The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review
Simple Summary
Abstract
1. Introduction
2. Cancer Risk in Obesity: Epidemiological Evidence
3. Tumor-Associated Inflammation: Friend and Foe
4. Obesity as a Chronic Inflammatory State
5. Insulin Resistance and Metabolic Dysregulation
6. Molecular Pathways Linking Obesity and Cancer
7. Inflammation-Induced EMT, Angiogenesis, and Metastasis
8. Interventions and Future Directions
8.1. Diet and Nutrition
8.2. Physical Activity
8.3. Anti-Inflammatory Agents
8.4. Metabolic Modulation Therapies
8.5. Emerging Molecular Targets
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sagliocchi, S.; Acampora, L.; Barone, B.; Crocetto, F.; Dentice, M. The impact of the tumor microenvironment in the dual burden of obesity-cancer link. Semin. Cancer Biol. 2025, 112, 36–42. [Google Scholar] [CrossRef]
- Abdulla, A.; Sadida, H.Q.; Jerobin, J.; Elfaki, I.; Mir, R.; Mirza, S.; Singh, M.; Macha, M.A.; Uddin, S.; Fakhro, K.; et al. Unraveling molecular interconnections and identifying potential therapeutic targets of significance in obesity-cancer link. J. Natl. Cancer Cent. 2025, 5, 8–27. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, C.; Chen, K.; Gu, Y.; Qiu, X.; Li, Q. Investigating the causal association between obesity and risk of hepatocellular carcinoma and underlying mechanisms. Sci. Rep. 2024, 14, 15717. [Google Scholar] [CrossRef]
- Cunha Junior, A.D.; Pericole, F.V.; Carvalheira, J.B.C. Metformin and blood cancers. Clinics 2018, 73, e412s. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; Garcia-Canaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866 e1826. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Rubinstein, M.M.; Brown, K.A.; Iyengar, N.M. Targeting obesity-related dysfunction in hormonally driven cancers. Br. J. Cancer 2021, 125, 495–509. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, P.; Liu, F.; Wang, X.; Si, C.; Gong, J.; Zhou, H.; Gu, J.; Qin, A.; Song, W.; et al. Metabolic dysfunction-associated steatotic liver disease and cancer risk: A cohort study. Diabetes Obes. Metab. 2025, 27, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Liu, Q.; Xu, C.; Ganesan, K.; Ye, Z.; Li, Y.; Wu, J.; Du, B.; Gao, F.; Song, C.; et al. Non-alcoholic fatty liver disease promotes breast cancer progression through upregulated hepatic fibroblast growth factor 21. Cell Death Dis. 2024, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Bhattacharjee, P. Clinical-exome sequencing unveils the genetic landscape of polycystic ovarian syndrome (PCOS) focusing on lean and obese phenotypes: Implications for cost-effective diagnosis and personalized treatment. Sci. Rep. 2024, 14, 24468. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Coe, P.O.; O’Reilly, D.A.; Renehan, A.G. Excess adiposity and gastrointestinal cancer. Br. J. Surg. 2014, 101, 1518–1531; discussion 1531, discussion 1531. [Google Scholar] [CrossRef]
- Adams, K.F.; Leitzmann, M.F.; Albanes, D.; Kipnis, V.; Moore, S.C.; Schatzkin, A.; Chow, W.H. Body size and renal cell cancer incidence in a large US cohort study. Am. J. Epidemiol. 2008, 168, 268–277. [Google Scholar] [CrossRef]
- An, C.; Pipia, I.; Ruiz, A.S.; Arguelles, I.; An, M.; Wase, S.; Peng, G. The molecular link between obesity and genomic instability in cancer development. Cancer Lett. 2023, 555, 216035. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 903800. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef]
- Bongartz, H.; Gille, K.; Hessenkemper, W.; Mandel, K.; Lewitzky, M.; Feller, S.M.; Schaper, F. The multi-site docking protein Grb2-associated binder 1 (Gab1) enhances interleukin-6-induced MAPK-pathway activation in an SHP2-, Grb2-, and time-dependent manner. Cell Commun. Signal 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, C.M.; Hovelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT 2013, 2, e23878. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Martinez, P.; Grant, W.B. Vitamin D: What role in obesity-related cancer? Semin. Cancer Biol. 2025, 112, 135–149. [Google Scholar] [CrossRef]
- Saitoh, M. Transcriptional regulation of EMT transcription factors in cancer. Semin. Cancer Biol. 2023, 97, 21–29. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J., 2nd; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 574667. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in cancer: Therapeutic opportunities from new insights. Mol. Cancer 2025, 24, 51. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, H.; Li, J.; Zeng, N.; Zhang, Q.; Hou, K.; Li, J.; Yu, J.; Wu, Y. Dissecting the emerging role of cancer-associated adipocyte-derived cytokines in remodeling breast cancer progression. Heliyon 2024, 10, e35200. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.K. alpha-Tocopherol and gamma-tocopherol decrease inflammatory response and insulin resistance during the interaction of adipocytes and macrophages. Nutr. Res. Pract. 2024, 18, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Bhanot, M.; Bolus, W.R.; Hasty, A.H. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol. Rev. 2020, 295, 101–113. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Rafaniello, C.; Giugliano, D. Metabolic syndrome and postmenopausal breast cancer: Systematic review and meta-analysis. Menopause 2013, 20, 1301–1309. [Google Scholar] [CrossRef]
- Esper, R.M.; Dame, M.; McClintock, S.; Holt, P.R.; Dannenberg, A.J.; Wicha, M.S.; Brenner, D.E. Leptin and Adiponectin Modulate the Self-renewal of Normal Human Breast Epithelial Stem Cells. Cancer Prev. Res. 2015, 8, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Akincilar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Cote, A.L.; Munger, C.J.; Ringel, A.E. Emerging insights into the impact of systemic metabolic changes on tumor-immune interactions. Cell Rep. 2025, 44, 115234. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef]
- Castro, A.V.; Kolka, C.M.; Kim, S.P.; Bergman, R.N. Obesity, insulin resistance and comorbidities? Mechanisms of association. Arq. Bras. Endocrinol. Metabol. 2014, 58, 600–609. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 2010, 21, 610–618. [Google Scholar] [CrossRef]
- Sachdev, D.; Yee, D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol. Cancer Ther. 2007, 6, 1–12. [Google Scholar] [CrossRef]
- Li, R.; Pourpak, A.; Morris, S.W. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J. Med. Chem. 2009, 52, 4981–5004. [Google Scholar] [CrossRef]
- Bowers, L.W.; Rossi, E.L.; O’Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef]
- Guerra, L.; Bonetti, L.; Brenner, D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep. 2020, 32, 107848. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Zhang, X.; Nguyen, M.H. Metabolic dysfunction-associated steatotic liver disease: A sexually dimorphic disease and breast and gynecological cancer. Metabolism 2025, 167, 156190. [Google Scholar] [CrossRef]
- Tanti, J.F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2012, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Mullen, M.; Gonzalez-Perez, R.R. Leptin-Induced JAK/STAT Signaling and Cancer Growth. Vaccines 2016, 4, 26. [Google Scholar] [CrossRef]
- Wiese, W.; Barczuk, J.; Racinska, O.; Siwecka, N.; Rozpedek-Kaminska, W.; Slupianek, A.; Sierpinski, R.; Majsterek, I. PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies-New Therapeutic Possibilities. Cancers 2023, 15, 5297. [Google Scholar] [CrossRef]
- da Cunha Junior, A.D.; Zanette, D.L.; Pericole, F.V.; Olalla Saad, S.T.; Barreto Campello Carvalheira, J. Obesity as a Possible Risk Factor for Progression from Monoclonal Gammopathy of Undetermined Significance Progression into Multiple Myeloma: Could Myeloma Be Prevented with Metformin Treatment? Adv. Hematol. 2021, 2021, 6615684. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, X.; Wu, X.; Lu, Y.; Chen, K.; Ye, Y. Fat Wasting Is Damaging: Role of Adipose Tissue in Cancer-Associated Cachexia. Front. Cell Dev. Biol. 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Schellerer, V.S.; Brunner, M.; Geppert, C.I.; Grutzmann, R.; Weber, K.; Merkel, S. The impact of body mass index on prognosis in patients with colon carcinoma. Int. J. Colorectal Dis. 2022, 37, 1107–1117. [Google Scholar] [CrossRef]
- Carrilho, L.A.O.; Juliani, F.L.; Moreira, R.C.L.; Dias Guerra, L.; Santos, F.S.; Padilha, D.M.H.; Branbilla, S.R.; Horita, V.N.; Novaes, D.M.L.; Antunes-Correa, L.M.; et al. Adipose tissue characteristics as a new prognosis marker of patients with locally advanced head and neck cancer. Front. Nutr. 2025, 12, 1472634. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, B.; Wang, C.; Chen, P.; Cheng, Y. The prognostic significance of metabolic syndrome and weight loss in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 10101. [Google Scholar] [CrossRef]
- Gabiatti, C.T.B.; Martins, M.C.L.; Miyazaki, D.L.; Silva, L.P.; Lascala, F.; Macedo, L.T.; Mendes, M.C.S.; Carvalheira, J.B.C. Myosteatosis in a systemic inflammation-dependent manner predicts favorable survival outcomes in locally advanced esophageal cancer. Cancer Med. 2019, 8, 6967–6976. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Suren Garg, S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res. Clin. Pract. 2023, 200, 110691. [Google Scholar] [CrossRef]
- Cuttica, C.M.; Briata, I.M.; DeCensi, A. Novel Treatments for Obesity: Implications for Cancer Prevention and Treatment. Nutrients 2023, 15, 3737. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective: A Summary of The Third Expert Report; World Cancer Research Fund International: London, UK, 2018; 112p. [Google Scholar]
- Isaksen, I.M.; Dankel, S.N. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 919–928. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, Microbiome, and Cancer Immunotherapy—A Comprehensive Review. Nutrients 2021, 13, 2217. [Google Scholar] [CrossRef]
- Bouras, E.; Tsilidis, K.K.; Triggi, M.; Siargkas, A.; Chourdakis, M.; Haidich, A.B. Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients 2022, 14, 1764. [Google Scholar] [CrossRef]
- Son, D.S.; Done, K.A.; Son, J.; Izban, M.G.; Virgous, C.; Lee, E.S.; Adunyah, S.E. Intermittent Fasting Attenuates Obesity-Induced Triple-Negative Breast Cancer Progression by Disrupting Cell Cycle, Epithelial-Mesenchymal Transition, Immune Contexture, and Proinflammatory Signature. Nutrients 2024, 16, 2101. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Huang, R.; Guo, M.; Zhou, Y.; Xu, L. The role and its mechanism of intermittent fasting in tumors: Friend or foe? Cancer Biol. Med. 2021, 18, 63–73. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]
- Krajewska, M.; Witkowska-Sedek, E.; Ruminska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrzak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef]
- Olukorode, J.O.; Orimoloye, D.A.; Nwachukwu, N.O.; Onwuzo, C.N.; Oloyede, P.O.; Fayemi, T.; Odunaike, O.S.; Ayobami-Ojo, P.S.; Divine, N.; Alo, D.J.; et al. Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders. Cureus 2024, 16, e72080. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Sig Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Lin, A.; Ding, Y.; Li, Z.; Jiang, A.; Liu, Z.; Wong, H.Z.; Cheng, Q.; Zhang, J.; Luo, P. Glucagon-like peptide 1 receptor agonists and cancer risk: Advancing precision medicine through mechanistic understanding and clinical evidence. Biomark. Res. 2025, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Agarwal, M.; Aggarwal, M.; Alexander, L.; Apovian, C.M.; Bindlish, S.; Bonnet, J.; Butsch, W.S.; Christensen, S.; Gianos, E.; et al. Nutritional priorities to support GLP-1 therapy for obesity: A joint Advisory from the American College of Lifestyle Medicine, the American Society for Nutrition, the Obesity Medicine Association, and The Obesity Society. Am. J. Clin. Nutr. 2025, 15, 100181. [Google Scholar] [CrossRef]
- Brzozowska, M.M.; Isaacs, M.; Bliuc, D.; Baldock, P.A.; Eisman, J.A.; White, C.P.; Greenfield, J.R.; Center, J.R. Effects of bariatric surgery and dietary intervention on insulin resistance and appetite hormones over a 3-year period. Sci. Rep. 2023, 13, 6032. [Google Scholar] [CrossRef]
- Madar, L.O.; Goldberg, N.; Netz, U.; Berenstain, I.F.; Abu Zeid, E.E.D.; Avital, I.; Perry, Z.H. Association between metabolic and bariatric surgery and malignancy: A systematic review, meta-analysis, trends, and conclusions. Surg. Obes. Relat. Dis. 2025, 21, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Christofk, H.; Metallo, C.; Liu, G.; Rabinowitz, J.; Sparks, L.; James, D. Metabolic heterogeneity in humans. Cell 2024, 187, 3821–3823. [Google Scholar] [CrossRef] [PubMed]
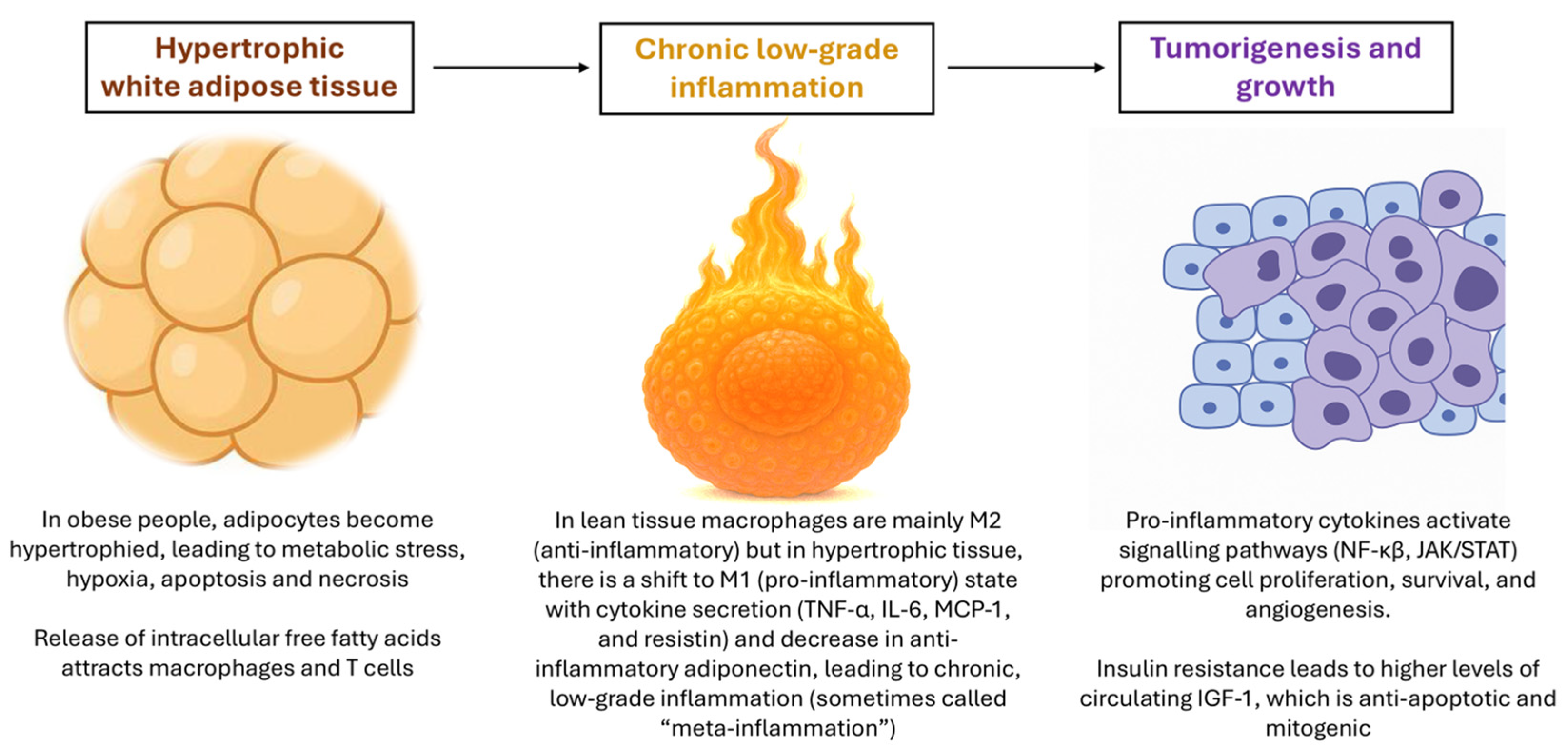
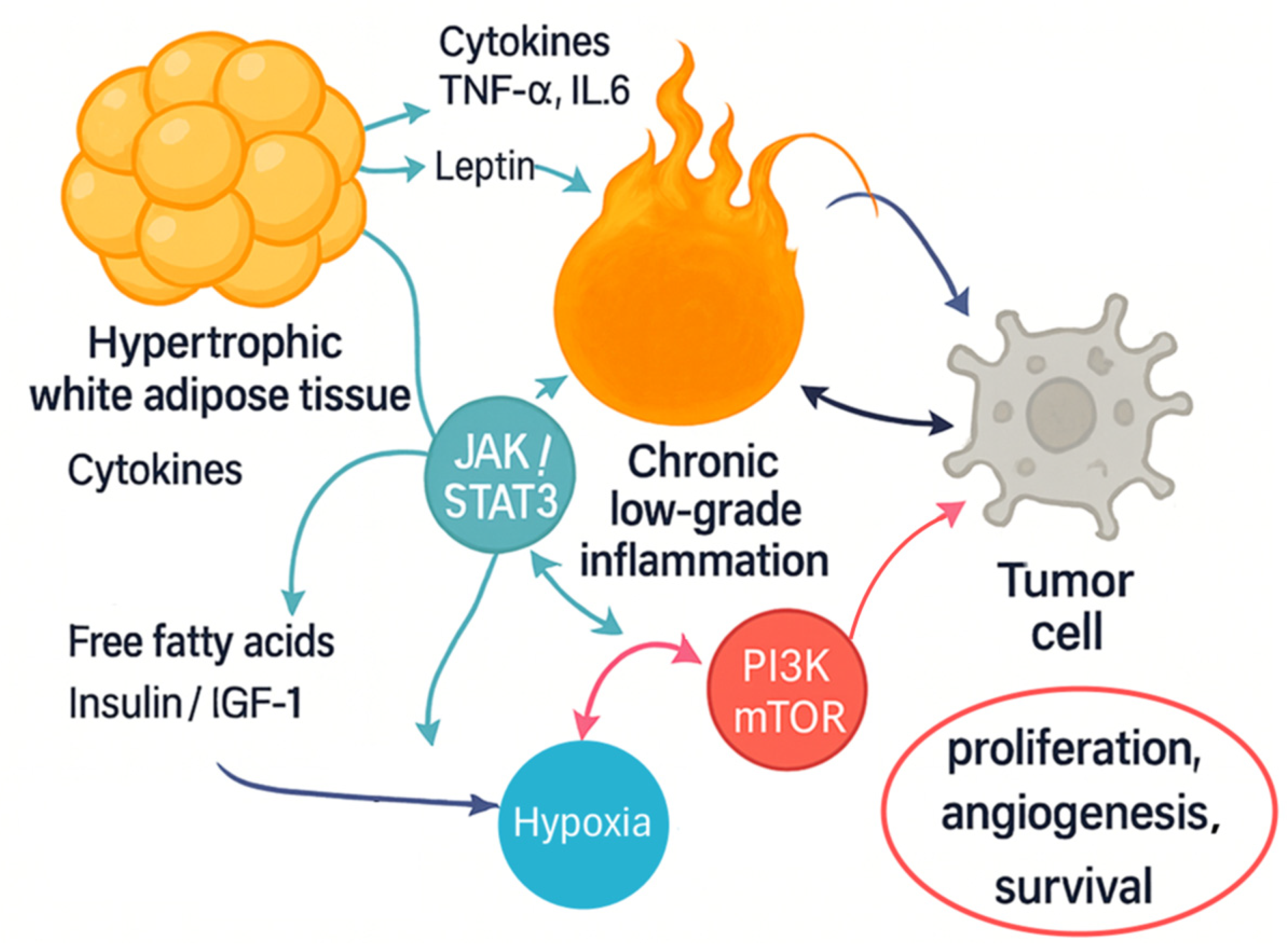
| Cell Type/Source | Key Mediators (Cytokines, Adipokines, Enzymes) | Main Pathways Activated | Functional Impact on Tumor |
|---|---|---|---|
| Adipocytes (hypertrophic WAT) | ↑ Leptin, ↓ Adiponectin, Free fatty acids | STAT3, PI3K/Akt/mTOR, TLR4–NF-κB | Proliferation, angiogenesis, reduced apoptosis, systemic insulin resistance |
| Tumor-Associated Macrophages (TAMs, M1 polarization) | TNF-α, IL-6, VEGF, MMPs | NF-κB, STAT3, HIF-1α | ECM remodeling, angiogenesis, immunosuppression, metastasis |
| Neutrophils | Pro-angiogenic chemokines, MMP-9 | NF-κB, MAPK | ECM degradation, angiogenesis, metastatic invasion |
| T Lymphocytes (Th1/Th17 skewing) | IL-17, IFN-γ, TNF-α | NF-κB, JAK/STAT | Chronic inflammation, enhanced tumor-promoting immune milieu |
| Adipose Tissue Fibroblasts/Stromal cells | TGF-β, ECM proteins | EMT pathways (Snail, Twist, ZEB), SMAD | Induction of epithelial-to-mesenchymal transition (EMT), invasion |
| Hepatocytes/Liver microenvironment | IL-6, C-reactive protein | JAK/STAT, NF-κB | Systemic inflammation, MASLD-associated tumorigenesis |
| Circulating factors in obesity | Hyperinsulinemia, IGF-1, Hyperglycemia, Dyslipidemia | PI3K/Akt/mTOR, MAPK, HIF-1α stabilization | Enhanced tumor metabolism (Warburg effect), DNA damage, survival advantage |
| Adipocytes, stromal and immune cells in obese TME | CCL2/CCR2, CCL5/CCR5, CXCL12/CXCR4 | NF-κB, JAK/STAT, MAPK | Recruitment of monocytes/TAMs, immunosuppressive polarization, EMT, metastatic dissemination, angiogenesis, matrix remodeling, immune evasion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cunha Junior, A.D.; Carrilho, L.A.O.; Nunes Filho, P.R.S.; Cantini, L.; Vidal, L.; Mendes, M.C.S.; Carvalheira, J.B.C.; Saini, K.S. The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review. Onco 2025, 5, 47. https://doi.org/10.3390/onco5040047
da Cunha Junior AD, Carrilho LAO, Nunes Filho PRS, Cantini L, Vidal L, Mendes MCS, Carvalheira JBC, Saini KS. The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review. Onco. 2025; 5(4):47. https://doi.org/10.3390/onco5040047
Chicago/Turabian Styleda Cunha Junior, Ademar Dantas, Larissa Ariel Oliveira Carrilho, Paulo Ricardo Santos Nunes Filho, Luca Cantini, Laura Vidal, Maria Carolina Santos Mendes, José Barreto Campello Carvalheira, and Kamal S. Saini. 2025. "The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review" Onco 5, no. 4: 47. https://doi.org/10.3390/onco5040047
APA Styleda Cunha Junior, A. D., Carrilho, L. A. O., Nunes Filho, P. R. S., Cantini, L., Vidal, L., Mendes, M. C. S., Carvalheira, J. B. C., & Saini, K. S. (2025). The Role of Metabolic Inflammation and Insulin Resistance in Obesity-Associated Carcinogenesis–A Narrative Review. Onco, 5(4), 47. https://doi.org/10.3390/onco5040047







