferroelectric Liquid Crystals. Material Properties and Applications. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1988, 165, 439–509. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F. Current Topics in Smectic Liquid Crystal Research. ChemPhysChem 2006, 7, 20–45. [Google Scholar] [CrossRef]
- Das, M.K.; Barman, B.; Das, B.; Hamplová, V.; Bubnov, A. Dielectric Properties of Chiral Ferroelectric Liquid Crystalline Compounds with Three Aromatic Rings Connected by Ester Groups. Crystals 2019, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Ważyńska, B.; Tykarska, M.; Okowiak-Chinalska, J. New Application of Thermotropic Liquid Crystals. Mol. Cryst. Liq. Cryst. 2011, 542, 213–220. [Google Scholar] [CrossRef]
- Castellano, J.A. Liquid Crystal Display Applications: Past, Present & Future. Liq. Cryst. Today 1991, 1, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Gauza, S.; Wen, C.-H.; Wu, B.; Wu, S.-T.; Spadlo, A.; Dąbrowski, R. Fast-response nematic liquid-crystal mixtures. J. Soc. Inf. Disp. 2006, 14, 241–246. [Google Scholar] [CrossRef]
- Tomilin, M.G.; Povzun, S.A.; Kurmashev, A.F.; Gribanova, E.V.; Efimova, T.A. The application of nematic liquid crystals for objective microscopic diagnosis of cancer. Liq. Cryst. Today 2001, 10, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Chilaya, G.; Chanishvili, A.; Petriashvili, G.; Barberi, R.; De Santo, M.P.; Matranga, M.A. Different Approaches of Employing Cholesteric Liquid Crystals in Dye Lasers. Mater. Sci. Appl. 2011, 2, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Manabe, T.; Sonoyama, K.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Toward practical application of cholesteric liquid crystals to tunable lasers. J. Mater. Chem. 2008, 18, 3040–3043. [Google Scholar] [CrossRef]
- Uchimura, M.; Watanabe, Y.; Araoka, F.; Watanabe, J.; Takezoe, H.; Konishi, G.-I. Development of Laser Dyes to Realize Low Threshold in Dye-Doped Cholesteric Liquid Crystal Lasers. Adv. Mater. 2010, 22, 4473–4478. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Dierking, I.; Neto, A.M.F. Novel Trends in Lyotropic Liquid Crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. BioMed Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef]
- Sonin, A.S.; Churochkina, N.A.; Kaznacheev, A.V.; Golovanov, A.V. Liquid Crystals of Clay Dispersions. Colloid J. 2018, 80, 593–614. [Google Scholar] [CrossRef]
- Kumar, H.; Sureshkumar, A.; Badduri, N.; Jain, V. A Review on Lyotropic Liquid Crystals and Its Potential Applications. Nanosci. Nanotechnol. Asia 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Marzal, V.; Caño, M.; Torres, J.C.; Quintana, X.; Pérez, I.; Garcia-Camara, B. Electrical Behavior of Liquid Crystal Devices with Dielectric Nanoparticles. J. Nanomater. 2020, 2020, 4515432. [Google Scholar] [CrossRef]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef] [Green Version]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2010, 40, 306–319. [Google Scholar] [CrossRef]
- Hegmann, T.; Qi, H.; Marx, V.M. Nanoparticles in liquid crystals:synthesis, self-assembly, defect formation and potential applications. J. Inorg. Organomet. Polym. 2007, 17, 483–508. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef] [Green Version]
- Scolari, L.; Gauza, S.; Xianyu, H.; Zhai, L.; Eskildsen, L.; Alkeskjold, T.T.; Wu, S.-T.; Bjarklev, A. Frequency tunability of solid-core photonic crystal fibers filled with nanoparticle-doped liquid crystals. Opt. Express 2009, 17, 3754–3764. [Google Scholar] [CrossRef] [Green Version]
- Gdovinová, V.; Tomašovičová, N.; Jeng, S.-C.; Zakutanská, K.; Kula, P.; Kopčanský, P. Memory effect in nematic phase of liquid crystal doped with magnetic and non-magnetic nanoparticles. J. Mol. Liq. 2019, 282, 286–291. [Google Scholar] [CrossRef]
- Budaszewski, D.; Siarkowska, A.; Chychłowski, M.; Jankiewicz, B.; Bartosewicz, B.; Dąbrowski, R.; Woliński, T.R. Nanoparticles-enhanced photonic liquid crystal fibers. J. Mol. Liq. 2018, 267, 271–278. [Google Scholar] [CrossRef]
- Siarkowska, A.; Chychłowski, M.; Budaszewski, D.; Jankiewicz, B.; Bartosewicz, B.; Wolinski, T.R. Thermo- and electro-optical properties of photonic liquid crystal fibers doped with gold nanoparticles. Beilstein J. Nanotechnol. 2017, 8, 2790–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodarte, A.L.; Nuno, Z.S.; Cao, B.H.; Pandolfi, R.J.; Quint, M.T.; Ghosh, S.; Hein, J.E.; Hirst, L.S. Tuning Quantum-Dot Organization in Liquid Crystals for Robust Photonic Applications. ChemPhysChem 2014, 15, 1413–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Sagar, L.K. CdSe quantum dots in a columnar matrix. Chem. Commun. 2011, 47, 12182–12184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Gupta, R.K.; Kumar, S.; Manjuladevi, V. Electro-Optic and Dielectric Studies on Quantum Dot Doped Nematic Liquid Crystal. Macromol. Symp. 2015, 357, 47–51. [Google Scholar] [CrossRef]
- Kurochkina, M.A.; Shcherbinin, D.P.; Konshina, E.A. Control of photoluminescence of CdSe/ZnS quantum dots in a nematic liquid crystal by an electric field. Opt. Spectrosc. 2015, 119, 812–815. [Google Scholar] [CrossRef]
- Yaroshchuk, O.; Kravchuk, R.; Dobrovolskyy, A.; Qiu, L.; Lavrentovich, O.D. Planar and tilted uniform alignment of liquid crystals by plasma-treated substrates. Liq. Cryst. 2004, 31, 859–869. [Google Scholar] [CrossRef]
- Nobles, J.E.; Melnyk, O.; Glushchenko, A.; Camley, R.E.; Celinski, Z. Effect of alignment methods on liquid crystal performance in millimeter wave devices. Eng. Res. Express 2020, 2, 025002. [Google Scholar] [CrossRef]
- Ishihara, S.; Mizusaki, M. Alignment control technology of liquid crystal molecules. J. Soc. Inf. Disp. 2019, 28, 44–74. [Google Scholar] [CrossRef]
- Kahn, F.; Taylor, G.; Schonhorn, H. Surface-produced alignment of liquid crystals. Proc. IEEE 1973, 61, 823–828. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, K.; Uchida, T. Relationship between Rubbing Strength and Surface Anchoring of Nematic Liquid Crystal. Jpn. J. Appl. Phys. 1992, 31, L579–L581. [Google Scholar] [CrossRef]
- Yokoyama, H. Surface Anchoring of Nematic Liquid Crystals. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1988, 165, 265–316. [Google Scholar] [CrossRef]
- Weng, L.; Liao, P.-C.; Lin, C.-C.; Ting, T.-L.; Hsu, W.-H.; Su, J.-J.; Chien, L.-C. Anchoring energy enhancement and pretilt angle control of liquid crystal alignment on polymerized surfaces. AIP Adv. 2015, 5, 097218. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Deng, H.; Wei, Y. Alignment of liquid crystals on ultrathin organized molecular films. Supramol. Sci. 1998, 5, 649–655. [Google Scholar] [CrossRef]
- Lu, M.; Yang, K.H.; Nakasogi, T.; Chey, S.J. 29.4: Homeotropic Alignment by Single Oblique Evaporation of SiO 2 and Its Application to High Resolution Microdisplays. SID Symp. Dig. Tech. Pap. 2000, 31, 446–449. [Google Scholar] [CrossRef]
- Yamahara, M.; Nakamura, M.; Koide, N.; Sasaki, T. Influence of rubbing conditions of polyimide alignment layer on optical anisotropy of immobilized liquid crystal film. Liq. Cryst. 2007, 34, 381–387. [Google Scholar] [CrossRef]
- Stöhr, J.; Samant, M. Liquid crystal alignment by rubbed polymer surfaces: A microscopic bond orientation model. J. Electron Spectrosc. Relat. Phenom. 1999, 98–99, 189–207. [Google Scholar] [CrossRef]
- Chen, C.; Bos, P.J.; Kim, J.; Li, Q.; Anderson, J.E. Improved liquid crystals for vertical alignment applications. J. Appl. Phys. 2006, 99, 123523. [Google Scholar] [CrossRef]
- Seo, D.; Kobayashi, S. Effect of high pretilt angle for anchoring strength in nematic liquid crystal on rubbed polyimide surface containing trifluoromethyl moieties. Appl. Phys. Lett. 1995, 66, 1202–1204. [Google Scholar] [CrossRef]
- Jeong, E.; Lim, Y.J.; Rhee, J.M.; Lee, S.H.; Lee, G.-D.; Park, K.H.; Choi, H.C. Viewing angle switching of vertical alignment liquid crystal displays by controlling birefringence of homogenously aligned liquid crystal layer. Appl. Phys. Lett. 2007, 90, 051116. [Google Scholar] [CrossRef]
- Hong, H.K.; Yoon, J.K.; Lim, M. Analysis of the dependence of optical response time of liquid crystal displays on the viewing direction. J. Soc. Inf. Disp. 2008, 16, 1063–1068. [Google Scholar] [CrossRef]
- Wu, S.-T. Phase retardation dependent optical response time of parallel-aligned liquid crystals. J. Appl. Phys. 1986, 60, 1836–1838. [Google Scholar] [CrossRef]
- Kundu, S.; Akimoto, M.; Hirayama, I.; Inoue, M.; Kobayashi, S.; Takatoh, K. Enhancement of Contrast Ratio by Using Ferroelectric Nanoparticles in the Alignment Layer of Liquid Crystal Display. Jpn. J. Appl. Phys. 2008, 47, 4751–4754. [Google Scholar] [CrossRef]
- Oh, S.-W.; Park, J.-H.; Yoon, T.-H. Near-zero pretilt alignment of liquid crystals using polyimide films doped with UV-curable polymer. Opt. Express 2015, 23, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yokoyama, H.; Gwag, J.S. Determination of surface nematic liquid crystal anchoring strength using nano-scale surface grooves. Opt. Express 2013, 21, 12135–12144. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.A. Surface Anchoring of Liquid Crystal Molecules on Various Substrates. Mol. Cryst. Liq. Cryst. 1983, 94, 33–41. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, F.K.; Tsui, O.K.C.; Sheng, P. Liquid Crystal Orientation Transition on Microtextured Substrates. Phys. Rev. Lett. 2003, 91, 215501. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.-S. Effect of pretilt angle on the polar anchoring energy in NLCs on weakly rubbed polyimide surfaces. Liq. Cryst. 1999, 26, 1615–1619. [Google Scholar] [CrossRef]
- Yokoyama, H.; van Sprang, H.A. A novel method for determining the anchoring energy function at a nematic liquid crystal-wall interface from director distortions at high fields. J. Appl. Phys. 1985, 57, 4520–4526. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Wang, C.-C.; Fuh, A.Y.-G. Controlling pre-tilt angles of liquid crystal using mixed polyimide alignment layer. Opt. Express 2008, 16, 17131–17137. [Google Scholar] [CrossRef]
- Varghese, S.; Crawford, G.P.; Bastiaansen, C.W.M.; de Boer, D.K.G.; Broer, D.J. Microrubbing technique to produce high pretilt multidomain liquid crystal alignment. Appl. Phys. Lett. 2004, 85, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.-R.; Chae, B.; Gil, E.K.; Bae, J.; Lee, S.W. Comparison of Liquid Crystal Alignments on Rubbed and Linearly Polarized UV-Irradiated Polyimide Surfaces. Mol. Cryst. Liq. Cryst. 2012, 563, 10–18. [Google Scholar] [CrossRef]
- Van Aerle, N.A.J.M.; Tol, A.J.W. Molecular Orientation in Rubbed Polyimide Alignment Layers Used for Liquid-Crystal Displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar] [CrossRef]
- Ishihara, S.; Wakemoto, H.; Nakazima, K.; Matsuo, Y. The effect of rubbed polymer films on the liquid crystal alignment. Liq. Cryst. 1989, 4, 669–675. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhuang, Z.; Patel, J.S. Effect of multidirection rubbing on the alignment of nematic liquid crystal. Appl. Phys. Lett. 2000, 77, 513–515. [Google Scholar] [CrossRef]
- Stôhr, J.; Samant, M.G.; Cossy-Favre, A.; Diaz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar] [CrossRef]
- Seo, D.; Kobayashi, S.; Mochizuki, A. Generation of the pretilt angles in nematic liquid crystal (5CB) aligned on the rubbed polypyrrole films. Appl. Phys. Lett. 1992, 60, 1025–1026. [Google Scholar] [CrossRef]
- Hatoh, H.; Yamamoto, T.; Morizumi, Y.; Okamoto, M.; Kubo, A. Dependence of pretilt angle on the topography of substrate in liquid crystal alignment brought about by rubbing technique. Appl. Phys. Lett. 1994, 64, 1103–1104. [Google Scholar] [CrossRef]
- Mosley, A.; Nicholas, B.; Gass, P. Surface alignment of liquid crystals by rubbed polymer layers. Displays 1987, 8, 17–21. [Google Scholar] [CrossRef]
- Janning, J.L. Thin film surface orientation for liquid crystals. Appl. Phys. Lett. 1972, 21, 173–174. [Google Scholar] [CrossRef]
- Johnson, M.; Penz, P. Low-tilt-angle nematic alignment compatible with frit sealing. IEEE Trans. Electron Devices 1977, 24, 805–807. [Google Scholar] [CrossRef]
- Urbach, W.; Boix, M.; Guyon, E. Alignment of nematics and smectics on evaporated films. Appl. Phys. Lett. 1974, 25, 479–481. [Google Scholar] [CrossRef]
- Armitage, D. Alignment of liquid crystals on obliquely evaporated silicon oxide films. J. Appl. Phys. 1980, 51, 2552. [Google Scholar] [CrossRef]
- Armitage, D. Ferroelectric liquid crystal alignment by oblique evaporation of SiO2. Ferroelectrics 1991, 122, 239–252. [Google Scholar] [CrossRef]
- Son, P.K.; Park, J.H.; Kim, J.C.; Yoon, T.-H. Control of liquid crystal alignment by deposition of silicon oxide thin film. Thin Solid Films 2006, 515, 3102–3106. [Google Scholar] [CrossRef]
- Oton, E.; Andres, S.L.; Bennis, N.; Oton, J.M.; Geday, M.A. Silicon oxides as alignment surfaces for vertically- aligned nematics in photonic devices. Opto-Electron. Rev. 2014, 22, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Heffner, W.R.; Berreman, D.W.; Sammon, M.; Meiboom, S. Liquid crystal alignment on surfactant treated obliquely evaporated surfaces. Appl. Phys. Lett. 1980, 36, 144–146. [Google Scholar] [CrossRef]
- Kaho, S.; Masumi, T.; Tahata, S.; Mizunuma, M.; Miyake, S. Alignment and Electro-Optic Properties of SSFLC Cells Aligned by Obliquely Evaporated SiO Films. Mol. Cryst. Liq. Cryst. 1991, 199, 87–95. [Google Scholar] [CrossRef]
- Yasuda, A.; Nito, K.; Matsui, E. Time-resolved FT-IR study of ferroelectric liquid crystals with SiO obliquely evaporated alignment layers. Liq. Cryst. 1993, 14, 1725–1734. [Google Scholar] [CrossRef]
- Liou, W.-R.; Chen, C.-Y.; Ho, J.-J.; Hsu, C.-K.; Chang, C.-C.; Hsiao, R.Y.; Chang, S.-H. An improved alignment layer grown by oblique evaporation for liquid crystal devices. Displays 2006, 27, 69–72. [Google Scholar] [CrossRef]
- Goodman, L.; McGinn, J.; Anderson, C.; DiGeronimo, F. Topography of obliquely evaporated silicon oxide films and its effect on liquid-crystal orientation. IEEE Trans. Electron Devices 1977, 24, 795–804. [Google Scholar] [CrossRef]
- Yamashita, M.; Amemiya, Y. Effect of Substrate Surface on Alignment of Liquid Crystal Molecules. Jpn. J. Appl. Phys. 1976, 15, 2087–2092. [Google Scholar] [CrossRef]
- Seki, T. New strategies and implications for the photoalignment of liquid crystalline polymers. Polym. J. 2014, 46, 751–768. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef] [PubMed]
- Yaroshchuk, O.; Reznikov, Y. Photoalignment of liquid crystals: Basics and current trends. J. Mater. Chem. 2012, 22, 286–300. [Google Scholar] [CrossRef]
- Ichimura, K.; Suzuki, Y.; Seki, T.; Hosoki, A.; Aoki, K. Reversible change in alignment mode of nematic liquid crystals regulated photochemically by command surfaces modified with an azobenzene monolayer. Langmuir 1988, 4, 1214–1216. [Google Scholar] [CrossRef]
- Fan, F.; Srivastava, A.K.; Du, T.; Tseng, M.C.; Chigrinov, V.; Kwok, H.S. Low voltage tunable liquid crystal lens. Opt. Lett. 2013, 38, 4116–4119. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Tseng, M.-C.; Srivastava, A.K.; Chigrinov, V.G.; Kwok, H.S. P-156: One Step Stabilized Azo Dye Photoalignment for Mass Production. SID Symp. Dig. Tech. Pap. 2017, 48, 1869–1872. [Google Scholar] [CrossRef]
- Chigrinov, V.; Muravski, A.; Kwok, H.S.; Takada, H.; Akiyama, H.; Takatsu, H. Anchoring properties of photoaligned azo-dye materials. Phys. Rev. E 2003, 68, 061702. [Google Scholar] [CrossRef] [Green Version]
- Barabanova, N.N.; Belyaev, V.V.; Bogdanov, D.L.; Bugrimov, A.L.; Chigrinov, V.G.; Dadivanyan, A.K.; Rodionova, Y.A. Distribution of Dye and Mesogen Molecules Orientation in Photoaligning Layer vs. Aperture of Polarized Light Beam. Mol. Cryst. Liq. Cryst. 2014, 596, 76–81. [Google Scholar] [CrossRef]
- Sheremet, N.; Kurioz, Y.; Senenko, A.; Trunov, M.; Reznikov, Y. Photoalignment in the isotropic phase of liquid crystal on chalcogenide glass film. Liq. Cryst. 2014, 42, 81–86. [Google Scholar] [CrossRef]
- Park, S.-K.; Jung, U.-S.; Kwon, S.-B.; Yi, M.; Ahn, T.; Kim, J.-S.; Kurioz, Y.; Reznikov, Y. Photoalignment of nematic liquid crystal on polyamic-acid-based soluble polyimide with no side fragments. J. Soc. Inf. Disp. 2010, 18, 199–205. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Hwang, J.-Y.; Seo, D.-S.; Nam, S.-H.; Han, J.-I. Control of High Pretilt Angle for Nematic Liquid Crystal on Homeotropic Alignment Layer by In-situ Photoalignment Method. Mol. Cryst. Liq. Cryst. 2004, 412, 269–275. [Google Scholar] [CrossRef]
- Seo, D.-S.; Kim, H.-K. Generation of Pretilt Angle for Nematic Liquid Crystal Using an In Situ Photoalignment Method on Polymer Surfaces. Jpn. J. Appl. Phys. 2000, 39, L993–L995. [Google Scholar] [CrossRef]
- Masaki, H. Photoalignment of nematic liquid crystal by polystyrene exposed to linearly polarized deep UV light. Jpn. J. Appl. Phys. 1999, 38, 255–257. [Google Scholar] [CrossRef]
- Gvozdovskyy, I.; Kurioz, Y.; Reznikov, Y. Exposure and temperature dependences of contact angle of liquid crystals on photoaligning surface. Opto-Electron. Rev. 2009, 17, 116–119. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Photoinduced surface alignment for liquid crystal displays. J. Phys. D Appl. Phys. 2000, 33, R67–R84. [Google Scholar] [CrossRef]
- Studer, P.; Bachels, T. Photoinduced Surface Alignment for Optical Thin Films and Liquid Crystal Displays. CHIMIA 2007, 61, 635–637. [Google Scholar] [CrossRef]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Ikeda, T.; Horiuchi, S.; Karanjit, D.B.; Kurihara, S.; Tazuke, S. Photochemical Image Storage in Polymer Liquid Crystals. Chem. Lett. 1988, 17, 1679–1682. [Google Scholar] [CrossRef]
- Akiyama, H.; Yoshida, M. Photochemically Reversible Liquefaction and Solidification of Single Compounds Based on a Sugar Alcohol Scaffold with Multi Azo-Arms. Adv. Mater. 2012, 24, 2353–2356. [Google Scholar] [CrossRef]
- Sato, M.; Nagano, S.; Seki, T. A photoresponsive liquid crystal based on (1-cyclohexenyl)phenyldiazene as a close analogue of azobenzene. Chem. Commun. 2009, 25, 3792–3794. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.-C.; Kuo, C.-W.; Wang, H.-L.; Liao, C.-C. Nanoparticles-induced vertical alignment in liquid crystal cell. Appl. Phys. Lett. 2007, 91, 061112. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Jeng, S.-C.; Wang, H.-L.; Liao, C.-C. Application of nanoparticle-induced vertical alignment in hybrid-aligned nematic liquid crystal cell. Appl. Phys. Lett. 2007, 91, 141103. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Jeng, S.-C.; Hsieh, I.-M. Nanoparticle-doped polyimide for controlling the pretilt angle of liquid crystals devices. Opt. Express 2010, 18, 16507–16512. [Google Scholar] [CrossRef]
- Qi, H.; Hegmann, T. Multiple Alignment Modes for Nematic Liquid Crystals Doped with Alkylthiol-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2009, 1, 1731–1738. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Jeng, S.-C.; Yang, C.-Y.; Kuo, C.-W.; Liao, C.-C. Characteristics of nanoparticle-doped homeotropic liquid crystal devices. J. Phys. D Appl. Phys. 2009, 42, 025102. [Google Scholar] [CrossRef]
- Vardanyan, K.K.; Daykin, A.; Kilmer, B. Study on cyanobiphenyl nematic doped by silver nanoparticles. Liq. Cryst. 2016, 44, 1240–1252. [Google Scholar] [CrossRef]
- Maleki, A.; Ara, M.M.; Saboohi, F. Dielectric properties of nematic liquid crystal doped with Fe3O4 nanoparticles. Phase Transit. 2016, 90, 371–379. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Misra, A.K.; Manohar, S.; Gupta, S.K.; Manohar, R. Improved dielectric and electro-optical parameters of ZnO nano-particle (8% Cu2+) doped nematic liquid crystal. J. Mol. Struct. 2013, 1035, 371–377. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, W.; Cui, X.; Tian, Y.; Guo, L.; Yang, H. Alignment of Liquid Crystals Doped with Nickel Nanoparticles Containing Different Morphologies. Adv. Mater. 2011, 23, 5779–5784. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Hegmann, T. Effects of functionalized metal and semiconductor nanoparticles in nematic liquid crystal phases. In Emerging Liquid Crystal Technologies III; SPIE: Bellingham, WA, USA, 2008; Volume 6911, pp. 1–11. [Google Scholar] [CrossRef]
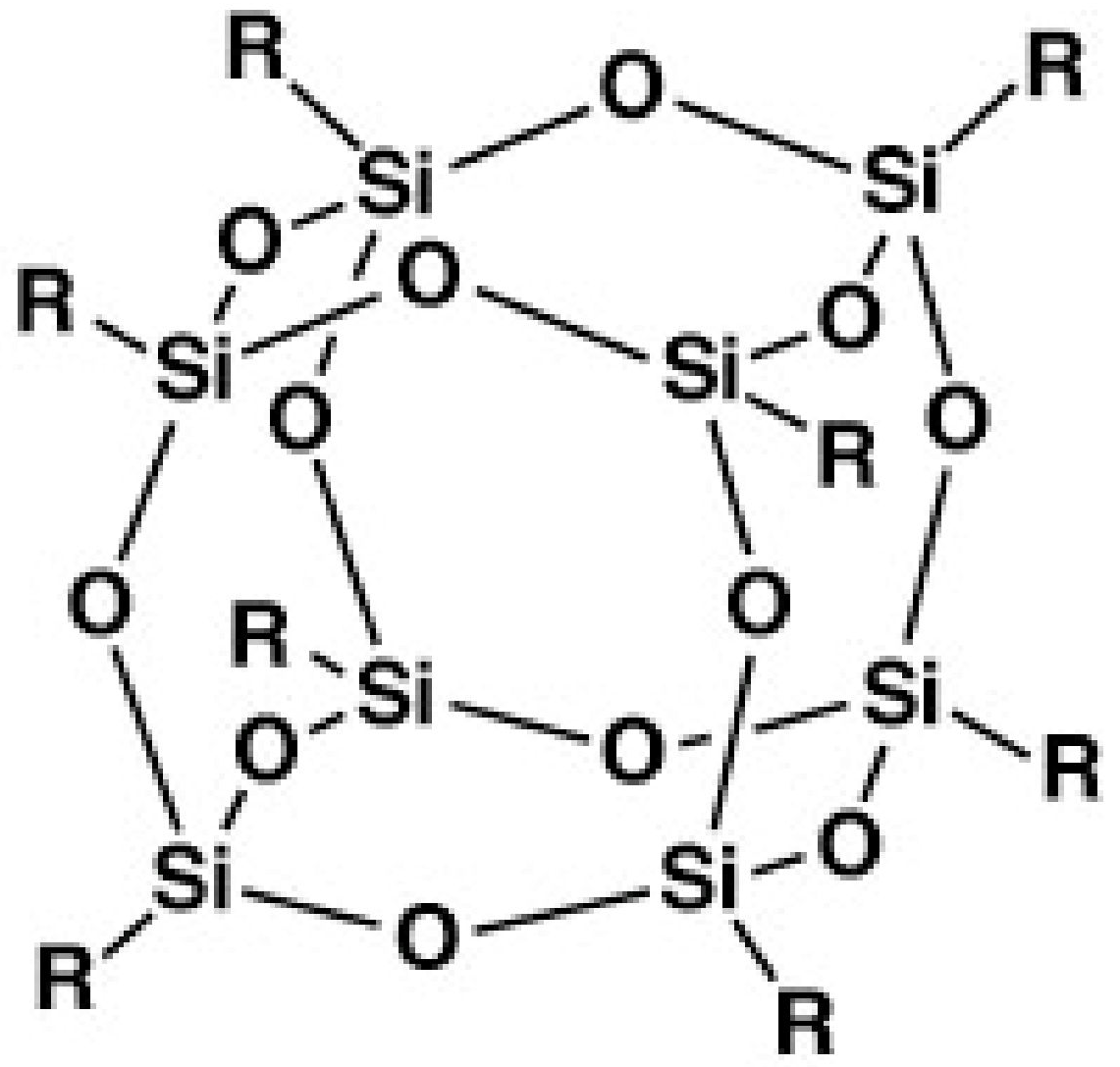
- Rabby, R.E.; Jeelani, S.; Rangari, V.K. Structural Analysis of Polyhedral Oligomeric Silsesquioxane Coated SiC Nanoparticles and Their Applications in Thermoset Polymers. J. Nanomater. 2015, 2015, 446. [Google Scholar] [CrossRef] [Green Version]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
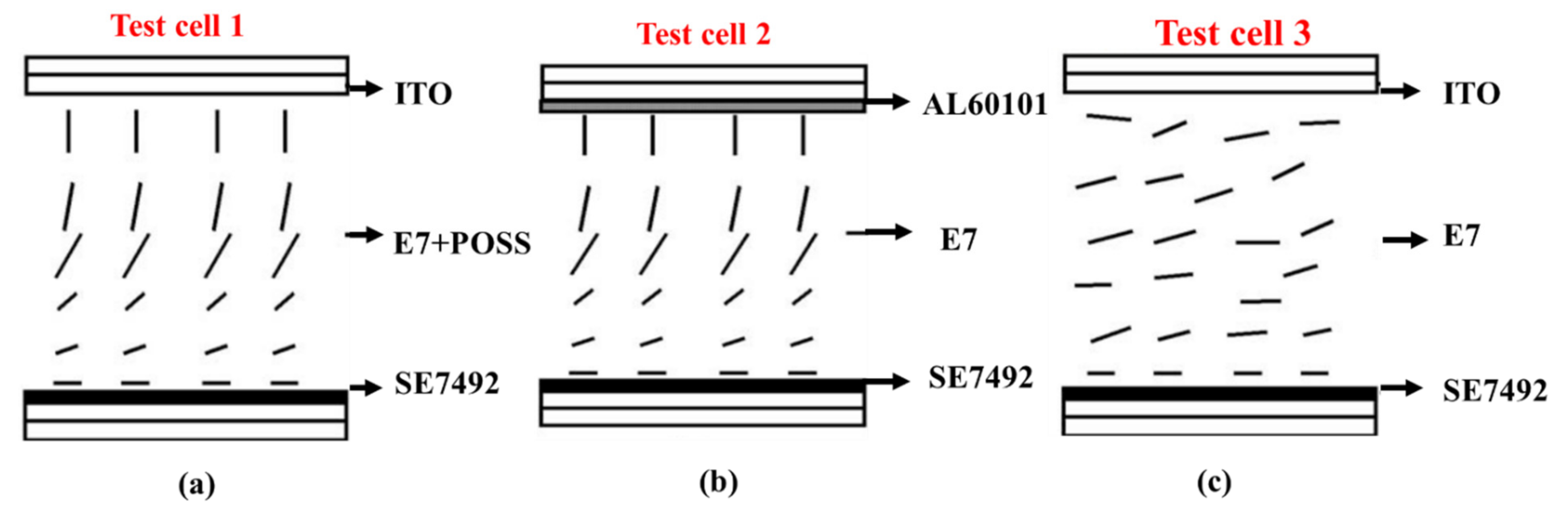
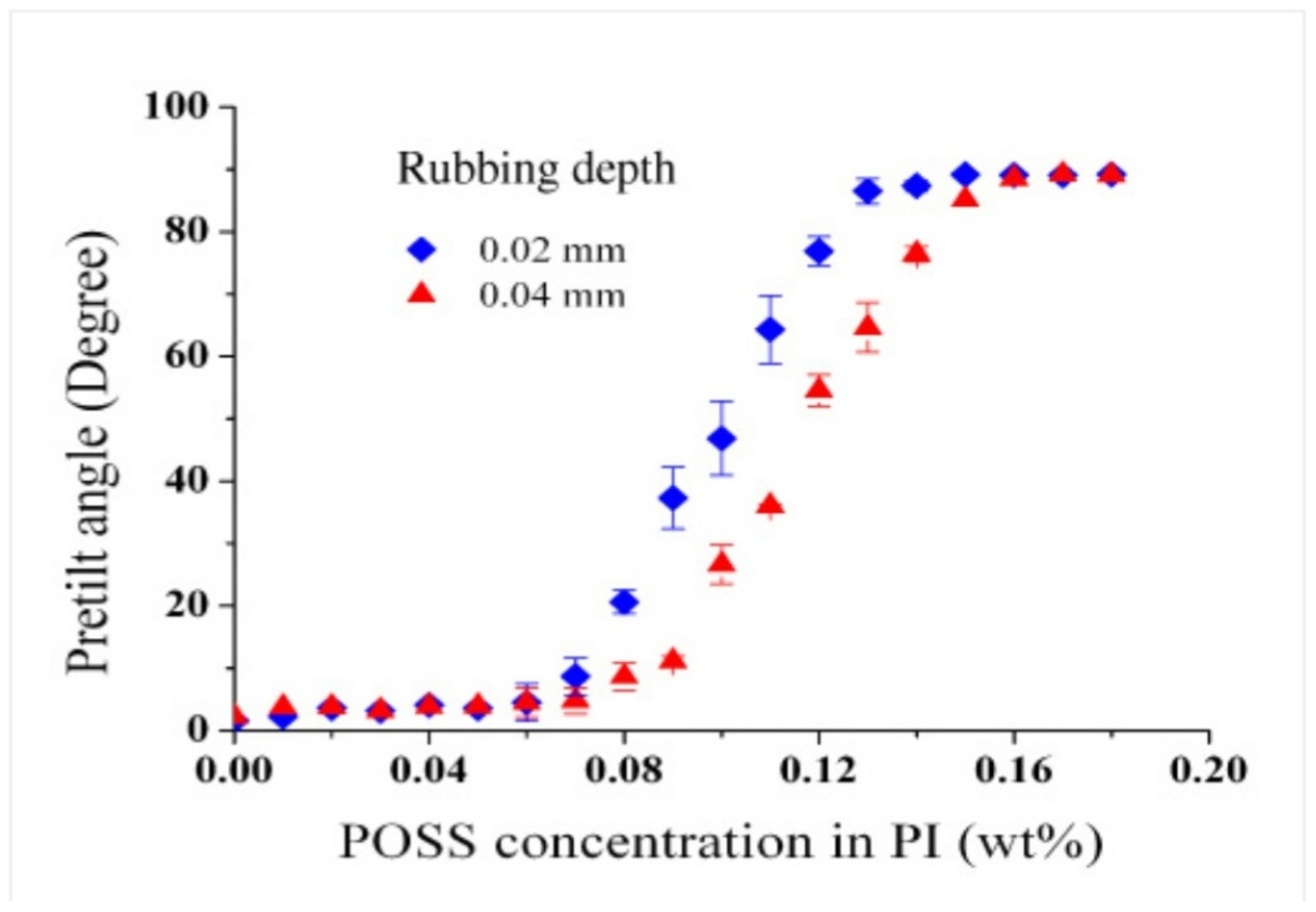
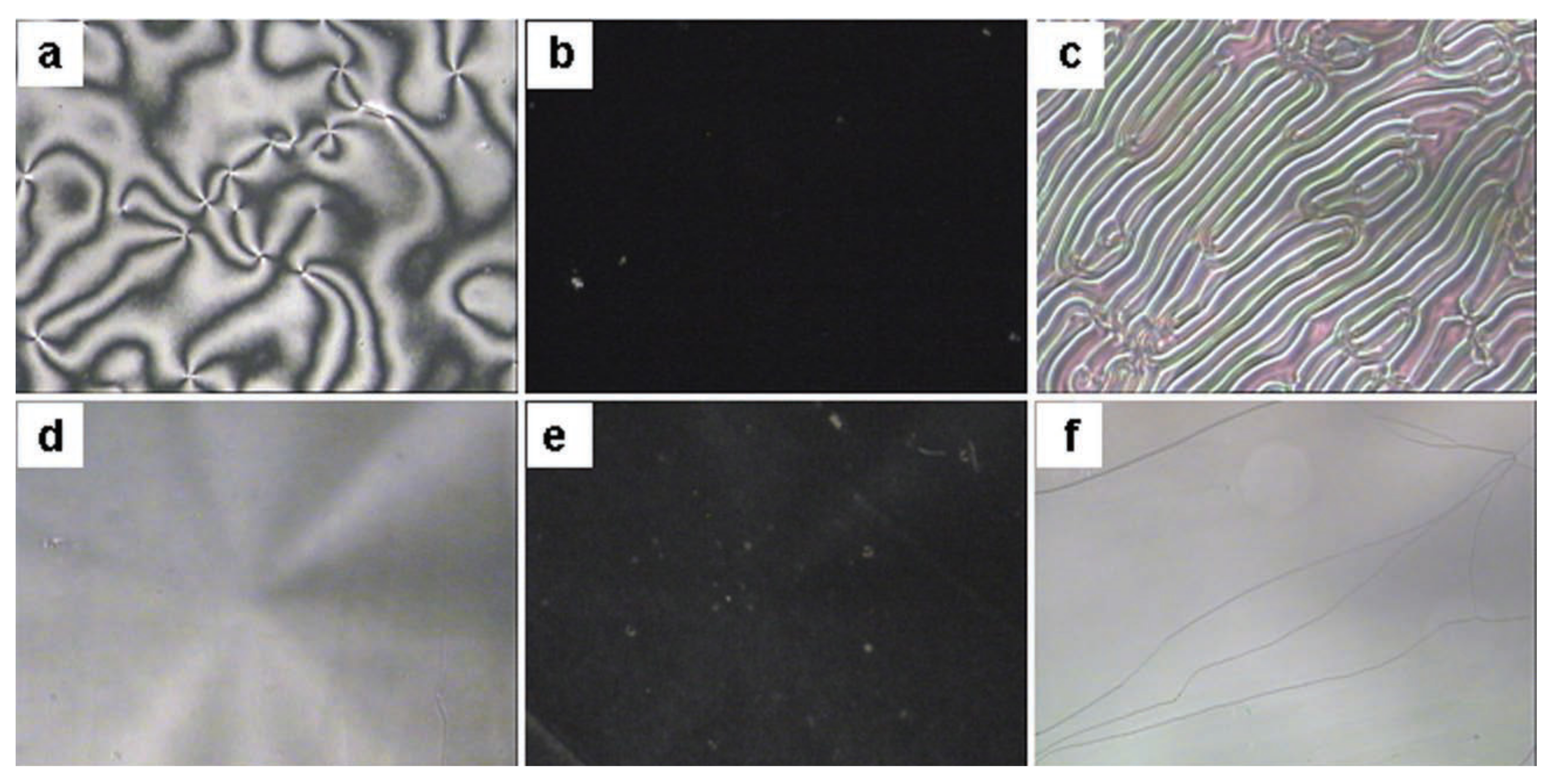
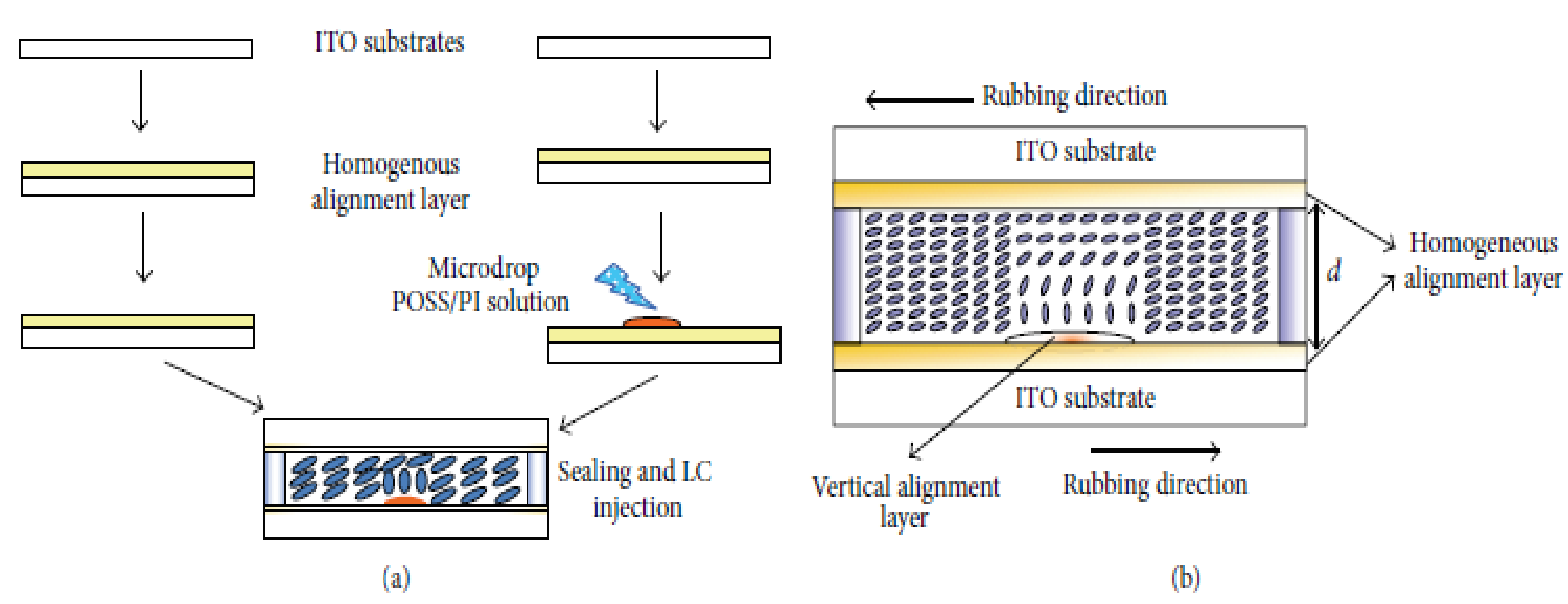
- Jeng, S.-C.; Hwang, S.-J.; Yang, C.-Y. Tunable pretilt angles based on nanoparticles-doped planar liquid-crystal cells. Opt. Lett. 2009, 34, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xiang, J.; Nemati, H.; Bisoyi, H.K.; Gutierrez-Cuevas, K.; Wang, L.; Gao, M.; Zhou, S.; Yang, D.-K.; Lavrentovich, O.D.; et al. Light-Driven Reversible Alignment Switching of Liquid Crystals Enabled by Azo Thiol Grafted Gold Nanoparticles. ChemPhysChem 2015, 16, 1852–1856. [Google Scholar] [CrossRef]
- Vimal, T.; Gupta, S.K.; Katiyar, R.; Srivastava, A.; Czerwinski, M.; Krup, K.; Kumar, S.; Manohar, R. Effect of metallic silver nanoparticles on the alignment and relaxation behaviour of liquid crystalline material in smectic C* phase. J. Appl. Phys. 2017, 122, 114102. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, J.; Goel, P.; Khan, T.; Dhawan, S.K.; Silotia, P.; Biradar, A.M. Polymeric-nanoparticles–induced vertical alignment in ferroelectric liquid crystals. Eur. Lett. 2009, 88, 1–6. [Google Scholar] [CrossRef]
- Qi, H.; Hegmann, T. Liquid crystal–gold nanoparticle composites. Liq. Cryst. Today 2011, 20, 102–114. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Bolisetty, S.; Mezzenga, R. Macroscopic Alignment of Lyotropic Liquid Crystals Using Magnetic Nanoparticles. Adv. Mater. 2011, 23, 3932–3937. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Marx, V.M.; Zhang, H.R.; Hegmann, T. Miscibility and Alignment Effects of Mixed Monolayer Cyanobiphenyl Liquid-Crystal-Capped Gold Nanoparticles in Nematic Cyanobiphenyl Liquid Crystal Hosts. ChemPhysChem 2009, 10, 1211–1218. [Google Scholar] [CrossRef]
- Chung, Y.-F.; Chen, M.-Z.; Yang, S.-H.; Jeng, S.-C. Tunable Surface Wettability of ZnO Nanoparticle Arrays for Controlling the Alignment of Liquid Crystals. ACS Appl. Mater. Interfaces 2015, 7, 9619–9624. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Hegmann, T. Unprecedented Dual Alignment Mode and Freedericksz Transition in Planar Nematic Liquid Crystal Cells Doped with Gold Nanoclusters. Adv. Funct. Mater. 2008, 18, 212–221. [Google Scholar] [CrossRef]
- Gardner, D.F.; Evans, J.S.; Smalyukh, I.I. Towards Reconfigurable Optical Metamaterials: Colloidal Nanoparticle Self-Assembly and Self-Alignment in Liquid Crystals. Mol. Cryst. Liq. Cryst. 2011, 545, 3–21. [Google Scholar] [CrossRef]
- Kim, K.-H.; Park, B.W.; Choi, S.-W.; Lee, J.-H.; Kim, H.; Shin, K.-C.; Kim, H.S.; Yoon, T.-H. Vertical alignment of liquid crystals without alignment layers. Liq. Cryst. 2013, 40, 391–395. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Zhang, Y.; Martinez, A.; Wang, S.; He, S.; White, T.J.; Smalyukh, I.I. Shape-dependent dispersion and alignment of nonaggregating plasmonic gold nanoparticles in lyotropic and thermotropic liquid crystals. Phys. Rev. E 2014, 89, 052505. [Google Scholar] [CrossRef]
- Kuang, Z.-Y.; Fan, Y.-J.; Tao, L.; Li, M.-L.; Zhao, N.; Wang, P.; Chen, E.-Q.; Fan, F.; Xie, H.-L. Alignment Control of Nematic Liquid Crystal using Gold Nanoparticles Grafted by the Liquid Crystalline Polymer with Azobenzene Mesogens as the Side Chains. ACS Appl. Mater. Interfaces 2018, 10, 27269–27277. [Google Scholar] [CrossRef] [PubMed]
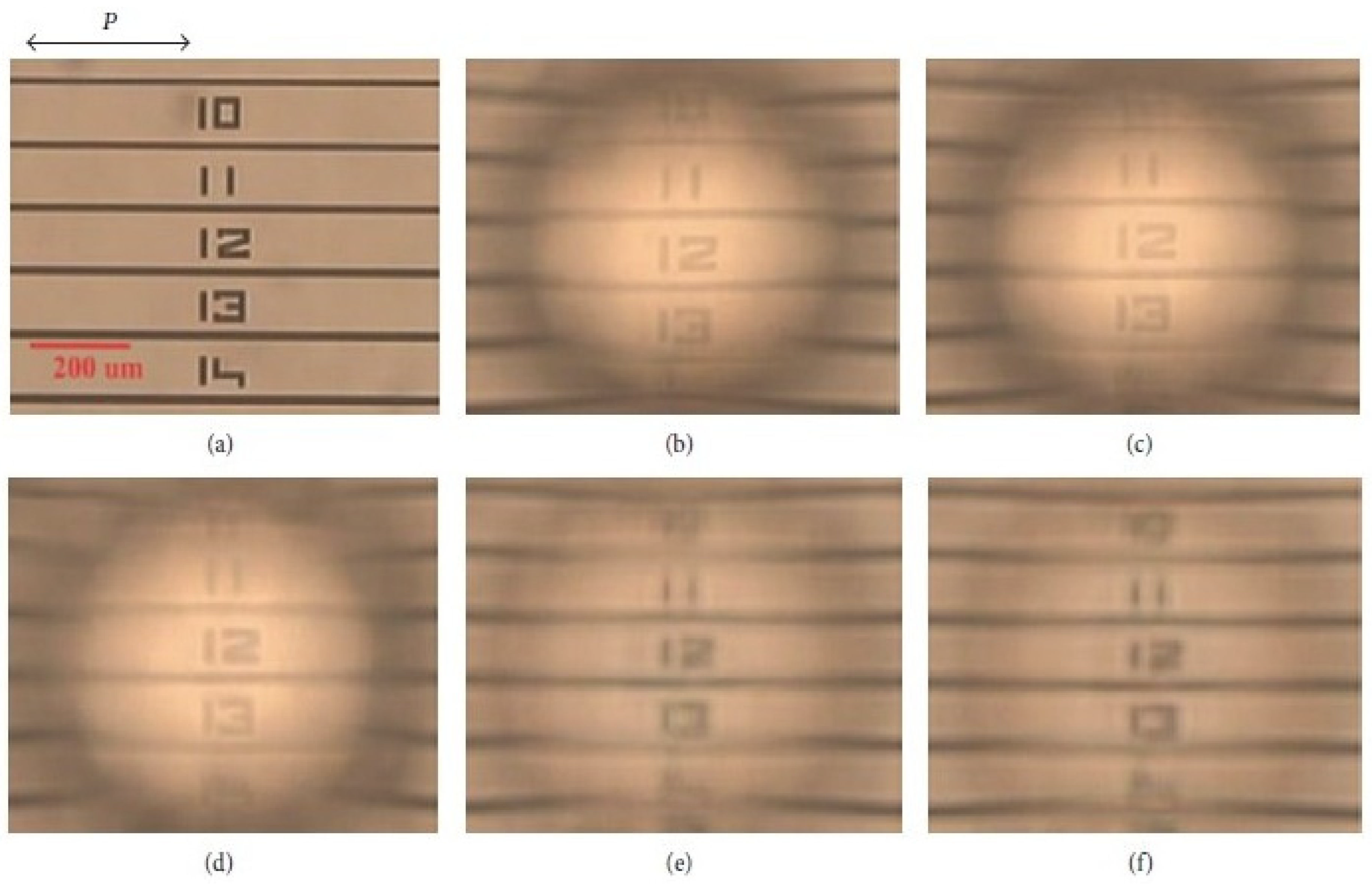
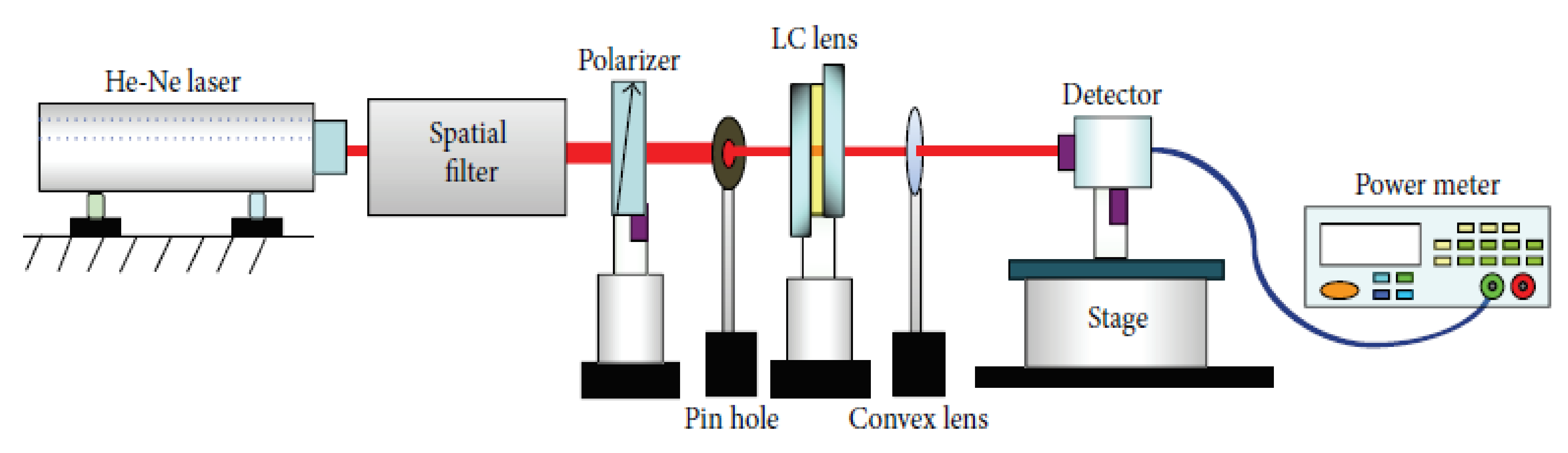
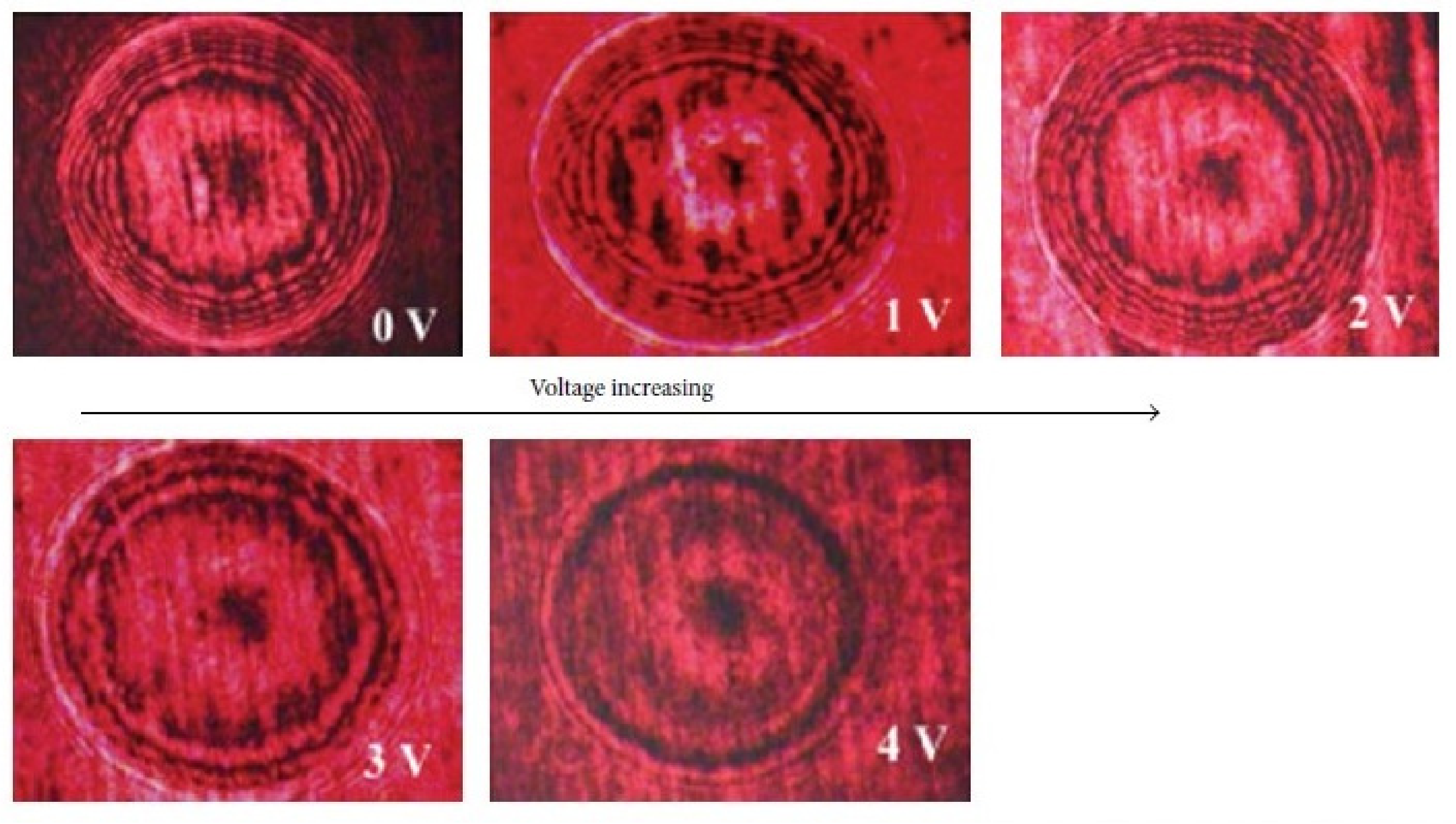
- Hwang, S.-J.; Shieh, Y.-M.; Lin, K.-R. Liquid Crystal Microlens Using Nanoparticle-Induced Vertical Alignment. J. Nanomater. 2015, 2015, 840182. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, J.; Kumar, A.; Chauhan, S. Aligning Liquid Crystal Materials through Nanoparticles: A Review of Recent Progress. Liquids 2022, 2, 50-71. https://doi.org/10.3390/liquids2020005
Prakash J, Kumar A, Chauhan S. Aligning Liquid Crystal Materials through Nanoparticles: A Review of Recent Progress. Liquids. 2022; 2(2):50-71. https://doi.org/10.3390/liquids2020005
Chicago/Turabian StylePrakash, Jai, Akash Kumar, and Shikha Chauhan. 2022. "Aligning Liquid Crystal Materials through Nanoparticles: A Review of Recent Progress" Liquids 2, no. 2: 50-71. https://doi.org/10.3390/liquids2020005





