Test of Ecogeographical Rules on Sparrows (Passer spp.) along the Elevation Gradient of the Himalaya in Central Nepal
Abstract
:1. Introduction
2. Materials and Methods
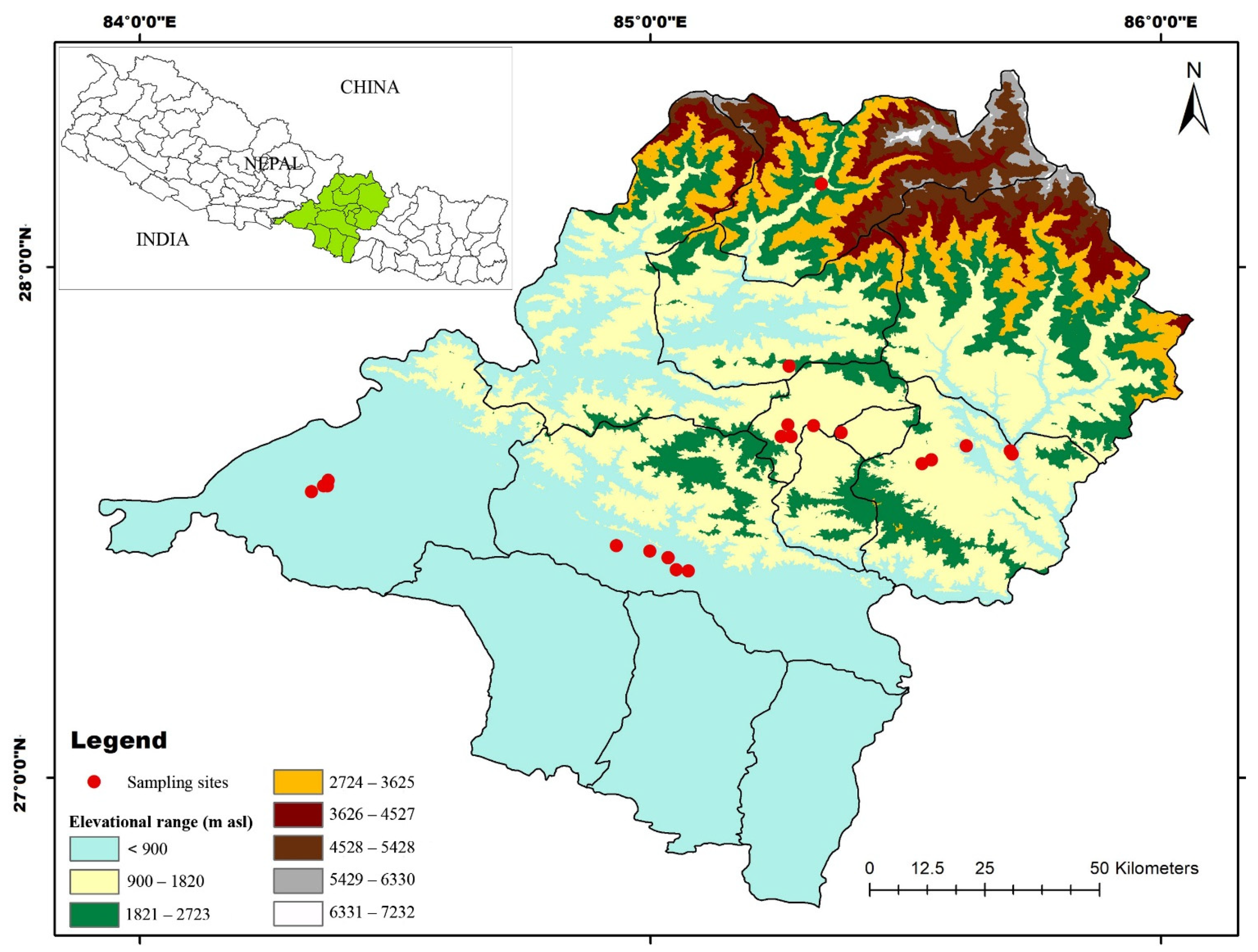
2.1. Study Area
2.2. Field Survey
2.3. Morphological Measurements
2.4. Environmental Variables
2.5. Data Analysis
3. Results
3.1. Elevational Distribution of Sparrows in Central Nepal
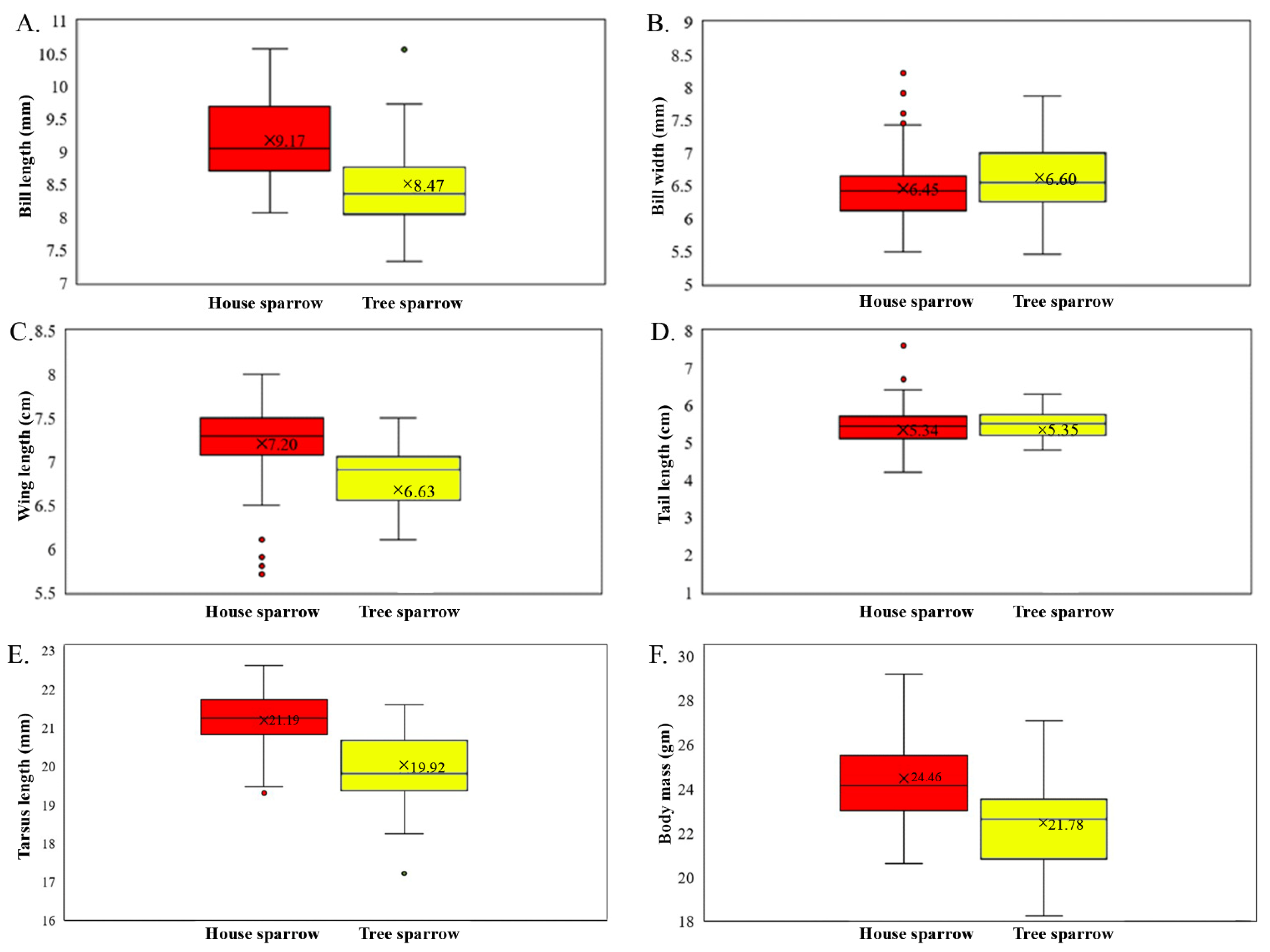
3.2. Inter-Species Morphological Variation
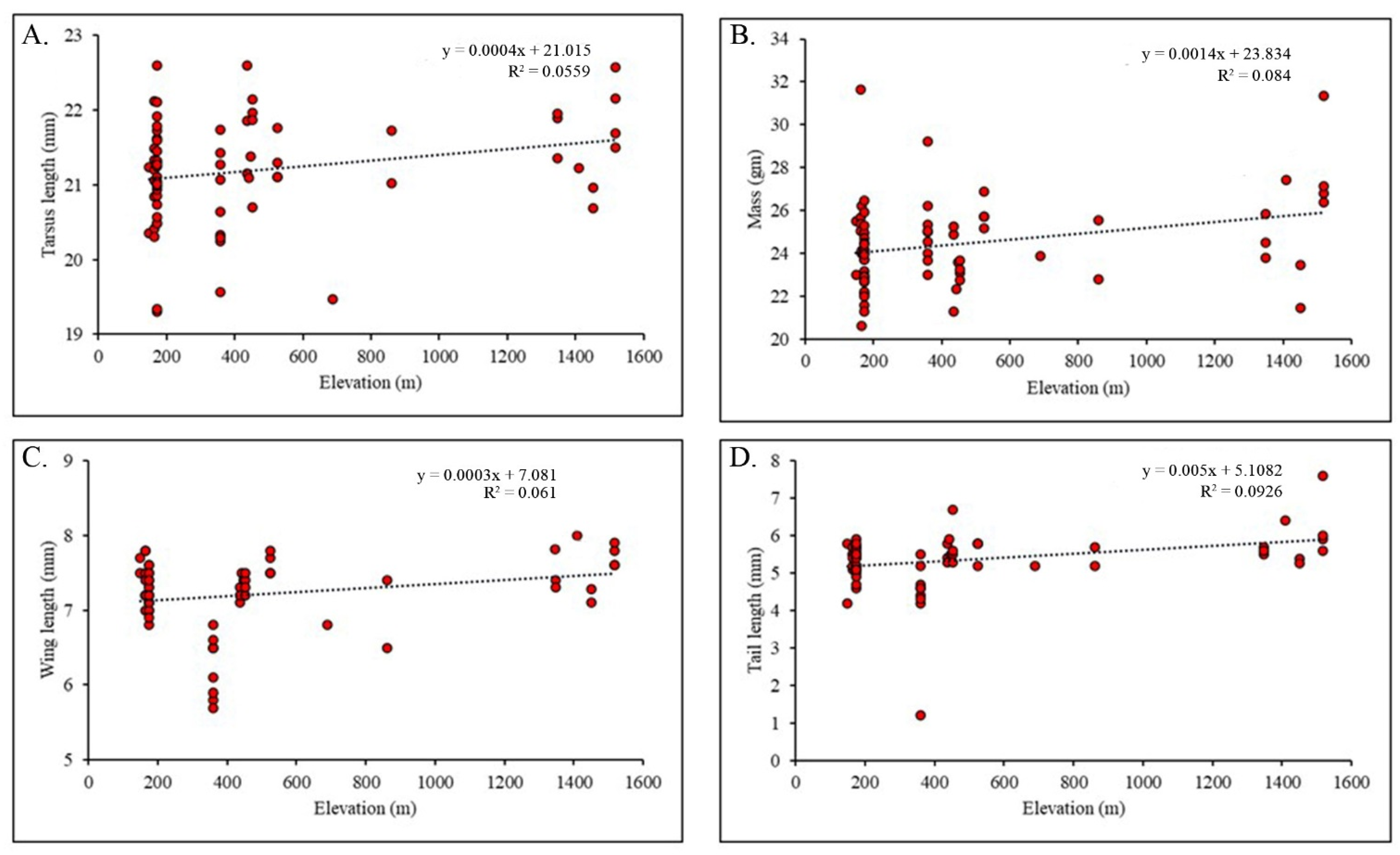
3.3. Morphological Variations along the Elevational Gradient
3.4. Association of Morphology with Climatic Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, T.; Monroe, M.; Lawson, B.; Phill, C.; Ewen, J. Body size changes in passerine birds introduced to New Zealand from the UK. NeoBiota 2013, 17, 1–18. [Google Scholar] [CrossRef]
- Gardner, J.L.; Heinsohn, R.; Joseph, L. Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proc. R. Soc. B Biol. Sci. 2009, 276, 3845–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Gott. Stud. 1848, 1, 585–708. [Google Scholar]
- Allen, J.A. The influence of physical conditions in the genesis of species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Salewski, V.; Watt, C. Bergmann’s rule: A biophysiological rule examined in birds. Oikos 2017, 126. [Google Scholar] [CrossRef]
- Watt, C.; Mitchell, S.; Salewski, V. Bergmann’s rule; a concept cluster? Oikos 2010, 119, 89–100. [Google Scholar] [CrossRef]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Hawkins, B.A. Bergmann’s rule and the mammal fauna of northern North America. Ecography 2004, 27, 715–724. [Google Scholar] [CrossRef]
- Ashton, K.G. Patterns of within-species body size variation of birds: Strong evidence for Bergmann’s rule. Global Ecol. Biogeogr. 2002, 11, 505–523. [Google Scholar] [CrossRef]
- Romano, A.; Séchaud, R.; Roulin, A. Geographical variation in bill size provides evidence for Allen’s rule in a cosmopolitan raptor. Global Ecol. Biogeogr. 2020, 29, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.M.; Redding, D.W.; Dyer, E.E. Bergmann’s rule in alien birds. Ecography 2019, 42, 102–110. [Google Scholar] [CrossRef]
- Mungee, M.; Pandit, R.; Athreya, R. Taxonomic scale dependency of Bergmann’s patterns: A cross-scale comparison of hawkmoths and birds along a tropical elevational gradient. J. Trop. Ecol. 2021, 37, 302–312. [Google Scholar] [CrossRef]
- Freeman, B.G. Little evidence for Bergmann’s rule body size clines in passerines along tropical elevational gradients. J. Biogeogr. 2017, 44, 502–510. [Google Scholar] [CrossRef]
- Fan, L.; Cai, T.; Xiong, Y.; Song, G.; Lei, F. Bergmann’s rule and Allen’s rule in two passerine birds in China. Avian Res. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Fu, Y.; Yeh, C.F.; Yeung, C.K.; Hung, H.; Yao, C.J.; Shaner, P.L.; Li, S.H. Morphological variations in a widely distributed Eastern Asian passerine cannot be consistently explained by ecogeographic rules. Ecol. Evol. 2021, 11, 15249–15260. [Google Scholar] [CrossRef]
- Esteban, L.; Campos, F.; Ariño, A.H. Biometrics amongst Dippers Cinclus cinclus in the north of Spain. Ringing Migr. 2000, 20, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Yom-Tov, Y.; Yom-Tov, S.; Wright, J.; JR Thorne, C.; Du Feu, R. Recent changes in body weight and wing length among some British passerine birds. Oikos 2006, 112, 91–101. [Google Scholar] [CrossRef]
- Hayes, F.E. Geographic variation, hybridization, and the leapfrog pattern of evolution in the Suiriri flycatcher (Suiriri suiriri) complex. Auk 2001, 118, 457–471. [Google Scholar] [CrossRef]
- Uddin, K.; Shrestha, H.L.; Murthy, M.; Bajracharya, B.; Shrestha, B.; Gilani, H.; Pradhan, S.; Dangol, B. Development of 2010 national land cover database for the Nepal. J. Environ. Manag. 2015, 148, 82–90. [Google Scholar] [CrossRef]
- Shaw, L.M.; Chamberlain, D.; Evans, M. The House Sparrow Passer domesticus in urban areas: Reviewing a possible link between post-decline distribution and human socioeconomic status. J. Ornithol. 2008, 149, 293–299. [Google Scholar] [CrossRef]
- Summers-Smith, J.D. The decline of the House Sparrow: A review. Br. Birds 2003, 96, 439–446. [Google Scholar]
- Inskipp, C.; Baral, H.; Phuyal, S.; Bhatt, T.; Khatiwada, M.; Inskipp, T.; Khatiwada, A.; Gurung, S.; Singh, P.; Murray, L. The Status of Nepal’s Birds: The National Red List Series; Zoological Society of London: London, UK, 2016. [Google Scholar]
- Kansakar, S.R.; Hannah, D.M.; Gerrard, J.; Rees, G. Spatial pattern in the precipitation regime of Nepal. Int. J. Climatol. A J. R. Meteorol. Soc. 2004, 24, 1645–1659. [Google Scholar] [CrossRef]
- Paudel, P.K.; Bhattarai, B.P.; Kindlmann, P. An overview of the biodiversity in Nepal. In Himalayan Biodiversity in the Changing World; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–40. [Google Scholar] [CrossRef]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A fixed-radius point count method for nonbreeding and breeding season use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Grimmett, R.; Inskipp, C.; Inskipp, T.; Baral, H.S. Birds of Nepal; Bloomsbury Publishing: London, UK, 2016; p. 368. [Google Scholar]
- FAO. Wild Birds and Avian Influenza: An Introduction to Applied Field Research and Disease Sampling Techniques; FAO Animal Production and Health Manual: Rome, Italy, 2007. [Google Scholar]
- Baldwin, S.P.; Oberholser, H.C.; Worley, L.G. Measurements of Birds; Cleveland Museum of Natural History: Cleveland, OH, USA, 1993; p. 165. [Google Scholar]
- Gosler, A.; Greenwood, J.; Baker, J.; Davidson, N. The field determination of body size and condition in passerines: A report to the British Ringing Committee. Bird Study 1998, 45, 92–103. [Google Scholar] [CrossRef]
- Andrew, S.C.; Awasthy, M.; Griffith, A.D.; Nakagawa, S.; Griffith, S.C. Clinal variation in avian body size is better explained by summer maximum temperatures during development than by cold winter temperatures. Auk Ornithol. Adv. 2018, 135, 206–217. [Google Scholar] [CrossRef] [Green Version]
- R-Core-Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 21 November 2021).
- Summers-Smith, J.D. Studies of West Palearctic birds. Br. Birds 1998, 91, 125. [Google Scholar]
- Chamberlain, D.E.; Toms, M.P.; Cleary-McHarg, R.; Banks, A.N. House sparrow (Passer domesticus) habitat use in urbanized landscapes. J. Ornithol. 2007, 148, 453–462. [Google Scholar] [CrossRef]
- Cordero, P.J.; Summers-Smith, J.D. Hybridization between house and tree sparrow (Passer domesticus, P. montanus). J. Für Ornithol. 1993, 134, 69–77. [Google Scholar] [CrossRef]
- Robinson, R.A.; Siriwardena, G.M.; Crick, H.Q. Size and trends of the House Sparrow Passer domesticus population in Great Britain. Ibis 2005, 147, 552–562. [Google Scholar] [CrossRef]
- von Post, M.; Smith, H.G. Effects on rural House Sparrow and Tree Sparrow populations by experimental nest-site addition. J. Ornithol. 2015, 156, 231–237. [Google Scholar] [CrossRef]
- Vepsäläinen, V.; Pakkala, T.; Tiainen, J. Population increase and aspects of colonization of the Tree Sparrow Passer montanus, and its relationships with the House Sparrow Passer domesticus, in the agricultural landscapes of Southern Finland. Ornis Fenn. 2005, 82, 117–128. [Google Scholar]
- Šálek, M.; Riegert, J.; Grill, S. House sparrows Passer domesticus and Tree sparrows Passer montanus: Fine-scale distribution, population densities, and habitat selection in a Central European city. Acta Ornithol. 2015, 50, 221–232. [Google Scholar] [CrossRef]
- Salewski, V.; Hochachka, W.M.; Fiedler, W. Global warming and Bergmann’s rule: Do central European passerines adjust their body size to rising temperatures? Oecologia 2010, 162, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Kirchman, J.J.; Schneider, K.J. Range expansion and the breakdown of Bergmann’s Rule in red-bellied woodpeckers (Melanerpes carolinus). Wilson J. Ornithol. 2014, 126, 236–248. [Google Scholar] [CrossRef]
- Nwaogu, C.J.; Tieleman, B.I.; Bitrus, K.; Cresswell, W. Temperature and aridity determine body size conformity to Bergmann’s rule independent of latitudinal differences in a tropical environment. J. Ornithol. 2018, 159, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Felemban, H.M.; Price, T.D. Morphological differences among populations of House Sparrows from different altitudes in Saudi Arabia. Wilson Bull. 1997, 109, 539–544. [Google Scholar]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic gradients in body size: A clarification of Bergmann’s rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Andrade, D.V.; Abe, A.S. Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 2009, 325, 468–470. [Google Scholar] [CrossRef] [Green Version]
- Symonds, M.R.; Tattersall, G.J. Geographical variation in bill size across bird species provides evidence for Allen’s rule. Am. Nat. 2010, 176, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, K.R.; Vetaas, O.R. Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Glob. Ecol. Biogeogr. 2003, 12, 327–340. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Song, G.; Lei, F.; Li, D.; Wu, Y. The role of climate factors in geographic variation in body mass and wing length in a passerine bird. Avian Res. 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.S.E. Behavioral thermoregulation in mammals and birds. Int. J. Biometeorol. 1964, 7, 231–240. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Geffen, E. Geographic variation in body size: The effects of ambient temperature and precipitation. Oecologia 2006, 148, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.S. Seasonality and patterns of natural selection for life histories. Am. Nat. 1979, 114, 569–583. [Google Scholar] [CrossRef]
- Khandelwal, S.; Goyal, R.; Kaul, N.; Mathew, A. Assessment of land surface temperature variation due to change in elevation of area surrounding Jaipur, India. Egypt. J. Remote Sens. Space Sci. 2018, 21, 87–94. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Wake, C.P.; Dibb, J.E.; Mayewski, P.A. Precipitation fluctuations in the Nepal Himalaya and its vicinity and relationship with some large scale climatological parameters. Int. J. Climatol. A J. R. Meteorol. Soc. 2000, 20, 317–327. [Google Scholar] [CrossRef]


| Species | Parameter | Body Mass | Tarsus Length | Wing Length | Tail Length | Bill Length | Bill Width |
|---|---|---|---|---|---|---|---|
| P. domesticus | P | 0.02 | 0.05 | 0.04 | 0.01 | 0.02 | 0.02 |
| r2 | 0.08 | 0.06 | 0.06 | 0.09 | 0.08 | 0.14 | |
| P. montanus | P | 0.88 | 0.96 | 0.26 | 0.96 | 0.88 | 0.16 |
| r2 | 0.001 | 0.0001 | 0.05 | 0.0001 | 0.001 | 0.07 |
| Traits | Parameter | Bio1 | Bio4 | Bio5 | Bio6 | Bio7 | Bio10 | Bio11 | Bio12 | Bio15 | Bio18 | Bio19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body mass | p | ns | ns | ns | ns | ns | ns | ns | 0.001 * | 0.01 * | ns | ns |
| r2 | ns | 0.144 | 0.08 | |||||||||
| Tarsus Length | p | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| r2 | ns | |||||||||||
| Wing length | p | ns | 0.01 * | ns | ns | ns | ns | ns | ns | 0.022 * | Ns | <0.01 * |
| r2 | ns | 0.08 | 0.075 | 0.19 | ||||||||
| Tail length | p | ns | ns | ns | ns | ns | ns | ns | ns | Ns | Ns | 0.02 * |
| r2 | ns | 0.07 | ||||||||||
| Bill length | p | <0.01 * | <0.01 * | <0.01 * | <0.01 * | <0.01 * | <0.01* | <0.01 * | ns | <0.01 * | <0.01 * | <0.01 * |
| r2 | 0.301 | 0.33 | 0.32 | 0.27 | 0.35 | 0.31 | 0.28 | 0.35 | 0.23 | 0.24 | ||
| Bill width | p | ns | ns | ns | ns | ns | ns | ns | <0.01 * | ns | ns | ns |
| r2 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dangol, D.; Khanal, L.; Pandey, N.; Ghimire, A.; Kyes, R.C. Test of Ecogeographical Rules on Sparrows (Passer spp.) along the Elevation Gradient of the Himalaya in Central Nepal. Ecologies 2022, 3, 480-491. https://doi.org/10.3390/ecologies3040034
Dangol D, Khanal L, Pandey N, Ghimire A, Kyes RC. Test of Ecogeographical Rules on Sparrows (Passer spp.) along the Elevation Gradient of the Himalaya in Central Nepal. Ecologies. 2022; 3(4):480-491. https://doi.org/10.3390/ecologies3040034
Chicago/Turabian StyleDangol, Deepa, Laxman Khanal, Naresh Pandey, Anuj Ghimire, and Randall C. Kyes. 2022. "Test of Ecogeographical Rules on Sparrows (Passer spp.) along the Elevation Gradient of the Himalaya in Central Nepal" Ecologies 3, no. 4: 480-491. https://doi.org/10.3390/ecologies3040034






