The Rotenone Models Reproducing Central and Peripheral Features of Parkinson’s Disease
Abstract
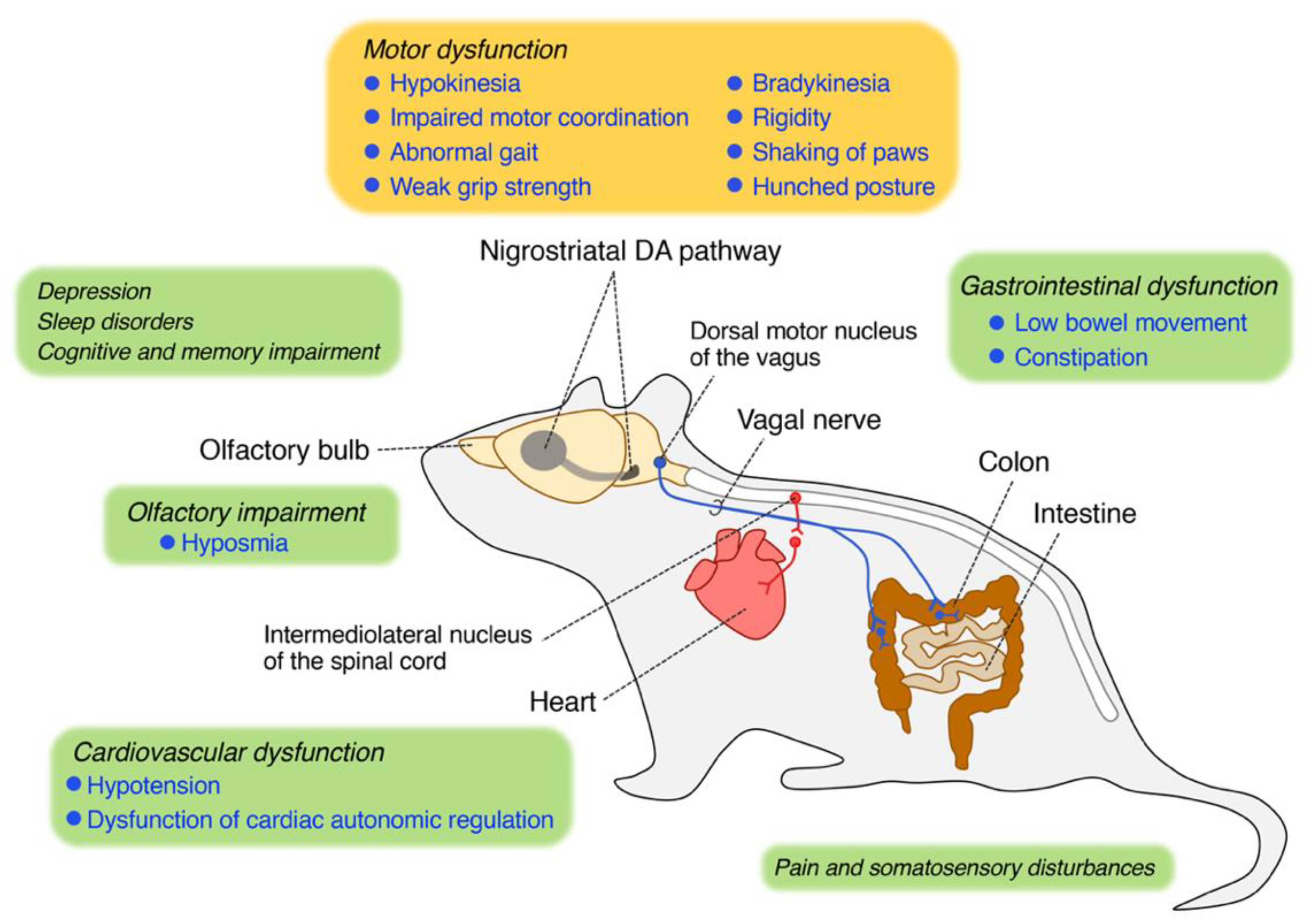
:1. Introduction
2. Rotenone-Induced Central and Peripheral Neuropathology
2.1. Neuropathology in the CNS
2.2. Neuropathology in the PNS
3. Rotenone Reproduced Motor Features of PD
4. Rotenone Reproduced Non-Motor Features of PD
4.1. Olfactory Impairment
4.2. Gastrointestinal Dysfunction
4.3. Cardiovascular Dysfunction
4.4. Depression
4.5. Sleep Disorders
4.6. Cognitive and Memory Impairment
4.7. Pain and Somatosensory Disturbances
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Otsuka, M. Life style risks of Parkinson’s disease: Association between decreased water intake and constipation. J. Neurol. 2004, 251 (Suppl. 7), vII18–vII23. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The gut-brain axis in Parkinson’s disease: Possibilities for food-based therapies. Eur. J. Pharmacol. 2017, 817, 86–95. [Google Scholar] [CrossRef]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [Green Version]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Kim, J.H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Tarbutton, G.L.; Levin, J.L.; Plotkin, G.M.; Lowry, L.K.; Nalbone, J.T.; Shepherd, S. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J. Agromed. 2008, 13, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Nandipati, S.; Litvan, I. Environmental Exposures and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2016, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Inden, M.; Kitamura, Y.; Abe, M.; Tamaki, A.; Takata, K.; Taniguchi, T. Parkinsonian rotenone mouse model: Reevaluation of long-term administration of rotenone in C57BL/6 mice. Biol. Pharm. Bull. 2011, 34, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 2015, 46, 101–116. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Imafuku, F.; Sun, J.; Kikuoka, R.; Furukawa, C.; Asanuma, M. Chronic Systemic Exposure to Low-Dose Rotenone Induced Central and Peripheral Neuropathology and Motor Deficits in Mice: Reproducible Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3254. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Miyazaki, I.; Miyoshi, K.; Asanuma, M. Long-Term Systemic Exposure to Rotenone Induces Central and Peripheral Pathology of Parkinson’s Disease in Mice. Neurochem. Res. 2015, 40, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, H.; Miyazono, S.; Noguchi, T.; Kashiwayanagi, M. Intranasal Administration of Rotenone to Mice Induces Dopaminergic Neurite Degeneration of Dopaminergic Neurons in the Substantia Nigra. Biol. Pharm. Bull. 2017, 40, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Yanagida, T.; Inden, M.; Takata, K.; Kitamura, Y.; Yamakawa, K.; Sawada, H.; Izumi, Y.; Yamamoto, N.; Kihara, T.; et al. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson’s disease models. J. Neurosci. Res. 2009, 87, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Zaitone, S.A.; Ahmed, E.; Elsherbiny, N.M.; Mehanna, E.T.; El-Kherbetawy, M.K.; ElSayed, M.H.; Alshareef, D.M.; Moustafa, Y.M. Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson’s disease therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, H.; Guo, X.; Ge, D.; Shi, Y.; Lu, X.; Lu, J.; Chen, J.; Ding, F.; Zhang, Q. Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models. Int. J. Environ. Res. Public Health 2019, 16, 3811. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, J.D.; Song, L.K.; Li, J.; Chu, S.F.; Yuan, Y.H.; Chen, N.H. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav. Brain Res. 2015, 294, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Hara, D.B.; Bicca, M.A.; Poli, A.; Takahashi, R.N. Early signs of colonic inflammation, intestinal dysfunction, and olfactory impairments in the rotenone-induced mouse model of Parkinson’s disease. Behav. Pharmacol. 2018, 29, 199–210. [Google Scholar] [CrossRef]
- Sasajima, H.; Miyazono, S.; Noguchi, T.; Kashiwayanagi, M. Intranasal administration of rotenone in mice attenuated olfactory functions through the lesion of dopaminergic neurons in the olfactory bulb. Neurotoxicology 2015, 51, 106–115. [Google Scholar] [CrossRef]
- Voronkov, D.N.; Kutukova, K.A.; Ivanov, M.V.; Khudoerkov, R.M. Immunomorphological Changes in the Olfactory Bulbs of Rats after Intranasal Administration of Rotenone. Bull. Exp. Biol. Med. 2017, 164, 203–206. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Jellinger, K.A. Neuropathology of Nonmotor Symptoms of Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 13–62. [Google Scholar] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.A.; Sauerbier, A.; Politis, M.; Carr, H.; Loehrer, P.; Chaudhuri, K.R. Presynaptic dopaminergic terminal imaging and non-motor symptoms assessment of Parkinson’s disease: Evidence for dopaminergic basis? Parkinsons Dis. 2017, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.F.; Silva, C.M.; D’Unhao, A.M.; Ferrari, M.F. Aged Lewis rats exposed to low and moderate doses of rotenone are a good model for studying the process of protein aggregation and its effects upon central nervous system cell physiology. Arq. Neuropsiquiatr. 2016, 74, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, G.M.; Holton, J.L.; Revesz, T.; Dickson, D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011, 122, 187–204. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Feger, J.; Prigent, A.; Michel, P.P.; Parain, K.; Champy, P.; Ruberg, M.; Oertel, W.H.; Hirsch, E.C. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 2003, 84, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Drolet, R.E.; Cannon, J.R.; Montero, L.; Greenamyre, J.T. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol. Dis. 2009, 36, 96–102. [Google Scholar] [CrossRef]
- Pan-Montojo, F.; Anichtchik, O.; Dening, Y.; Knels, L.; Pursche, S.; Jung, R.; Jackson, S.; Gille, G.; Spillantini, M.G.; Reichmann, H.; et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 2010, 5, e8762. [Google Scholar] [CrossRef] [Green Version]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.M.; Rodrigo-Angulo, M.; et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef] [Green Version]
- Lebouvier, T.; Chaumette, T.; Damier, P.; Coron, E.; Touchefeu, Y.; Vrignaud, S.; Naveilhan, P.; Galmiche, J.P.; Bruley des Varannes, S.; Derkinderen, P.; et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 2008, 57, 1741–1743. [Google Scholar] [CrossRef] [Green Version]
- Shannon, K.M.; Keshavarzian, A.; Mutlu, E.; Dodiya, H.B.; Daian, D.; Jaglin, J.A.; Kordower, J.H. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 2012, 27, 709–715. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Binienda, Z.K.; Sarkar, S.; Mohammed-Saeed, L.; Gough, B.; Beaudoin, M.A.; Ali, S.F.; Paule, M.G.; Imam, S.Z. Chronic exposure to rotenone, a dopaminergic toxin, results in peripheral neuropathy associated with dopaminergic damage. Neurosci. Lett. 2013, 541, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; Dodiya, H.B.; Broersen, L.M.; Douna, H.; van Wijk, N.; Lopes da Silva, S.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. Gut-brain and brain-gut axis in Parkinson’s disease models: Effects of a uridine and fish oil diet. Nutr. Neurosci. 2018, 21, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonia Angeline, M.; Chaterjee, P.; Anand, K.; Ambasta, R.K.; Kumar, P. Rotenone-induced parkinsonism elicits behavioral impairments and differential expression of parkin, heat shock proteins and caspases in the rat. Neuroscience 2012, 220, 291–301. [Google Scholar] [CrossRef]
- Richter, F.; Hamann, M.; Richter, A. Chronic rotenone treatment induces behavioral effects but no pathological signs of parkinsonism in mice. J. Neurosci. Res. 2007, 85, 681–691. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Aurich, M.F.; Rodrigues, L.S.; Targa, A.D.S.; Noseda, A.C.D.; Cunha, F.D.W.; Lima, M.M.S. Olfactory impairment is related to REM sleep deprivation in rotenone model of Parkinson’s disease. Sleep Sci. 2017, 10, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.S.; Targa, A.D.; Noseda, A.C.; Aurich, M.F.; Da Cunha, C.; Lima, M.M. Olfactory impairment in the rotenone model of Parkinson’s disease is associated with bulbar dopaminergic D2 activity after REM sleep deprivation. Front. Cell. Neurosci. 2014, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.D.; Petrovitch, H.; White, L.R.; Masaki, K.H.; Tanner, C.M.; Curb, J.D.; Grandinetti, A.; Blanchette, P.L.; Popper, J.S.; Ross, G.W. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001, 57, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, E.; Navarro-Otano, J.; Tolosa, E.; Gaig, C.; Compta, Y.; Rey, M.J.; Marti, M.J.; Hernandez, I.; Valldeoriola, F.; Rene, R.; et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. 2014, 29, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Lebouvier, T.; Neunlist, M.; Bruley des Varannes, S.; Coron, E.; Drouard, A.; N’Guyen, J.M.; Chaumette, T.; Tasselli, M.; Paillusson, S.; Flamand, M.; et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE 2010, 5, e12728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasselli, M.; Chaumette, T.; Paillusson, S.; Monnet, Y.; Lafoux, A.; Huchet-Cadiou, C.; Aubert, P.; Hunot, S.; Derkinderen, P.; Neunlist, M. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol. Motil. 2013, 25, e183–e193. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.G.; Noorian, A.R.; Srinivasan, S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp. Neurol. 2009, 218, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Orimo, S.; Uchihara, T.; Nakamura, A.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008, 131, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Fukumitsu, N.; Suzuki, M.; Fukuda, T.; Kiyono, Y.; Kajiyama, S.; Saji, H. Reduced 125I-meta-iodobenzylguanidine uptake and norepinephrine transporter density in the hearts of mice with MPTP-induced parkinsonism. Nucl. Med. Biol. 2006, 33, 37–42. [Google Scholar] [CrossRef]
- Takatsu, H.; Nishida, H.; Matsuo, H.; Watanabe, S.; Nagashima, K.; Wada, H.; Noda, T.; Nishigaki, K.; Fujiwara, H. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: Clinical and experimental studies with radiolabeled MIBG. J. Nucl. Med. 2000, 41, 71–77. [Google Scholar]
- Zhang, Z.; Du, X.; Xu, H.; Xie, J.; Jiang, H. Lesion of medullary catecholaminergic neurons is associated with cardiovascular dysfunction in rotenone-induced Parkinson’s disease rats. Eur. J. Neurosci. 2015, 42, 2346–2355. [Google Scholar] [CrossRef]
- Shiba, M.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Anxiety disorders and depressive disorders preceding Parkinson’s disease: A case-control study. Mov. Disord. 2000, 15, 669–677. [Google Scholar] [CrossRef]
- Madiha, S.; Haider, S. Curcumin restores rotenone induced depressive-like symptoms in animal model of neurotoxicity: Assessment by social interaction test and sucrose preference test. Metab. Brain Dis. 2019, 34, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Lima, M.M.; Martynhak, B.J.; Santiago, R.; Takahashi, T.T.; Ariza, D.; Barbiero, J.K.; Andreatini, R.; Vital, M.A. Characterization of motor, depressive-like and neurochemical alterations induced by a short-term rotenone administration. Pharmacol. Rep. 2012, 64, 1081–1090. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Kim, T.W.; Lee, J.M.; Sung, Y.H.; Lim, B.V. Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J. Exerc. Rehabil. 2017, 13, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Zaminelli, T.; Gradowski, R.W.; Bassani, T.B.; Barbiero, J.K.; Santiago, R.M.; Maria-Ferreira, D.; Baggio, C.H.; Vital, M.A. Antidepressant and antioxidative effect of Ibuprofen in the rotenone model of Parkinson’s disease. Neurotox Res. 2014, 26, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Hu, M.T.M. Sleep Disturbance as Potential Risk and Progression Factor for Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, P.L.; Tsai, C.H.; Lu, M.K.; Liu, H.J.; Chen, Y.C.; Chang, F.C. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 2007, 30, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Darweesh, S.K.L.; Wolters, F.J.; Postuma, R.B.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.K.; Ikram, M.A. Association Between Poor Cognitive Functioning and Risk of Incident Parkinsonism: The Rotterdam Study. JAMA Neurol. 2017, 74, 1431–1438. [Google Scholar] [CrossRef]
- Huang, X.; Chen, P.C.; Poole, C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology 2004, 62, 2198–2202. [Google Scholar] [CrossRef] [Green Version]
- Ghebremedhin, E.; Del Tredici, K.; Vuksic, M.; Rub, U.; Thal, D.R.; Burbach, G.J.; Rosenberger, A.; Bickeboller, H.; Deller, T.; de Vos, R.A.; et al. Relationship of apolipoprotein E and age at onset to Parkinson disease neuropathology. J. Neuropathol. Exp. Neurol. 2006, 65, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.K.; Ji, Z.S.; Dodson, S.E.; Miranda, R.D.; Rosenblum, C.I.; Reynolds, I.J.; Freedman, S.B.; Weisgraber, K.H.; Huang, Y.; Mahley, R.W. Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J. Biol. Chem. 2011, 286, 5215–5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, S.; Madiha, S.; Batool, Z. Amelioration of motor and non-motor deficits and increased striatal APoE levels highlight the beneficial role of pistachio supplementation in rotenone-induced rat model of PD. Metab. Brain Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, R.; Shifrin, A.; Rogowski, Z.; Sprecher, E.; Melamed, E.; Yarnitsky, D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 2004, 62, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Valek, L.; Auburger, G.; Tegeder, I. Sensory neuropathy and nociception in rodent models of Parkinson’s disease. Dis. Model. Mech. 2019, 12, dmm039396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, A.V.; Tankisi, H.; Finnerup, N.B.; Fuglsang-Frederiksen, A.; Jennum, P.; Svendsen, K.B.; Kirov, F.I.; Otto, M. Somatosensory function is impaired in patients with idiopathic REM sleep behaviour disorder. Sleep Med. 2018, 42, 83–89. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chung, J.M.; Chung, K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008, 447, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Farombi, E.O.; Awogbindin, I.O.; Olorunkalu, P.D.; Ogbuewu, E.; Oyetunde, B.F.; Agedah, A.E.; Adeniyi, P.A. Kolaviron protects against nigrostriatal degeneration and gut oxidative damage in a stereotaxic rotenone model of Parkinson’s disease. Psychopharmacol. (Berl) 2020. [Google Scholar] [CrossRef]
- Bassani, T.B.; Gradowski, R.W.; Zaminelli, T.; Barbiero, J.K.; Santiago, R.M.; Boschen, S.L.; da Cunha, C.; Lima, M.M.; Andreatini, R.; Vital, M.A. Neuroprotective and antidepressant-like effects of melatonin in a rotenone-induced Parkinson’s disease model in rats. Brain Res. 2014, 1593, 95–105. [Google Scholar] [CrossRef]
- Cicchetti, F.; Drouin-Ouellet, J.; Gross, R.E. Environmental toxins and Parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 2009, 30, 475–483. [Google Scholar] [CrossRef]
| Animal | Dose Route | Central Neuropathology | Peripheral Neuropathology | Motor Symptom | Non-motor Symptom | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | OB | Other | DMV | ENS | Other | OI | GI | Other | ||||
| SD, Lewis rat | 2–3 mg/kg i.v. | + | + | [12] | ||||||||
| Lewis rat | 1–3 mg/kg s.c. | + | Hip, LC | [11,13,34] | ||||||||
| Lewis rat | 2.75–3 mg/kg i.p. | + | + | [16] | ||||||||
| Wistar rat | 2.5 mg/kg i.n. | + | [29] | |||||||||
| Wistar rat | 12 ug/site intranigral | + | + | Hip | + | + | Dep | [49,50,63] | ||||
| Lewis rat | 2.5 mg/kg i.v. | + | PP, LC Hip Cb, Cx | + | [36] | |||||||
| Lewis rat | 1, 2 mg/kg i.p. | + | + | [37] | ||||||||
| Lewis rat | 3 mg/kg s.c. | - | + | [55] | ||||||||
| SD rat | 2 mg/kg s.c. | + | ScN | + | [43] | |||||||
| Wistar rat | 1, 2.5 mg/kg i.p. | + | RVLM | + | CV Dep | [59,62,65] | ||||||
| Wistar rat | 1.5 mg/kg s.c. | + | Hip | Dep | [61] | |||||||
| SD rat | 3 mg/kg s.c. | + | DR | + | Dep Sleep | [64,67] | ||||||
| Wistar rat | 1.5, 3 mg/kg s.c. | + | + | CI | [72,73] | |||||||
| C57BL mouse | 10, 30 mg/kg p.o. | + | + | + | + | [17,23,44,54] | ||||||
| Swiss mouse | 1 mg/kg s.c. | + | + | [24,45] | ||||||||
| ICR mouse | 1, 3 mg/kg i.p. | + | + | [25] | ||||||||
| C57BL mouse | 5 mg/kg s.c. | - | + | [46] | ||||||||
| BALB/c mouse | 0.35 mg/kg i.n. | + | + | + | + | [22,28] | ||||||
| C57BL mouse | 50 mg/kg s.c. | + | + | + | + | [21] | ||||||
| C57BL mouse | 5 mg/kg contact | + | + | + | + | + | + | [26] | ||||
| Swiss mouse | 30 mg/kg p.o. | + | + | + | + | + | [27] | |||||
| C57BL mouse | 5 mg/kg p.o. | + | + | + | IML | + | + | [38,39] | ||||
| C57BL mouse | 2.5 mg/kg s.c. | + | + | + | + | + | [19,20] | |||||
| C57BL mouse | 5.4 µg/site intrastriatal | + | + | + | + | CI | [7,44] | |||||
| C57BL mouse | 50–2500 pmol intrathecal | Pain | [77] | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, I.; Asanuma, M. The Rotenone Models Reproducing Central and Peripheral Features of Parkinson’s Disease. NeuroSci 2020, 1, 1-14. https://doi.org/10.3390/neurosci1010001
Miyazaki I, Asanuma M. The Rotenone Models Reproducing Central and Peripheral Features of Parkinson’s Disease. NeuroSci. 2020; 1(1):1-14. https://doi.org/10.3390/neurosci1010001
Chicago/Turabian StyleMiyazaki, Ikuko, and Masato Asanuma. 2020. "The Rotenone Models Reproducing Central and Peripheral Features of Parkinson’s Disease" NeuroSci 1, no. 1: 1-14. https://doi.org/10.3390/neurosci1010001





