Green Synthesised Silver Nanocomposite for Thermoregulating E-Textiles †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of Silver Nanoparticles
2.3. Plackett–Burman Optimisation of Synthesis
2.4. In Situ Polymerisation of AgNP Pyrrole on Linen
2.5. Characterisation
3. Results and Discussion
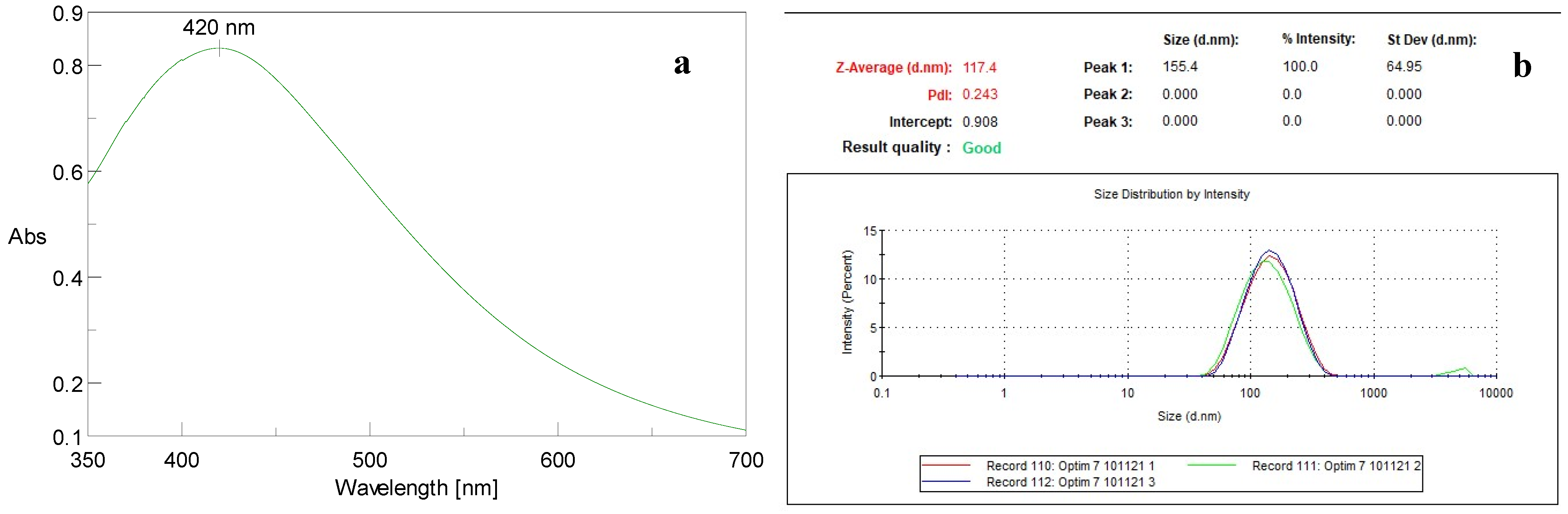
3.1. Silver Nanoparticle Morphological Analyses
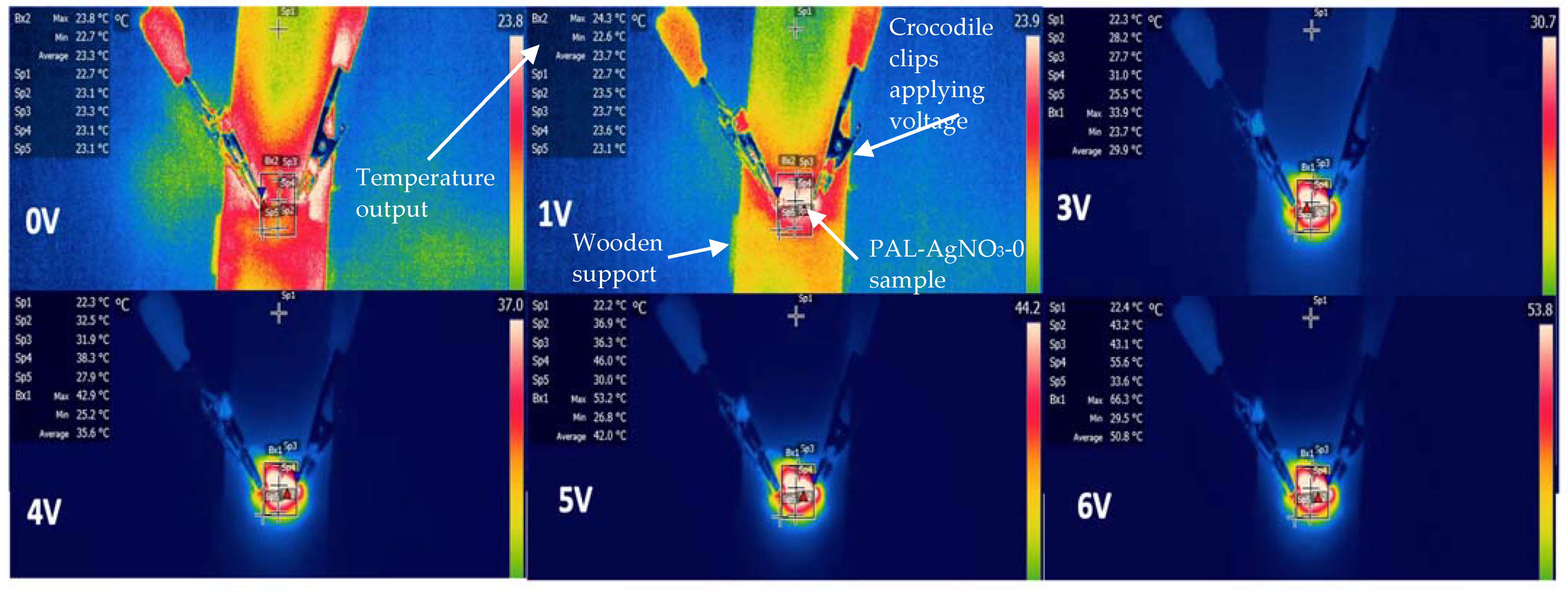
3.2. Thermoelectric Performance of Polypyrrole Silver Nanoparticle Linen
3.3. EDX-XRF Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Smith, A. A Net Zero Climate-Resilient Future: Science, Technology and the Solutions for Change; The Royal Society: London, UK, 2021. [Google Scholar]
- Kou, Y.; Sun, K.; Luo, J.; Zhou, F.; Huang, H.; Wu, Z.-S.; Shi, Q. An intrinsically flexible phase change film for wearable thermal managements. Energy Storage Mater. 2021, 34, 508–514. [Google Scholar] [CrossRef]
- Hughes-Riley, T.; Jobling, P.; Dias, T.; Faulkner, S.H. An investigation of temperature-sensing textiles for temperature monitoring during sub-maximal cycling trials. Text. Res. J. 2020, 91, 624–645. [Google Scholar] [CrossRef]
- Hughes-Riley, T.; Dias, T.; Cork, C. A Historical Review of the Development of Electronic Textiles. Fibers 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Morris, N.B.; Jay, O.; Flouris, A.D.; Casanueva, A.; Gao, C.; Foster, J.; Havenith, G.; Nybo, L. Sustainable solutions to mitigate occupational heat strain—An umbrella review of physiological effects and global health perspectives. Environ. Health 2020, 19, 95. [Google Scholar] [CrossRef]
- Lugoda, P.; Hughes-Riley, T.; Oliveira, C.; Morris, R.; Dias, T. Developing Novel Temperature Sensing Garments for Health Monitoring Applications. Fibers 2018, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Han, D.-C.; Shin, H.-J.; Yeom, S.-H.; Ju, B.-K.; Lee, W. PEDOT:PSS-Based Temperature-Detection Thread for Wearable Devices. Sensors 2018, 18, 2996. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Cheng, H.; Zhu, J.; Yuan, Y.; Wang, C. A flexible and stretchable polypyrrole/knitted cotton for electrothermal heater. Org. Electron. 2020, 85, 105819. [Google Scholar] [CrossRef]
- Zhang, L.; Baima, M.; Andrew, T.L. Transforming Commercial Textiles and Threads into Sewable and Weavable Electric Heaters. ACS Appl. Mater. Interfaces 2017, 9, 32299–32307. [Google Scholar] [CrossRef]
- Ahmed, A.; Jalil, M.A.; Hossain, M.M.; Moniruzzaman, M.; Adak, B.; Islam, M.T.; Parvez, M.S.; Mukhopadhyay, S. A PEDOT:PSS and graphene-clad smart textile-based wearable electronic Joule heater with high thermal stability. J. Mater. Chem. C 2020, 8, 16204–16215. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, C.; Zhao, J.; Cai, Z.; Ge, F. Low-Voltage Electrical Heater Based on One-Step Fabrication of Conductive Cu Nanowire Networks for Application in Wearable Devices. Adv. Mater. Interfaces 2020, 8, 2001695. [Google Scholar] [CrossRef]
- Ke, F.; Song, F.; Zhang, H.; Xu, J.; Wang, H.; Chen, Y. Layer-by-layer assembly for all-graphene coated conductive fibers toward superior temperature sensitivity and humidity independence. Compos. Part B: Eng. 2020, 200, 108253. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Effect of Relative Humidity Condition on Electrical Heating Textile Coated with Graphene-based on Cotton Fabric. Fibers Polym. 2021, 22, 276–284. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Montazer, M.; Gao, W. Low temperature welding of graphene on PET with silver nanoparticles producing higher durable electro-conductive fabric. Carbon 2017, 118, 443–451. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Kim, D.Y.; Park, Y.-B.; Park, H.W. Smart gating of the flexible Ag@CoxMo1-xP and rGO-loaded composite based personal thermal management device inspired by the neuroanatomic circuitry of endotherms. Chem. Eng. J. 2020, 421, 127746. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, L.; Lu, Y. Electromagnetic interference shielding of layered linen fabric/polypyrrole/nickel (LF/PPy/Ni) composites. Mater. Des. 2016, 95, 97–106. [Google Scholar] [CrossRef]
- Shen, L.; Patel, M.K. Life Cycle Assessment of Polysaccharide Materials: A Review. J. Polym. Environ. 2008, 16, 154. [Google Scholar] [CrossRef] [Green Version]
- Werf, H.M.G.v.d.; Turunen, L. The environmental impacts of the production of hemp and flax textile yarn. Ind. Crops Prod. 2008, 27, 1–10. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Murugesan, S.; Muneeswaran, T.; Suresh, S.; Kandasamy, M.; Valdés, H.; Selvaraj, M.; Dennyson Savariraj, A.; Mangalaraja, R.V. Biocidal activity of citrus limetta peel extract mediated green synthesized silver quantum dots against MCF-7 cancer cells and pathogenic bacteria. J. Environ. Chem. Eng. 2021, 9, 105089. [Google Scholar] [CrossRef]
- Dutta, T.; Ghosh, N.N.; Das, M.; Adhikary, R.; Mandal, V.; Chattopadhyay, A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: Experimental and theoretical studies. J. Environ. Chem. Eng. 2020, 8, 104019. [Google Scholar] [CrossRef]
- Minitab. Available online: https://app.minitab.com (accessed on 16 December 2021).
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the Size of Silver Nanoparticles from Their Optical Properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, N.; Divya, D.; Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926–34948. [Google Scholar] [CrossRef] [Green Version]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; Luca, G.D.; et al. Structural and morphological characterizations of MWCNTs hybrid coating onto cotton fabric as potential humidity and temperature wearable sensor. Sens. Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naysmith, A.; Mian, N.S.; Rana, S. Green Synthesised Silver Nanocomposite for Thermoregulating E-Textiles. Eng. Proc. 2022, 15, 15. https://doi.org/10.3390/engproc2022015015
Naysmith A, Mian NS, Rana S. Green Synthesised Silver Nanocomposite for Thermoregulating E-Textiles. Engineering Proceedings. 2022; 15(1):15. https://doi.org/10.3390/engproc2022015015
Chicago/Turabian StyleNaysmith, Ashleigh, Naeem S. Mian, and Sohel Rana. 2022. "Green Synthesised Silver Nanocomposite for Thermoregulating E-Textiles" Engineering Proceedings 15, no. 1: 15. https://doi.org/10.3390/engproc2022015015




