Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Measurement of Photosynthetic Pigments
2.3. Leaf Photosynthetic Gas Exchange Rates
2.4. Pulse-Amplitude-Modulated Chlorophyll a Fluorescence (PAM)
2.5. Redox State of P700
2.6. SDS–PAGE Electrophoresis and Western Blot Analysis
2.7. Data Analysis
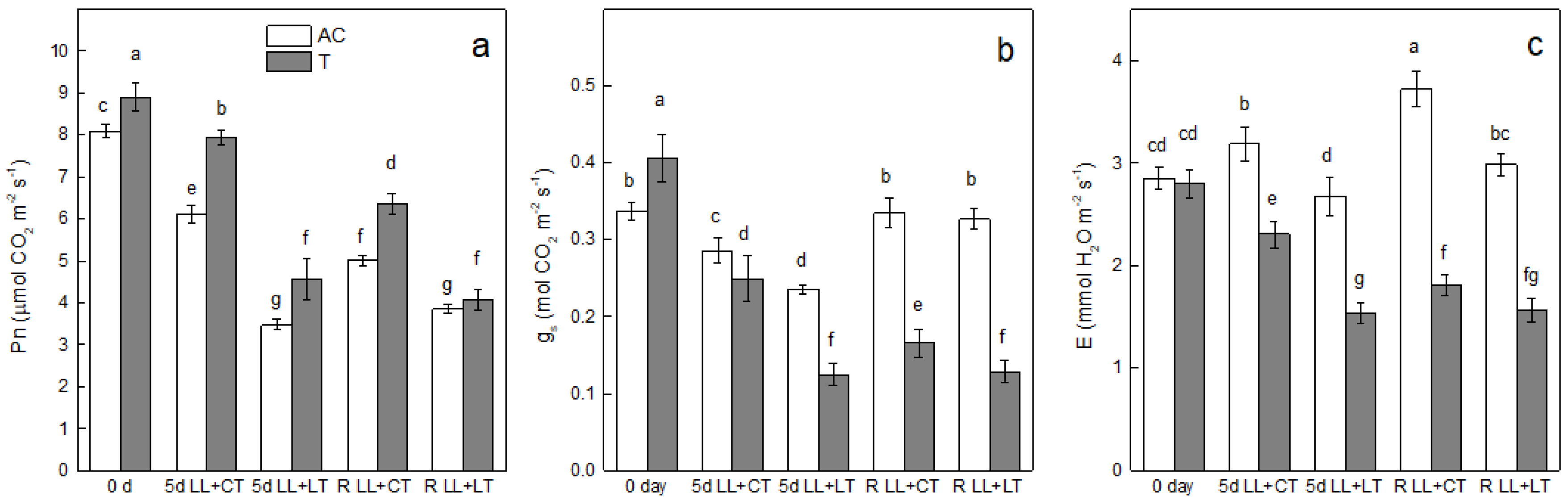
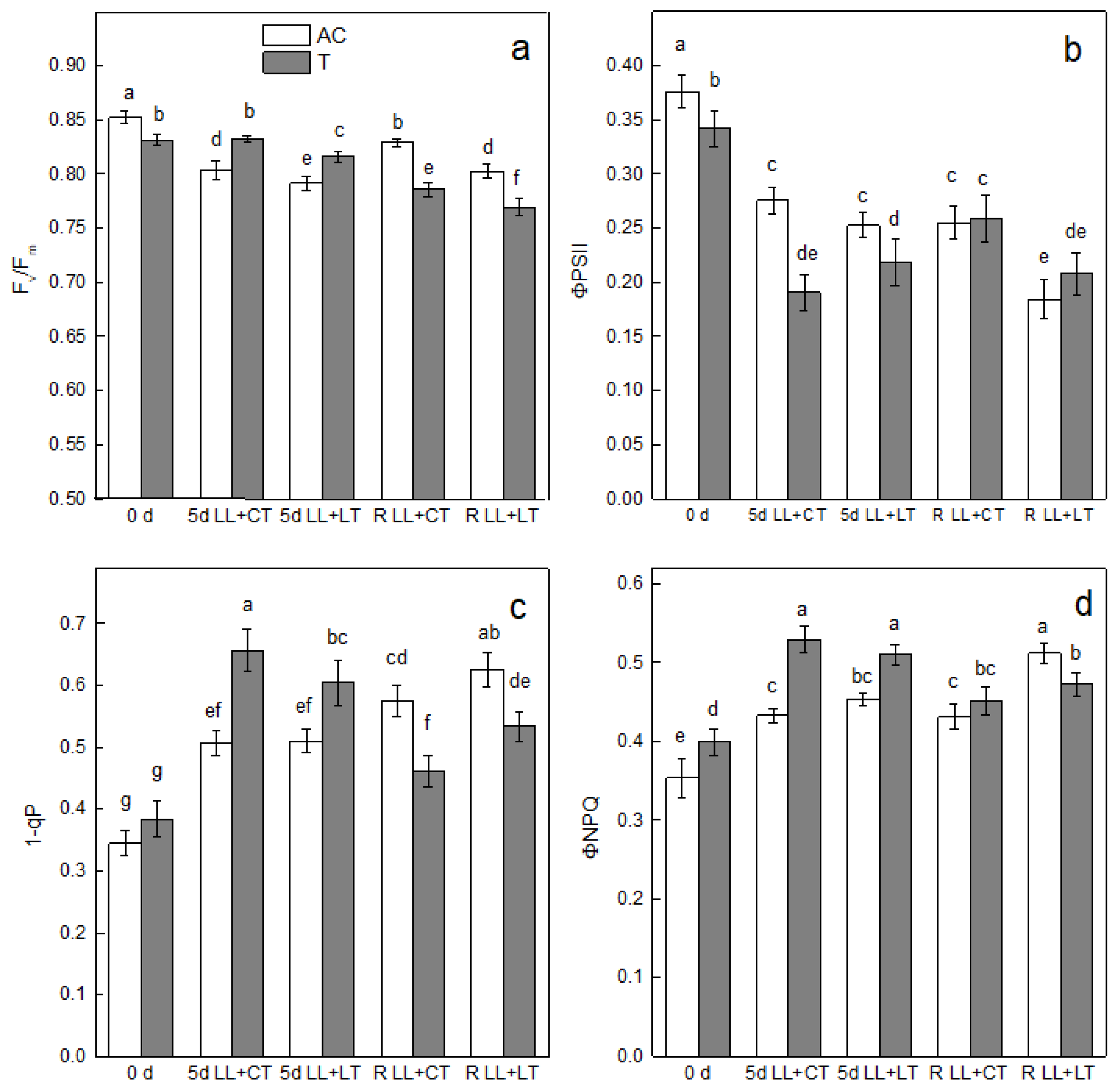
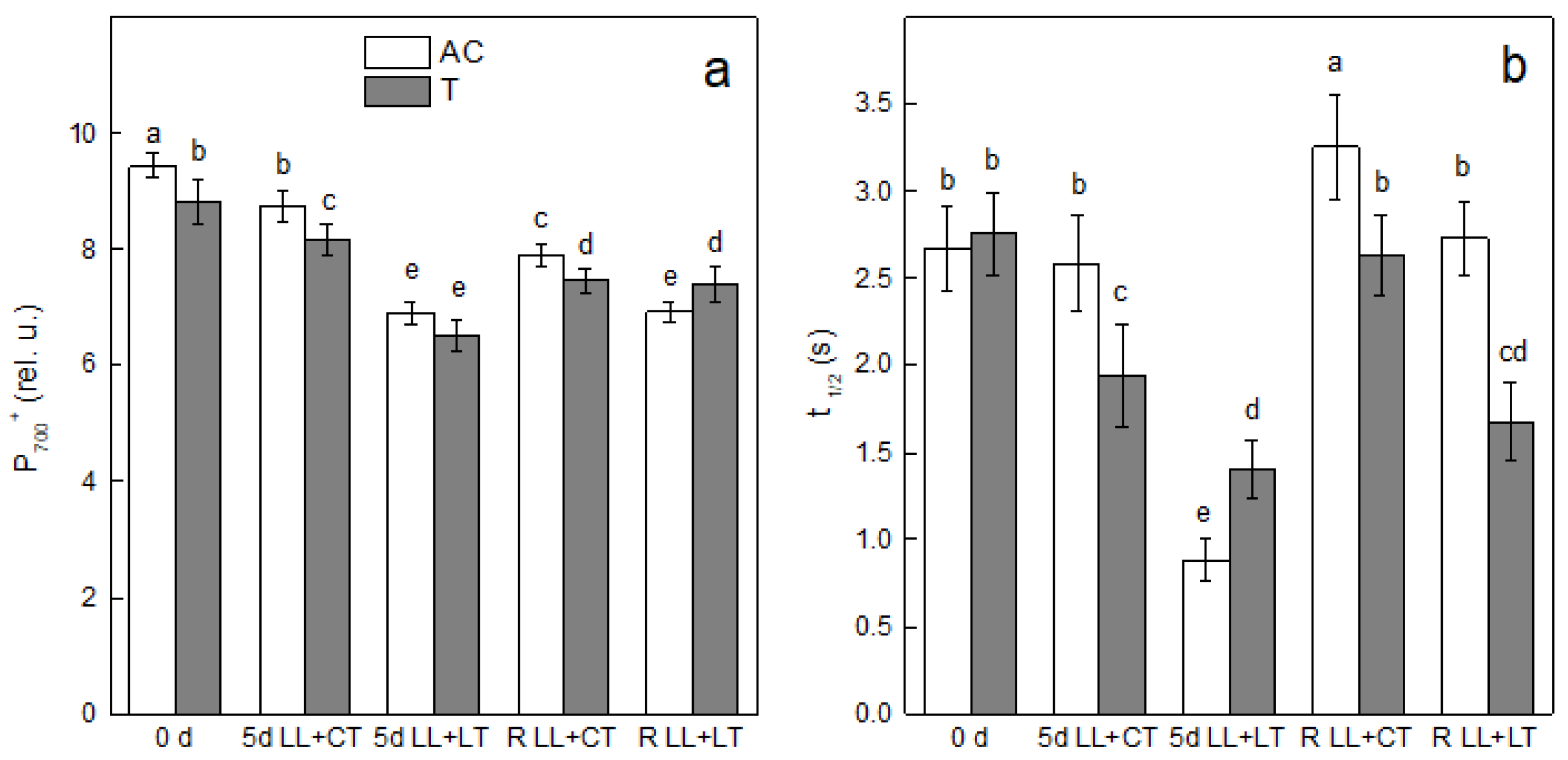
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Ailsa Craig |
| CT | Control temperature |
| LL | Low light intensity |
| LT | Low temperature |
| PSI and PSII | Photosystem I and Photosystem II |
| T | tangerine |
References
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Responses of leaf photosynthesis, plant growth and fruit production to periodic alteration of plant density in winter produced single-truss tomatoes. Hortic. J. 2017, 86, 511–518. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-I. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Xiaoa, F.; Yang, Z.; Zhua, L. Low temperature and weak light affect greenhouse tomato growth and fruit quality. J. Plant Sci. 2018, 6, 16–24. [Google Scholar]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum L.) seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef]
- Popova, A.V.; Stefanov, M.; Mihailova, G.; Borisova, P.; Georgieva, K. Response of tomato plants, Ailsa Craig and carotenoid mutant tangerine, to simultaneous treatment by low light and low temperature. Plants 2024, 13, 1929. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kalaji, H.M. Photosynthetic responses of sun and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosyn. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef]
- Li, X.-G.; Meng, Q.-W.; Jiang, G.-Q.; Zou, Q. The susceptibility of cucumber and sweet pepper to chilling under low irradiance is related to energy dissipation and water-water cycle. Photosynthetica 2003, 41, 259–265. [Google Scholar] [CrossRef]
- Wu, X.; Khan, R.; Gao, H.; Liu, H.; Zhang, J.; Ma, X. Low light alters the photosynthesis process in cigar tobacco via modulation of the chlorophyll content, chlorophyll fluorescence, and gene expression. Agriculture 2021, 11, 755. [Google Scholar] [CrossRef]
- Velitchkova, M.; Stefanov, M.; Popova, A.V. Effect of low light on photosynthetic performance of tomato plants—Ailsa Craig and carotenoid mutant tangerine. Plants 2023, 12, 3000. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, J.; Xie, J.M.; Yu, J.; Dawuda, M.M.; Lyv, J.; Tang, Z.Q.; Zhang, J.; Zhang, X.D.; Tang, C.N. Changes in photosynthesis and carotenoid composition of pepper (Capsicum annuum L.) in response to low-light stress and low temperature combined with low-light stress. Photosynthetica 2020, 58, 125–136. [Google Scholar] [CrossRef]
- Popova, A.V.; Dobrev, K.; Velitchkova, M.; Ivanov, A.G. Differential temperature effects on dissipation of excess light energy and energy partitioning in lut2 mutant of Arabidopsis thaliana under photoinhibitory conditions. Photosyn. Res. 2019, 139, 367–385. [Google Scholar] [CrossRef]
- Popova, A.V.; Borisova, P.; Mihailova, G.; Georgieva, K. Antioxidative response of Arabidopsis thaliana to combined action of low temperature and high light illumination when lutein is missing. Acta Physiol. Plant. 2022, 44, 10. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Telegina, T.A.; Vechtomova, Y.L.; Aybush, A.V.; Buglaks, A.A.; Kritsky, M.S. Isomerization of carotenoids in photosynthesis and metabolic adaptation. Biophys. Rev. 2023, 15, 887–906. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The Regulation of carotenoid formation in tomato fruit. Plant J. 2017, 89, 774–788. [Google Scholar] [CrossRef]
- Yu, Q.; Ghisla, S.; Hirschberg, J.; Mann, V.; Beyer, P. Plant carotene cis-trans isomerase CRTISO. J. Biol. Chem. 2011, 286, 8666–8676. [Google Scholar] [CrossRef]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef]
- Tanambell, H.; Bishop, K.S.; Quek, S.Y. Tangerine tomatoes: Origin, biochemistry, potential health benefits and future prospects. Crit. Rev. Food Sci. Nutr. 2021, 61, 2237–2248. [Google Scholar] [CrossRef]
- Gerganova, M.; Popova, A.V.; Stanoeva, D.; Velitchkova, M. Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol. Biochem. 2016, 104, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- van Kooten, O.V.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosyn. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Morgan, R.M.; Gray, G.R.; Velitchkova, M.Y.; Huner, N.P.A. Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Let. 1998, 430, 288–292. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef]
- Khanal, N.; Bray, G.E.; Grisnich, A.; Moffatt, B.A.; Gray, G.R. Differential mechanisms of photosynthetic acclimation to light and low temperature in Arabidopsis and the extremophile Eutrema salsugineum. Plants 2017, 6, 32. [Google Scholar] [CrossRef]
- Yuan, L.; Shu, S.; Sun, J.; Guo, S.; Tezuka, T. Effects of 24-Epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth. Res. 2012, 112, 205–214. [Google Scholar] [CrossRef]
- Lu, D.; Liu, B.; Ren, M.; Wu, C.; Ma, J.; Shen, Y. Light deficiency inhibits growth by affecting photosynthesis efficiency as well as JA and ethylene signaling in endangered plant Magnolia sinostellata. Plants 2021, 10, 2261. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta-Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Bukov, N.; Carpentier, R. Alternative photosystem I-driven electron transport routes: Mechanisms and functions. Photosyn. Res. 2004, 82, 17–33. [Google Scholar] [CrossRef]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated rubisco expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Sekhar, S.; Panda, D.; Kumar, J.; Mohanty, N.; Biswal, M.; Baig, M.J.; Kumar, A.; Umakanta, N.; Samantaray, S.; Pradhan, S.K.; et al. Comparative transcriptome profiling of low light tolerant and sensitive rice varieties induced by low light stress at active tillering stage. Sci. Rep. 2019, 9, 5753. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, A.V.; Stefanov, M.; Tsonev, T.; Velikova, V.; Velitchkova, M. Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment. Crops 2025, 5, 77. https://doi.org/10.3390/crops5060077
Popova AV, Stefanov M, Tsonev T, Velikova V, Velitchkova M. Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment. Crops. 2025; 5(6):77. https://doi.org/10.3390/crops5060077
Chicago/Turabian StylePopova, Antoaneta V., Martin Stefanov, Tsonko Tsonev, Violeta Velikova, and Maya Velitchkova. 2025. "Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment" Crops 5, no. 6: 77. https://doi.org/10.3390/crops5060077
APA StylePopova, A. V., Stefanov, M., Tsonev, T., Velikova, V., & Velitchkova, M. (2025). Differential Photosynthetic Response of Tomato Plants—Ailsa Craig and Carotenoid Mutant tangerine—To Low Light Intensity and Low Temperature Treatment. Crops, 5(6), 77. https://doi.org/10.3390/crops5060077






