Valorization of Expired Milk into Protein Hydrolysate as a Plant Biostimulant: Characterization and Application on Hydroponically Grown Cos Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk-Derived Protein Hydrolysate Preparation and Characteristics
2.2. Amino Acid Profiles of MPH
2.3. Plant Materials and Growth Conditions
2.4. Lettuce Biomass and Plant Canopy
2.5. Determination of Bioactive Compounds
2.6. Analysis of Phenolic and Flavonoid Profiles
2.7. Determination of Antioxidant Capacity and Ascorbic Acid Content
2.8. Determination of Soluble Nitrate Content
2.9. Mineral Profiles of MPH and Lettuce Leaves
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Properties and Amino Acid Composition of Milk-Derived Protein Hydrolysate
3.2. Effect of MPH on Lettuce Biomass and Plant Canopy
3.3. Effect of MPH on Antioxidant Capacity and Ascorbic Acid Content
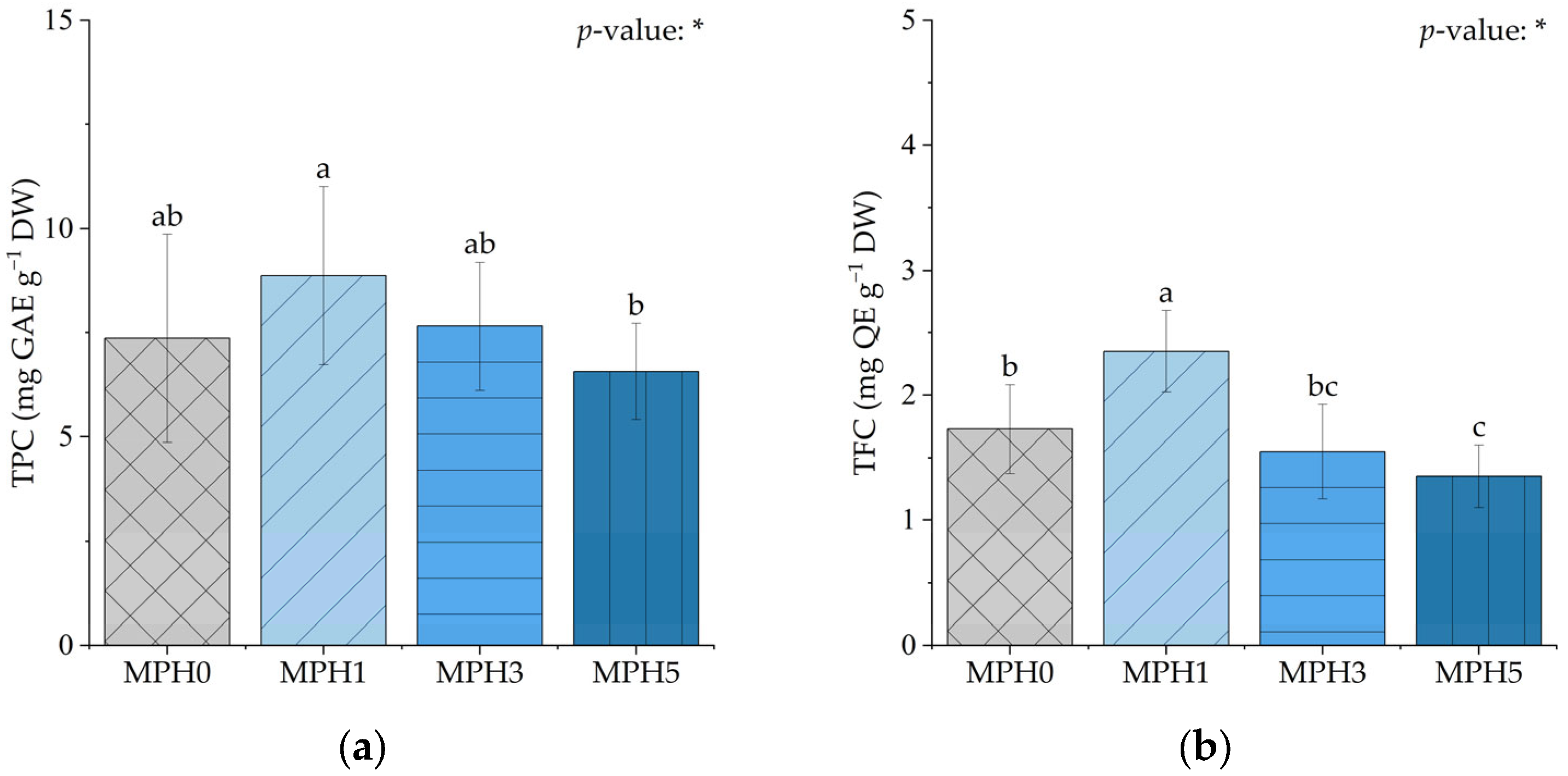
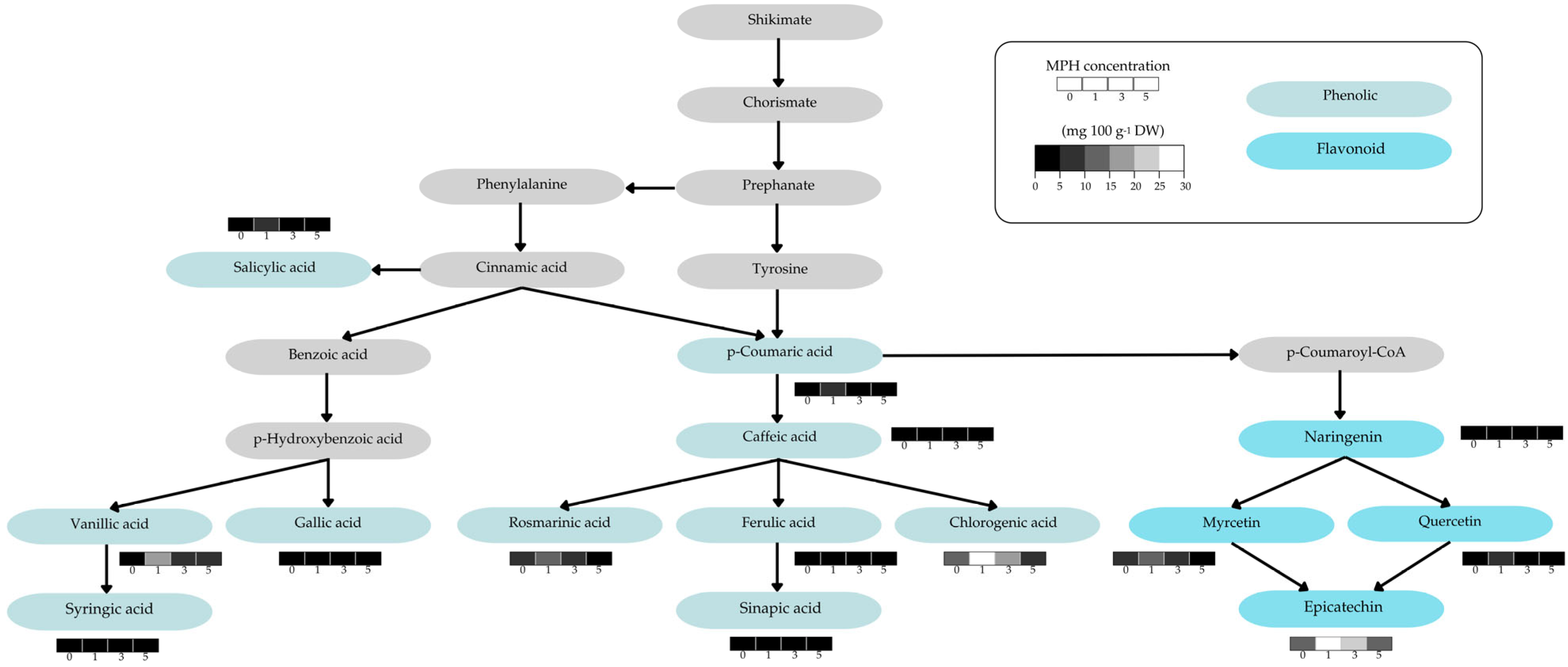
3.4. Effect of MPH on Bioactive Compounds and Phenolic and Flavonoid Content Profiles
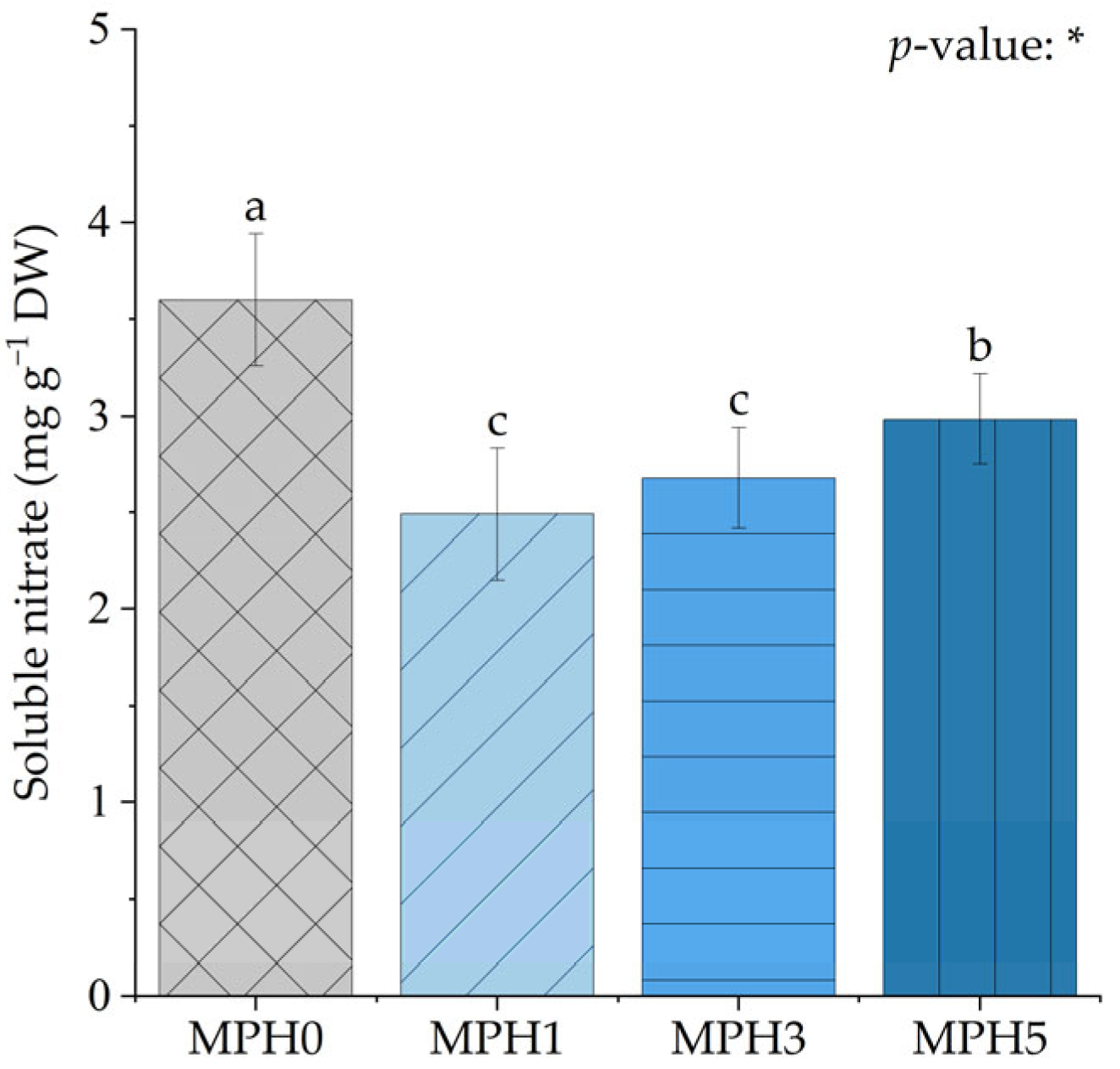
3.5. Effect of MPH on Soluble Nitrate Content
3.6. Effect of MPH on Mineral Content Profiles
4. Discussion
4.1. Implications of Physicochemical Characteristics and Amino Acid Profiles for Biostimulant Potential
4.2. MPH-Induced Changes in Cos Lettuce Biomass and Canopy
4.3. MPH-Induced Changes in Antioxidant Capacity and Ascorbic Acid Content
4.4. MPH-Induced Changes and Alterations in Bioactive Compounds and Phenolic and Flavonoid Content Profiles
4.5. MPH-Induced Changes in Soluble Nitrate Content
4.6. MPH-İnduced Changes in Mineral Content Profiles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Bruin, S.; Dengerink, J.; van Vliet, J. Urbanisation as driver of food system transformation and opportunities for rural livelihoods. Food Secur. 2021, 13, 781–798. [Google Scholar] [CrossRef]
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Agricultural, industrial, municipal, and forest wastes. In Sustainable Resource Recovery and Zero Waste Approaches; Mohammad, J., Kim, B., Jonathan, W., Ashok, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. [Google Scholar]
- Zhang, X.; Yin, J.; Ma, Y.; Peng, Y.; Fenton, O.; Wang, W.; Zhang, W.; Chen, Q. Unlocking the potential of biostimulants derived from organic waste and by-product sources: Improving plant growth and tolerance to abiotic stresses in agriculture. Environ. Technol. Innov. 2024, 34, 103571. [Google Scholar] [CrossRef]
- Campbell, C.G.; Feldpausch, G.L. Invited review: The consumer and dairy food waste: An individual plus policy, systems, and environmental perspective. J. Dairy Sci. 2022, 105, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, N.N.; Gomah, N.H.; Aly, M.M.; Abdel-Aleem, J.A.; Hammam, A.R.A.; Osman, D.M.; El-Derwy, Y.M.A. Compositional characteristics of dairy products and their potential nondairy applications after shelf-life. Curr. Res. Food Sci. 2022, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Dass, S.M.; Chai, T.T.; Cao, H.; Ooi, A.L.; Wong, F.C. Application of enzyme-digested soy protein hydrolysate on hydroponic-planted lettuce: Effects on phytochemical contents, biochemical profiles and physical properties. Food Chem. X 2021, 12, 100132. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 552. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Lv, H.; Wang, Q.; Liang, B.; Li, J. Foliar application of pig blood-derived protein hydrolysates improves antioxidant activities in lettuce by regulating phenolic biosynthesis without compromising yield production. Sci. Hortic. 2022, 291, 110602. [Google Scholar] [CrossRef]
- Tayi, F.; Metomo, F.N.N.N.; Essamlali, Y.; Akil, A.; Amadine, O.; Aboulhrouz, S.; Sair, S.; Zahouily, M. Preparation and characterization of fish-derived protein hydrolysate and assessment of its effect on tomato and sorghum plants growth and productivity. Sustain. Chem. Pharm. 2025, 43, 101877. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- El-Sanatawy, A.; Ash-Shormillesy, S.; El-Yazied, A.; El-Gawad, H.; Azab, E.; Gobouri, A.; Sitohy, M.; Osman, A. Enhancing grain yield and nitrogen accumulation in wheat plants grown under a Mediterranean arid environment by foliar spray with papain-released whey peptides. Agronomy 2021, 11, 1913. [Google Scholar] [CrossRef]
- Osman, A.; Merwad, A.M.; Mohamed, A.H.; Sitohy, M. Foliar spray with pepsin-and papain-whey protein hydrolysates promotes the productivity of pea plants cultivated in clay loam soil. Molecules 2021, 26, 2805. [Google Scholar] [CrossRef]
- Bělonožníková, K.; Černý, M.; Hýsková, V.; Synková, H.; Valcke, R.; Hodek, O.; Křížek, T.; Kavan, D.; Vaňková, R.; Dobrev, P.; et al. Casein as protein and hydrolysate: Biostimulant or nitrogen source for Nicotiana tabacum plants grown in vitro? Physiol. Plant 2023, 175, e13973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Karthik, C.; Govindan, P.; Sharma, R.K.; Padikasan, I.A. Evaluating the biostimulating potential of whey protein hydrolysate (WPH) in Solanum lycopersicum: A comprehensive investigation. Waste Biomass Valori. 2025, 2025, 1–12. [Google Scholar] [CrossRef]
- Buňka, F.; Kříž, O.; Veličková, A.; Buňková, L.; Kráčmar, S. Effect of acid hydrolysis time on amino acid determination in casein and processed cheeses with different fat content. J. Food Compos. Anal. 2009, 22, 224–232. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Kasampalis, D.S.; Aktsoglou, D.-C.; Barbayiannis, N.; Siomos, A.S. Effect of reduced nitrogen and supplemented amino acids nutrient solution on the nutritional quality of baby green and red lettuce grown in a floating system. Agronomy 2020, 10, 922. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, R.; Liang, Y.; Zhang, S.; Zhang, Z.; Sun, C.; Li, J.; Qi, Z.; Yang, Q. Comparing efficacy of different biostimulants for hydroponically grown lettuce (Lactuca sativa L.). Agronomy 2022, 12, 786. [Google Scholar] [CrossRef]
- Rajaseger, G.; Chan, K.L.; Yee Tan, K.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Kadamb Haribhai, P. Hydroponics: Current trends in sustainable crop production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Horowitz, A. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemist: Rockville, MD, USA, 2008. [Google Scholar]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Physicochemical and flavor characteristics of flavoring agent from mungbean protein hydrolyzed by bromelain. J. Agric. Food Chem. 2011, 59, 8475–8483. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular 1950, 347, 1–32. [Google Scholar]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C.; Duru, M.E. Chemical composition and nutraceutical potential of indian borage (Plectranthus amboinicus) stem extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol inhibition of lipid peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.F.; Hall, J.W. Ascorbic acid, dehydroascorbic acid and diketogulonic acid in frozen green peppers. J. Food Sci. 1978, 43, 532–534. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Farrell Jr, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Izquierdo-Ramos, M.J.; Garcia-Huertas, C.; Rodriguez-Alcantara, M.; Navarro-Morillo, I.; Navarro-Leon, E. An evaluation of the effectivity of the green leaves biostimulant on lettuce growth, nutritional quality, and mineral element efficiencies under optimal growth conditions. Plants 2024, 13, 917. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the degree of hydrolysis on functional properties and antioxidant activity of enzymatic soybean protein hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef] [PubMed]
- GenÇ, E.; Atici, Ö. Chicken feather protein hydrolysate as a biostimulant improves the growth of wheat seedlings by affecting biochemical and physiological parameters. Turk. J. Bot. 2019, 43, 67–79. [Google Scholar] [CrossRef]
- Carillo, P.; De Micco, V.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Giordano, M.; Colla, G.; Rouphael, Y. Morpho-anatomical, physiological, and mineral composition responses induced by a vegetal-based biostimulant at three rates of foliar application in greenhouse lettuce. Plants 2022, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Canellas, N.A.; Val, F.; Spaccini, R.; Mazzei, P.; Olivares, F.L. Changes in amino acids profile and uptake on maize seedlings treated with protein hydrolysates and humic substances. Nitrogen 2024, 5, 439–454. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, M.; Yang, G.; Sun, M.; Yang, A.; Sun, C.; Zhao, H.; Ao, X. Root morphology, nitrogen metabolism and amino acid metabolism in soybean under low phosphorus stress. Sci. Rep. 2024, 14, 28583. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ruan, Y.; Gao, W. Combinatorial effects of glycine and inorganic nitrogen on root growth and nitrogen nutrition in maize (Zea mays L.). Sustainability 2023, 15, 14122. [Google Scholar] [CrossRef]
- Pérez-Aguilar, H.; Lacruz-Asaro, M.; Ruzafa-Silvestre, C.; Arán-Ais, F. Protein recovery from wastewater animal processing by-products of rendering plants for biostimulant applications in agriculture. Sustain. Chem. Pharm. 2023, 32, 101009. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, M.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Kim, Y.B.; Sankaranarayanan, S.; Sathasivam, R.; Park, S.U. Influence of different types of carbon sources on glucosinolate and phenolic compounds in radish sprouts. Horticulturae 2023, 9, 679. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Casadesus, A.; Perez-Llorca, M.; Munne-Bosch, S.; Polo, J. An enzymatically hydrolyzed animal protein-based biostimulant (pepton) increases salicylic acid and promotes growth of tomato roots under temperature and nutrient stress. Front. Plant Sci. 2020, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Aktsoglou, D.C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Protein hydrolysates supplement in the nutrient solution of soilless grown fresh peppermint and spearmint as a tool for improving product quality. Agronomy 2021, 11, 317. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Sagharyan, M.; Sharifi, M. Metabolic and physiological changes induced by exogenous phenylalanine in linum album cells. J. Plant Growth Regul. 2024, 43, 2785–2801. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. J. EFSA 2017, 15, e04787. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce: A comprehensive review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]


| Properties | MPH Compositions |
|---|---|
| Degree of hydrolysis (%) | 94.55 ± 1.75 |
| Soluble protein (g L−1) | 2.43 ± 0.03 |
| Total salt (%) | 14.27 ± 0.22 |
| Specific gravity (g cm−3) | 1.10 ± 0.00 |
| Electrical conductivity (dS m−1) | 192.00 ± 2.53 |
| pH | 6.51 ± 0.04 |
| Total nitrogen (g L−1) | 6.10 ± 0.00 |
| P (g L−1) | 0.10 ± 0.01 |
| K (g L−1) | 0.31 ± 0.00 |
| Ca (g L−1) | 8.01 ± 0.12 |
| Fe (mg L−1) | 0.90 ± 0.04 |
| Mg (g L−1) | 0.64 ± 0.01 |
| Amino Acids | Total Amino Acids (g 100 g−1 Protein) | Free Amino Acids (g 100 g−1 Protein) |
|---|---|---|
| Essential amino acid | ||
| Lysine | 4.05 ± 0.03 | 4.07 ± 0.01 |
| Histidine | 1.48 ± 0.01 | 0.88 ± 0.01 |
| Threonine | 3.06 ± 0.02 | 1.94 ± 0.03 |
| Valine | 4.43 ± 0.01 | 1.52 ± 0.01 |
| Leucine | 6.35 ± 0.01 | 4.11 ± 0.01 |
| Isoleucine | 3.54 ± 0.01 | 1.42 ± 0.01 |
| Phenylalanine | 3.91 ± 0.01 | 2.47 ± 0.03 |
| Methionine | ND * | 1.39 ± 0.01 |
| Non-essential amino acid | ||
| Aspartic Acid | 7.09 ± 0.01 | 6.37 ± 0.04 |
| Glutamic Acid | 13.81 ± 0.01 | 11.30 ± 0.01 |
| Arginine | 3.58 ± 0.01 | 2.66 ± 0.01 |
| Glycine | 3.00 ± 0.01 | 2.57 ± 0.03 |
| Tyrosine | 1.69 ± 0.04 | 1.67 ± 0.02 |
| Serine | 4.35 ± 0.01 | 3.31 ± 0.01 |
| Cystine | ND * | 3.42 ± 0.01 |
| Alanine | 2.98 ± 0.02 | 2.55 ± 0.01 |
| Proline | 5.30 ± 0.01 | 3.78 ± 0.01 |
| Treatments | Shoot FW (g) | Root FW (g) | Plant Canopy (cm3) |
|---|---|---|---|
| MPH0 | 92.86 ± 5.83 b | 14.47 ± 0.77 c | 899.25 ± 97.89 bc |
| MPH1 | 118.76 ± 8.65 a | 17.83 ± 0.88 a | 1140.98 ± 164.49 a |
| MPH3 | 97.66 ± 11.17 b | 16.09 ± 0.78 b | 964.11 ± 125.95 b |
| MPH5 | 81.46 ± 9.55 c | 14.32 ± 1.07 c | 876.72 ± 113.78 c |
| p-value | * | * | * |
| Parameters | Phenolic and Flavonoid Profiles (mg 100 g−1 DW) | p-Value | |||
|---|---|---|---|---|---|
| MPH0 | MPH1 | MPH3 | MPH5 | ||
| Phenolic profiles | |||||
| Gallic acid | 0.61 ± 0.40 b | 1.28 ± 0.67 a | 0.90 ± 0.37 ab | 0.58 ± 0.19 b | * |
| Chlorogenic acid | 14.02 ± 13.71 ab | 27.55 ± 30.23 a | 18.84 ± 19.74 ab | 6.84 ± 6.76 b | * |
| Caffeic acid | 1.01 ± 0.60 ab | 1.98 ± 1.40 a | 1.56 ± 0.86 ab | 0.80 ± 0.47 b | * |
| Vanillic acid | 3.22 ± 1.67 b | 15.2 ± 12.83 a | 8.88 ± 7.69 ab | 5.15 ± 5.04 b | * |
| Para-coumaric acid | 2.05 ± 0.99 b | 6.46 ± 3.97 a | 3.71 ± 1.79 b | 1.97 ± 0.71 b | * |
| Syringic acid | 0.61 ± 0.24 b | 1.27 ± 0.83 a | 0.87 ± 0.43 ab | 0.52 ± 0.22 b | * |
| Ferulic acid | 1.02 ± 0.41 b | 2.49 ± 2.06 a | 1.92 ± 1.17 ab | 0.92 ± 0.29 b | * |
| Sinapic acid | 0.72 ± 0.45 ab | 1.42 ± 1.15 a | 0.92 ± 0.47 ab | 0.43 ± 0.24 b | * |
| Rosmarinic acid | 5.70 ± 4.37 ab | 13.52 ± 14.73 a | 7.96 ± 5.13 ab | 4.39 ± 2.57 b | * |
| Salicylic acid | 2.58 ± 1.23 b | 7.43 ± 7.52 a | 3.99 ± 2.02 ab | 2.10 ± 1.12 b | * |
| Flavonoid profiles | |||||
| Quercetin | 2.80 ± 1.35 b | 6.01 ± 4.43 a | 4.36 ± 3.19 ab | 2.65 ± 1.56 b | * |
| Epicatechin | 14.87 ± 10.28 ab | 29.52 ± 24.25 a | 24.32 ± 15.53 ab | 11.02 ± 7.14 b | * |
| Myricetin | 5.08 ± 2.30 b | 10.88 ± 7.79 a | 8.17 ± 6.09 ab | 4.42 ± 2.59 b | * |
| Naringenin | 1.33 ± 0.79 b | 2.62 ± 1.45 a | 1.78 ± 1.02 ab | 1.05 ± 0.45 b | * |
| Mineral Composition | Nutrient Solutions | t-Test | |
|---|---|---|---|
| MPH0 | MPH1 | ||
| P (mg g−1 DW) | 0.22 ± 0.10 | 0.39 ± 0.14 | * |
| K (mg g−1 DW) | 4.79 ± 0.29 | 5.65 ± 0.18 | * |
| Ca (mg g−1 DW) | 0.86 ± 0.04 | 1.25 ± 0.04 | * |
| Fe (µg g−1 DW) | 2.70 ± 0.60 | 5.10 ± 2.40 | * |
| Mg (mg g−1 DW) | 0.40 ± 0.01 | 0.59 ± 0.16 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, A.M.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N.; Tira-Umphon, A. Valorization of Expired Milk into Protein Hydrolysate as a Plant Biostimulant: Characterization and Application on Hydroponically Grown Cos Lettuce. Crops 2025, 5, 56. https://doi.org/10.3390/crops5050056
Zahra AM, Uthairatanakij A, Laohakunjit N, Jitareerat P, Kaisangsri N, Tira-Umphon A. Valorization of Expired Milk into Protein Hydrolysate as a Plant Biostimulant: Characterization and Application on Hydroponically Grown Cos Lettuce. Crops. 2025; 5(5):56. https://doi.org/10.3390/crops5050056
Chicago/Turabian StyleZahra, Aryanis Mutia, Apiradee Uthairatanakij, Natta Laohakunjit, Pongphen Jitareerat, Nattapon Kaisangsri, and Arak Tira-Umphon. 2025. "Valorization of Expired Milk into Protein Hydrolysate as a Plant Biostimulant: Characterization and Application on Hydroponically Grown Cos Lettuce" Crops 5, no. 5: 56. https://doi.org/10.3390/crops5050056
APA StyleZahra, A. M., Uthairatanakij, A., Laohakunjit, N., Jitareerat, P., Kaisangsri, N., & Tira-Umphon, A. (2025). Valorization of Expired Milk into Protein Hydrolysate as a Plant Biostimulant: Characterization and Application on Hydroponically Grown Cos Lettuce. Crops, 5(5), 56. https://doi.org/10.3390/crops5050056









