1. Introduction
Alkaline water-splitting electrolysis is a mature technology, with a long track-record of producing hydrogen for industrial applications going back over many decades. Despite this, its use for energy capture and storage applications has been severely limited by its high cost. Hydrogen is inevitably cheaper to produce by the reforming of low-cost hydrocarbons such as methane, rather than via the splitting of a thermodynamically stable compound such as water. However, as a result of the inexorable and readily apparent consequences of global warming, the case for modern societies with decarbonised energy systems continues to grow. This case has been strengthened further by the coronavirus lockdown of 2020, which within weeks led to drastic reductions in the levels of carbon-related air pollution normally experienced by many of the world’s urban inhabitants.
An example of such decarbonisation is the commitment made by the city of Copenhagen to achieve carbon neutrality by 2025, largely by further extension of existing initiatives. Currently, 36% of all journeys within the city are made by bicycle, and there are plans to bring 85% of the population within 600
of a subway station. However, the bulk of the reductions will be achieved by alternative methods for the generation of heat and electricity, particularly the concept of combined heat and power. Thus, the city boasts not just the world’s largest district heating system, but also a district cooling system based on seawater that, compared with conventional air-conditioning, results in a reduction in carbon emissions of 70% (sources:
https://www.theguardian.com/environment/2013/apr/12/copenhagen-push-carbon-neutral-2025,
https://www.weforum.org/agenda/2019/05/the-copenhagen-effect-how-europe-can-become-heat-efficient/).
Amongst the buildings in Copenhagen that already benefit from such low-carbon cooling are data centres, which globally have a carbon footprint that is of great concern. The combined carbon output of all the world’s communication technology is already on a par with that of the aviation industry [
1], but unlike the aviation industry, its emissions are set to triple in the next 10 years [
2]. Within that, the electricity consumption of data centres and the associated wired networks to connect them is predicted to quintuple by 2030, accounting for nearly 70% of total Information and Communications Technology (ICT) consumption [
2]. With energy usage doubling every four years, by 2040 if unchecked the consumption would be equal to that of the US (source:
https://www.computerworld.com/article/3431148).
Meanwhile, conventional forms of fossil-derived energy have experienced reduced levels of demand. At the peak of the lockdown the price of aviation fuel fell to 80% below pre-2020 levels at just
$16 per barrel (source:
https://www.iata.org/en/publications/economics/fuel-monitor/ viewed using Wayback Machine
https://web.archive.org for 11 May 2020). Three weeks earlier, as a result of contractual obligations by traders to buy oil even though they had nowhere to store it, the benchmark price of US crude oil fell to minus
$37 a barrel. Although in the short term this may make it harder for renewable energy to compete on economic terms with legacy generation, in the longer term it highlights the decreasing return on investment available within the oil and gas sector.
If water-splitting electrolysis is to be competitive, efficiency must be increased and costs reduced, and to this end many technologies and materials have been investigated [
3,
4,
5]. However, it is often the case that increases in efficiency have only been achieved by large increases in cost. This particularly applies to acidic PEM electrolysis, which has remained stubbornly dependent on noble-metal catalysts, particularly for oxygen evolution [
6]. Not only does this raise cost, but the scarcity of such metals means that no such technology could ever be scaled. High temperature electrolysis similarly trades electrical efficiency against formidable challenges regarding choice of materials and system longevity. This therefore leaves alkaline electrolysis as the technology still most likely to make up the bulk of future capacity. Meanwhile, if the cost of renewable generation continues to halve every few years, (source:
https://www.gov.uk/government/news/offshore-wind-energy-revolution-to-provide-a-third-of-all-uk-electricity-by-2030) the economic case for high efficiency electrolysis could disappear faster than it can be developed.
This brings older technologies back to the fore, and there are few more venerable than Raney nickel. The material was invented in 1926 by American engineer Murray Raney, when seeking an improved method for the hydrogenation of vegetable oils, and is created by dissolving nickel in molten aluminium. Zinc or chromium is then added whilst quenching to produce an alloy, which subsequently undergoes a process called ‘activation’. This refers specifically to the reaction of the alloy with sodium hydroxide:
which selectively leaches out the more reactive alloying components, such as Al, to leave just the nickel behind. This creates a sponge-like material, the catalytic properties of which are based not just on its very much increased surface area, but also on nickel’s ability to adsorb hydrogen. Of all the first-row transition elements, nickel adheres most closely to Sabatier’s Principle, which states:
the best catalysts should bind atoms and molecules with an intermediate strength: not too weakly in order to be able to activate the reactants, and not too strongly to be able to desorb the products [
7]
This is equivalent to stating that the optimum binding energy occurs close to the thermoneutral state, i.e., where
[
8]. At this balance point, the entropy change to and from hydrogen gas is equalised by the enthalpy of adsorption, and hydrogen is thus able to adsorb and desorb at the fastest possible rate. This balance point is exemplified by platinum, which consequently has the second highest exchange current density of any known material. Once incorporated as platinum black, a form with a larger surface area, its ability to adsorb and desorb hydrogen, and thus resist changes in potential, is precisely what makes it a key component of the ultimate reference electrode. It is also why platinum appears close to the top of volcano plots for hydrogen evolution activity [
9]. However, in the absence of abundant supplies of platinum, nickel constitutes a far more practical choice.
The activation of Raney nickel relies on the amphoteric properties of aluminium hydroxide. This means that even though it is almost completely insoluble in water, it is able to dissolve in strong sodium hydroxide by producing sodium aluminate:
Thus the hydroxide has reacted with another hydroxide, as if it were an acid. Less concentrated NaOH will result in the precipitation of Al(OH)
3, thus blocking the pores and reducing the surface area of the catalyst. By contrast, decreasing the temperature of the solute slows the reaction down, and tends to increase the surface area [
10]. An alternative employed by Schiller et al. with Raney nickel deposited by vacuum plasma spraying was 30 wt% KOH at 80
. However, the precipitation of aluminium hydroxide still had to be avoided by the inclusion of 10 wt% K-Na-tartrate-tetrahydrate [
11].
Alloying is not the only method by which Raney nickel can be produced, and electrodeposition methods have been investigated commercially since the 1950’s [
12], and in academia since the 1980’s [
12,
13,
14,
15], in addition to other methods such as pressed powders and plasma spraying [
16,
17]. Although typically investigated for hydrogen evolution [
18,
19,
20,
21,
22,
23,
24,
25], as well as in combination with non-abundant compounds [
26,
27,
28], the coating is also known to perform well for oxygen evolution [
29,
30].
With electrodeposited Raney nickel, the aluminium is exchanged for zinc, but is otherwise completely analogous. The deposition of zinc not just with nickel, but with many ferrous metals, is regarded as an example of Anomalous Co-Deposition (ACD). The anomaly is based on the observation that the less noble metal can be deposited preferentially, and at a higher percentage than is available in the electrolyte [
31]. Several theories have been proposed to explain this anomaly, although without any conclusive success [
32]. It is known that a lower deposition current density leads to a higher mass fraction of deposited zinc, and thus after activation the potential for a higher surface area of the finished coating. For example, at a density of 10
−2 the proportion of zinc was measured as 93 wt% [
32]. Despite this, deposits which are 50 wt% zinc are thought to be the most effective for electrolysis [
19], therefore it is expected that a higher current density will produce the best results.
It is known that pure, polished nickel electrodes are vulnerable to a decrease in activity of up to 400
when used for alkaline hydrogen evolution, which has been attributed to hydride formation [
33]. It is also known that nickel is more likely to form hydrides in NaOH than KOH [
34], as well as at high current density and strong electrolyte [
35]. However, this effect was not observed with aluminium-based Raney nickel until the fraction of aluminium fell to very low levels [
36]. This loss of Al/Zn is driven by the sort of reduced duty-cycle which is typical of renewable energy, with each current interruption causing the cathode to become briefly anodic, thus leaching out the less noble components. The Zn is therefore able to protect the nickel cathode from current interruption corrosion by acting sacrificially. This finding has been confirmed over long-term intermittent experiments on Raney nickel electrodes, where it was observed that Zn was selectively removed from the cathode, but the levels of nickel remained unaffected [
37].
Here we investigate the remarkable properties of the ‘Raney 2.0’ variant of the Raney nickel coating, as deposited on plain 316-grade stainless steel, and show that it has an overpotential for hydrogen evolution of just 28 in 1 KOH, making it one of the highest performing electrocatalysts yet reported. In combination with its modest overpotential for oxygen evolution, this makes it one of the highest performing bifunctional electrocatalysts reported, and certainly the simplest. We show that this activity can be related to its high capacitance and surface area, and also show that a novel technique for the fitting of a transient simulation of an RQ network produced an extremely convincing match to the measured CV waveforms, thus providing a useful theoretical basis for all high surface area research.
2. Materials and Methods
All procedures were conducted in standard laboratory 100 mL beakers. Such beakers are large enough to accommodate up to two
paddle-shaped 316-grade stainless-steel (316SS) electrodes, as shown in
Figure 1a. All chemicals were standard reagent grade. For Raney1 the counter-electrode was made from a graphite rod. For Raney2 the counter-electrode was made from 316SS, which was partially consumed during the deposition, thereby progressively altering the composition of the coating. The typical appearance of the Raney2 coating, and the three-electrode cell into which it was assembled, were as shown in
Figure 1b.
Electrodeposition: The 4 cm × 4 cm 316SS electrode was degreased in hot 25 wt% NaOH for 1 min, then submerged in 18 wt% HCl for 1 min at room temperature, before being placed in 70 wt% H
2SO
4 for 3 min at an anodic current of 108 mA m
−2. The electrode was then placed in a nickel strike solution consisting of 240 g L
−1 NiCl
2·6H
2O and 120 mL L
−1 HCl for 5 min at a cathodic current of
mA cm
−2. Between each step the electrode was rinsed with deionised water, and the air-exposure time minimised. At this point the electrode was covered in a thin, adherent coating of nickel that was able to act as a base for any subsequent functional coating [
20]. The electrode was then immersed in a modified Watt’s Bath consisting of 330 g L
−1 NiSO
4·6H
2O, 45 g L
−1 NiCl
2·6H
2O, 37 g L
−1 H
3BO
3 and 20 g L
−1 ZnCl
2 at 50
for 60 min at a cathodic current of
mA cm
−2. Lastly the coating was activated by immersion in 6
NaOH at 50
for 48 h.
Potentiostat: All electrochemical experiments were performed on an Ivium n-Stat potentiostat. The electrolyte was NaOH, and the reference electrode was a commercial Ag/AgCl device containing 3 KCl, which was routinely calibrated against a standard calomel electrode. CV was performed within a 100 range around OCP at rates of 10 −1 or less. The potential was held for 10 s between changes of direction to allow diffusion gradients within the electrolyte to disperse. EIS was performed at frequencies below 10 around OCP, starting at low frequency. Method: impedance; technique: constant E; amplitude: between 10 and 100. The electrode was pretreated for 120 s at OCP to reduce initial transient currents. All EIS results were analysed within the IviumSoft software package, wherein RCR and RQ equivalent circuits were fitted to the results (where Q is the symbol that represents a CPE).
Equivalent Capacitance: According to Brug et al. the double-layer capacitance for the series connection of a resistor and a constant-phase element can be calculated using:
where
,
and
n are the best-fit parameters of an RQ network to either the EIS or CV results [
38,
39]. It is worth noting that in the Brug paper
is referred to as
, and
n is referred to as
.
can be expressed in units of
−1 and
in
, or as an area pseudo-capacitance
Ω
−1 cm
−2 in which case
must be in
. From this the roughness factor can be calculated using:
where
is the capacitance of a completely smooth surface, which is known to be 40
−2 in alkaline conditions [
40].
RQ Transient Best-fit: As a function of time, the voltage across a CPE is given by a convolution integral:
where
is the magnitude of the CPE (or its ‘pseudo-capacitance’),
n is the phase of the CPE, and
is the gamma function [
41]. For cyclic voltammetry, where it is the voltage that is controlled and the current that is measured, solving this involves expressing the electrical network as a differential equation.
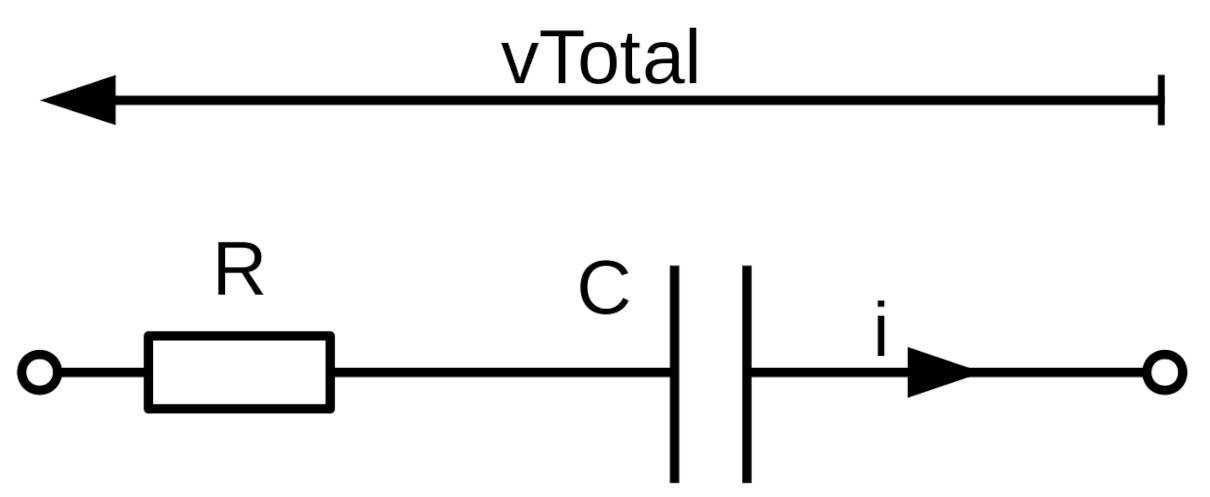
For example, for the basic RC network shown in
Figure 2 the equation can be expressed as:
where
q is electrical charge, such that
, and
is equal to the voltage on the capacitor
. The Euler method can be used to approximate a solution to this equation, based on the first-order simplification that:
where
h is the step size in time. Replacing the capacitor by a CPE, and therefore
by
, produces:
This iterative scheme can be converted into a computer program, permitting the simulation of the response of the network to any arbitrary voltage waveform (see SI of Reference [
42]).
If the values R, and n are taken to be the axes of a three-dimensional solution space, then standard gradient descent techniques can be employed to find the position of the best-fit, i.e., the position that minimises a suitable cost-function, for example one defined as the square of the difference between the measured and simulated waveforms.
4. Comparisons
Hydrogen evolution: A limited survey of recently published results for earth-abundant hydrogen evolution catalysts is as presented in
Table 4. (Abbreviations: CC: carbon cloth; CCH: cobaltous carbonate hydroxide; CFP: carbon fibre paper; CNT: carbon nanotubes; DO: derived oxide; FTO: fluorine-doped tin oxide; GCE: glassy carbon electrode; LDH: layered double hydroxide; MNA: mesoporous nanorod array; MS: microsphere; NA: nanorod array; NF: nickel foam; NP: nanoplates; NR: nanorods; NrGO: nitrogenated reduced graphene oxide; NSh: nanosheets; Nst: nanostructures; NA: nanowire arrays). The table shows that the overpotential, at least at 10
−2, can be almost arbitrarily small. As a result, some authors only quote the overpotential at 100
−2, by which point hydrogen evolution is very well established. Therefore, the table has been generated by taking measurements from published diagrams, so as to establish a common baseline. Many papers also cite a well-known commercial catalyst called ‘PtC’ (platinum on carbon) as a baseline for comparison, therefore it has been included in the table, even though it is not earth-abundant. It is heartening to note that many of the catalysts are able to outperform it, including the Raney2 catalyst studied in this paper.
Oxygen evolution: A limited survey of recently published results for earth-abundant oxygen evolution catalysts is as presented in
Table 5.
Although it can be difficult to make direct comparisons between catalysts, a picture has been emerging over recent years of the limits to which transition metals can be taken. At present this looks to be an overpotential of about 200
at a current density of 10
−2. This current density is chosen because it is the most widely quoted figure, and because it is described as the most significant for solar fuel synthesis [
40]. However, it is not the most significant figure for a commercial electrolyser, which benefits from attaining figures about two orders of magnitude higher. As an illustration of what is possible, figures of 690
−2 at an overpotential of 281
are quoted for the FeP/Ni
2P catalyst, whereas the NiFe-N catalyst has achieved 360
−2 at an overpotential of 255
, fully 220
less than the equivalent figure measured for IrO
2.
The table shows that the Raney2 catalyst is certainly not exceptional, being about 100 behind the leading edge. However, it is unusual in being one of only two bifunctional catalysts listed in the table. It should also be noted that the developers of the leading catalyst, FeCoW, discovered that any annealing of their catalyst had a destructive effect on its catalytic ability. This they ascribed to the phase separation of the ternary catalyst into discrete crystalline particles of Fe3O4, Co3O4 and CoWO4. This therefore highlights the difficulty of producing highly active catalysts that are not subsequently prone to some form of instability.
As a bifunctional catalyst the Raney2 coating is not just highly stable, but also achieves a combined overpotential of just 320
at 10
−2. In addition, since 10 of the higher-performing catalysts in
Table 5 feature nickel or nickel-iron, the scope for increasing its specificity for OER appears promising.