Structural Investigation of the Carbon Deposits on Ni/Al2O3 Catalyst Modified by CaO-MgO for the Biogas Dry Reforming Reaction †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Materials Characterization
2.3. Catalysts Tests
3. Results and Discussion
3.1. Characterization of Spent Catalysts
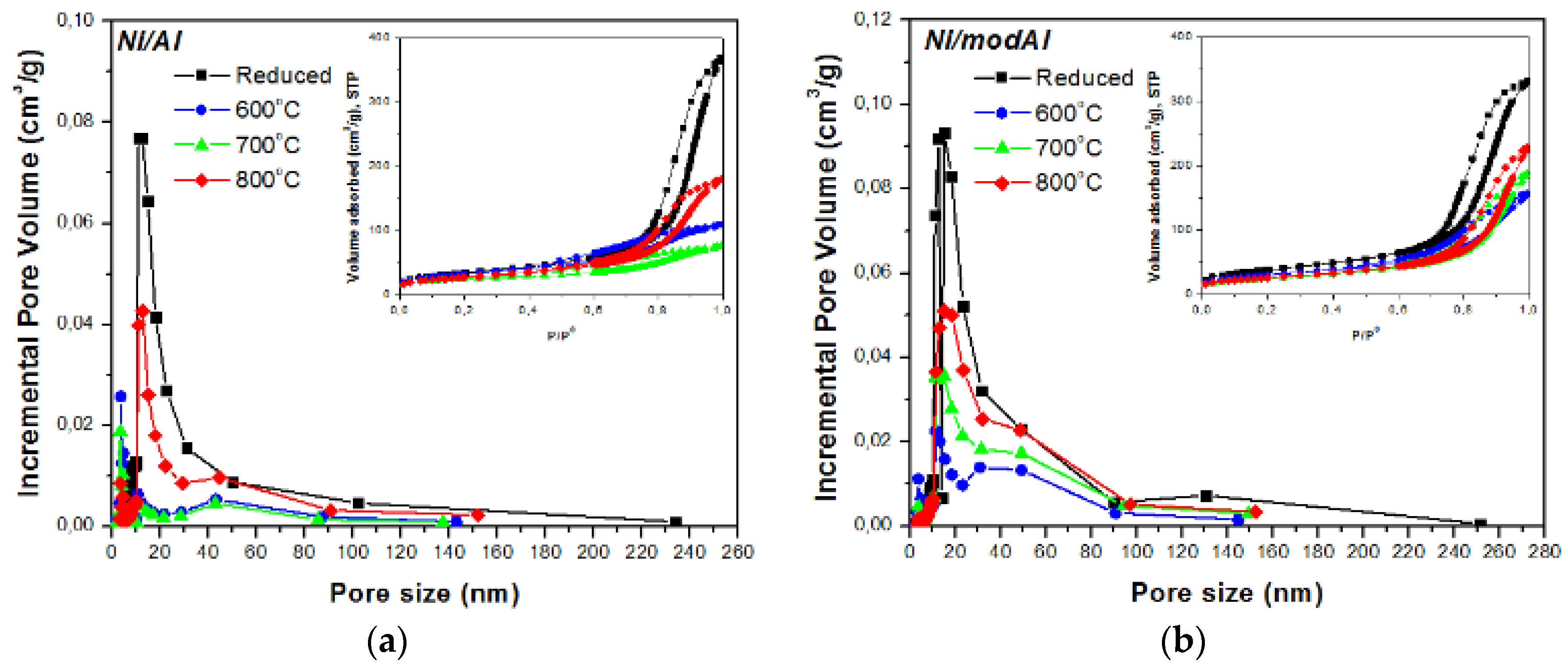
3.1.1. Textural Characterization
3.1.2. TGA Analysis
3.1.3. Raman Spectroscopy Analysis
3.1.4. Electron Microscopy Analysis
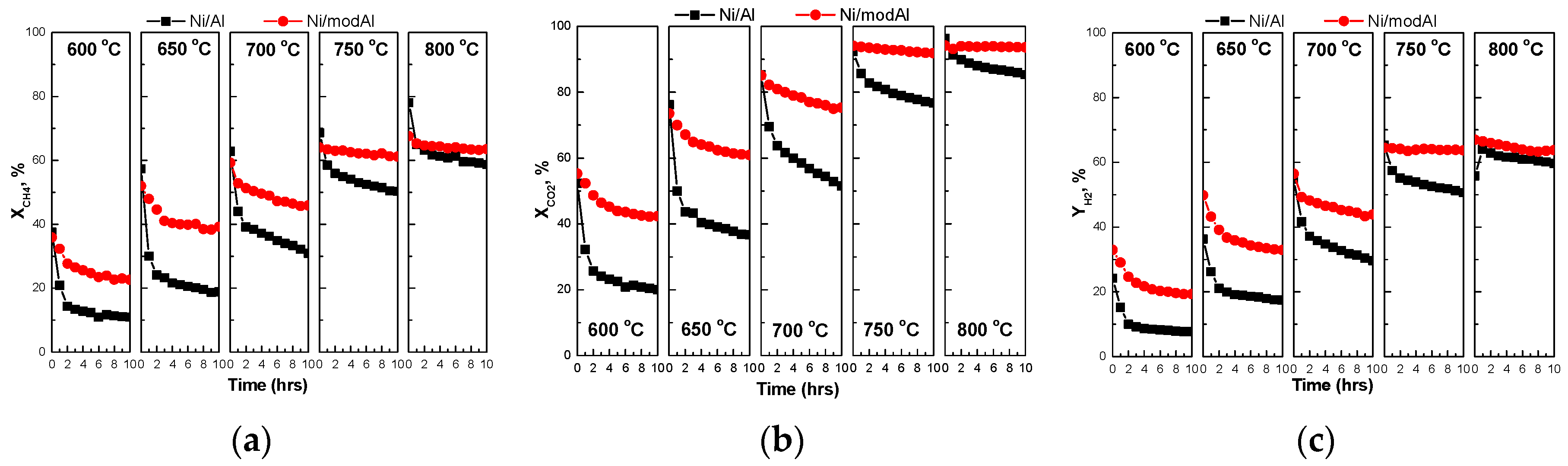
3.2. Catalytic Stability
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Italiano, C.; Balzarotti, R.; Vita, A.; Latorrata, S.; Fabiano, C.; Pino, L.; Cristiani, C. Preparation of structured catalysts with Ni and Ni-Rh/CeO2 catalytic layers for syngas production by biogas reforming processes. Catal. Today 2016, 273, 3–11. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Roman-Martinez, M.C.; Illan-Gomez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane: Effect of pretreatments. Appl. Catal. A-Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzon, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Recent advances in dry reforming of methane over Ni-based catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Ang, M.L.; Mo, L.; Li, Z.; Oemar, U.; Kawi, S. Highly active and coke resistant Ni/SiO2 catalysts for oxidative reforming of model biogas: Effect of low ceria loading. J. CO2 Util. 2017, 19, 284–295. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Makri, M.M.; Djinovic, P.; Erjavec, B.; Pintar, A.; Efstathiou, A.M. Dry reforming of methane over 5wt% Ni/Ce1-xPrxO2-δ catalysts: Performance and characterisation of active and inactive carbon by transient isotopic techniques. Appl. Catal. B-Environ. 2016, 197, 168–183. [Google Scholar] [CrossRef]
- Bellido, J.D.A.; Assaf, E.M. Effect of the Y2O3-ZrO2 support composition on nickel catalyst evaluated in dry reforming of methane. Appl. Catal. A-Gen. 2009, 352, 179–187. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. Effects of K2O promoter on the activity and stability of nickel catalysts supported on mesoporous nanocrystalline zirconia in CH4 reforming with CO2. Energy Fuel 2008, 22, 2195–2202. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; AlKetbi, M.; Polychronopoulou, K.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar] [CrossRef]
- Velasquez, M.; Batiot-Dupeyrat, C.; Gallego, L.; Santamaria, A. Chemical and morphological characterization of multi-walled-carbon nanotubes synthesized by carbon deposition from an ethanol-glycerol blend. Diam. Relat. Mater. 2014, 50, 38–48. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; El-Desouki, D.S.; Aboul-Gheit, A.K. Catalytic thermal decomposition of methane to COx-free hydrogen and carbon nanotubes over MgO supported bimetallic group VIII catalysts. Appl. Surf. Sci. 2014, 296, 100–107. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charisiou, N.D.; Siakavelas, G.I.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Papadakis, V.G.; Wang, W.; Polychronopoulou, K.; Goula, M.A. Structural Investigation of the Carbon Deposits on Ni/Al2O3 Catalyst Modified by CaO-MgO for the Biogas Dry Reforming Reaction. Chem. Proc. 2020, 2, 15. https://doi.org/10.3390/ECCS2020-07569
Charisiou ND, Siakavelas GI, Sebastian V, Hinder SJ, Baker MA, Papadakis VG, Wang W, Polychronopoulou K, Goula MA. Structural Investigation of the Carbon Deposits on Ni/Al2O3 Catalyst Modified by CaO-MgO for the Biogas Dry Reforming Reaction. Chemistry Proceedings. 2020; 2(1):15. https://doi.org/10.3390/ECCS2020-07569
Chicago/Turabian StyleCharisiou, Nikolaos D., Georgios I. Siakavelas, Victor Sebastian, Steven J. Hinder, Mark A. Baker, Vagelis G. Papadakis, Wen Wang, Kyriaki Polychronopoulou, and Maria A. Goula. 2020. "Structural Investigation of the Carbon Deposits on Ni/Al2O3 Catalyst Modified by CaO-MgO for the Biogas Dry Reforming Reaction" Chemistry Proceedings 2, no. 1: 15. https://doi.org/10.3390/ECCS2020-07569




