Impact of Solvent Type on Total Phenol and Flavonoid Content and Sun Protection Factor of Crude Cashew Nutshell Liquid
Abstract
:1. Introduction
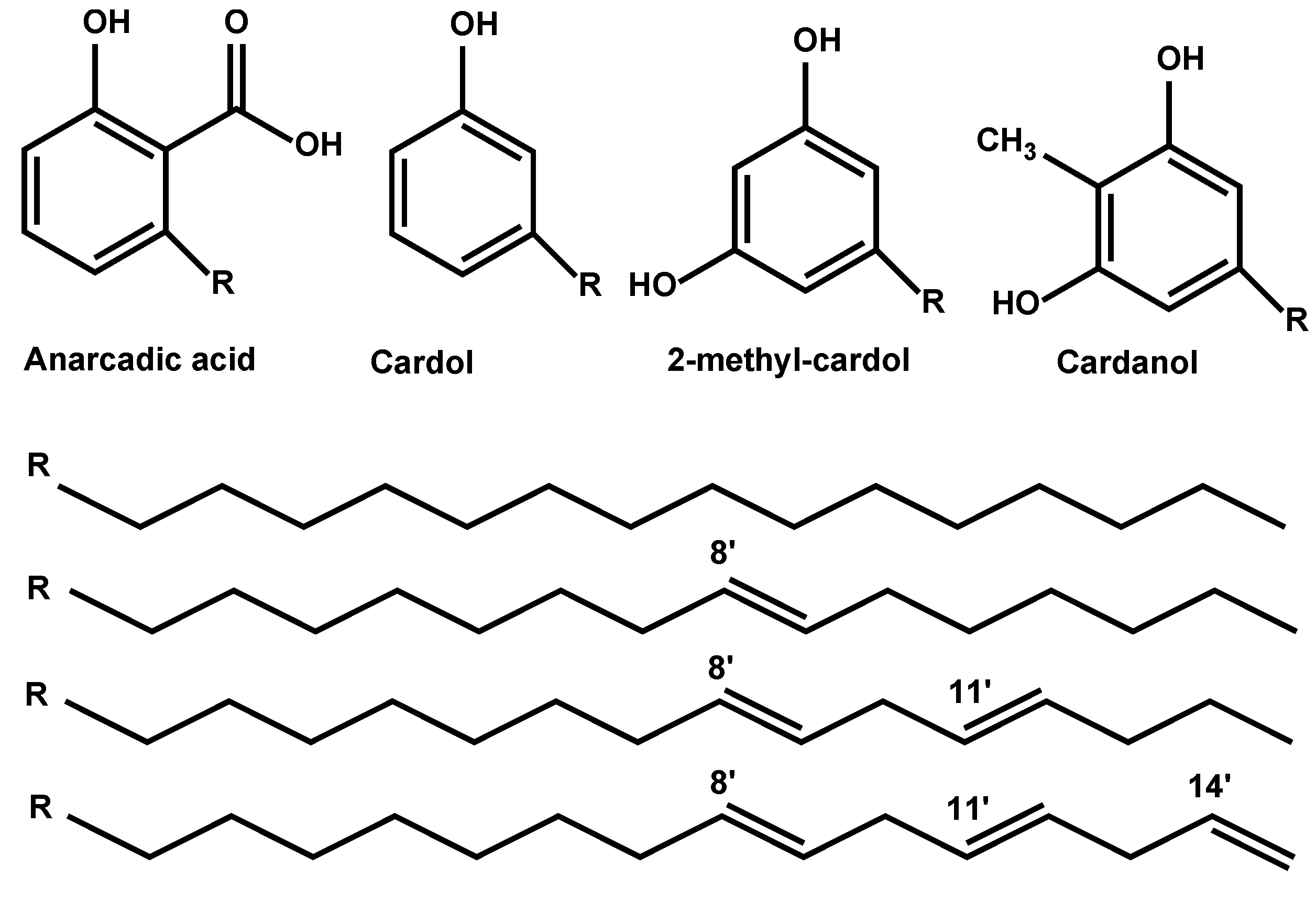
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
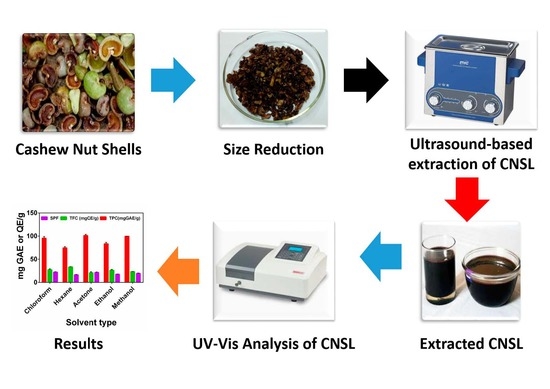
2.3. Sample Collection and Preparation
2.4. Extraction of CNSL
2.5. Determination of Total Phenol Content (TPC)
2.6. Determination of Total Flavonoid Content (TFC)
2.7. Determination of the Sun Protection Factor (SPF)
- CF = correction factor (10);
- EE = erythemogenic effect of radiation with wavelength;
- I = solar intensity spectrum;
- Abs (λ) = spectrophotometric absorbance values at wavelength.
2.8. Statistical Analyses
3. Results and Discussion
3.1. Percent Yield (%)
3.2. Total Phenolic Content (TPC)
3.3. Total Flavonoid Content (TFC)
3.4. Sun Protection Factor (SPF)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorrell, S.; Speirs, J.; Bentley, R.; Brandt, A.; Miller, R. An Assessment of the Evidence for a Near-Term Peak in Global Oil Production; UK Energy Research Centre: London, UK, 2009; p. 228. [Google Scholar]
- Vörös, Z. EU–China Relations:“Race” for Raw Materials. In Tuka, Ágnes–Tarrósy, István: Borderless Europe–Challenges–Opportunities; Publikon Kiadó: Pécs, Hungary, 2010. [Google Scholar]
- Akram, Q.F. Commodity prices, interest rates and the dollar. Energy Econ. 2009, 31, 838–851. [Google Scholar] [CrossRef] [Green Version]
- Pathak, C.; Mandalia, H.C. Petroleum industries: Environmental pollution effects, management and treatment methods. Int. J. Sep. Environ. Sci. 2012, 1, 55. [Google Scholar]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luque, R.; Sepulveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.K.; Bhatia, S. The current status and perspectives of biofuel production via catalytic cracking of edible and non-edible oils. Energy 2010, 35, 111–119. [Google Scholar] [CrossRef]
- Murugesan, A.; Umarani, C.; Chinnusamy, T.; Krishnan, M.; Subramanian, R.; Neduzchezhain, N. Production and analysis of bio-diesel from non-edible oils—A review. Renew. Sustain. Energy Rev. 2009, 13, 825–834. [Google Scholar] [CrossRef]
- Bragoni, V.; Rit, R.K.; Kirchmann, R.; Trita, A.S.; Gooßen, L.J. Synthesis of bio-based surfactants from cashew nutshell liquid in water. Green Chem. 2018, 20, 3210–3213. [Google Scholar] [CrossRef]
- Panda, H. The Complete Book on Cashew (Cultivation, Processing & By-Products): Cashew Nut Processing Industry in India, Cashew Nut Processing Plant, Cashew Nut Processing Projects, Cashew Nut Processing with CNSL Business, Cashew Nut Shell Liquid Product and Uses, Cashew Nut Small Business Manufacturing, Cashew Nuts Processing Small Business Project, Cashew Processing Unit, Food Processing Business List; Asia Pacific Business Press Inc.: New Delhi, India, 2013. [Google Scholar]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Banchhor, M.; Baid, R. CNSL (Cashew Nut Shell Liquid)—A versatile renewable natural resource. Plant Arch. 2007, 7, 497–501. [Google Scholar]
- Wadgaonkar, P.P. Cashew Nut Shell Liquid (CNSL): A Versatile Renewable Raw Material of Great Industrial Promise. Available online: https://ibmm.umontpellier.fr/IMG/pdf/Wadgaonkar_Total_06-06_abstract.pdf (accessed on 13 May 2022).
- Kubo, J.; Lee, J.R.; Kubo, I. Anti-Helicobacter pylori agents from the cashew apple. J. Agric. Food Chem. 1999, 47, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Kanyaboon, P. The Antiviral Activity of Phenolic Lipids Group against Dengue Virus; Chulalongkorn University: Bangkok, Thailand, 2017. [Google Scholar]
- Kamble, K.; Thakor, N. Effect of Temperature on Physical Properties of CNSL based Termiticides. Int. J. Environ. Agric. Res. 2017, 3, 28–34. [Google Scholar]
- Raja, K. Comparative performance on insecticidal and oviposition deterrence of cashew nut shell liquid (CNSL) on bruchids (Callosobruchus chinensis L.) in cowpea (Vigna unguiculata (L.) Walp.) seed. J. Biopestic. 2015, 8, 147. [Google Scholar]
- Kubo, I.; Komatsu, S.; Ochi, M. Molluscicides from the cashew Anacardium occidentale and their large-scale isolation. J. Agric. Food Chem. 1986, 34, 970–973. [Google Scholar] [CrossRef]
- Ademola, I.; Eloff, J.N. Anthelmintic efficacy of cashew (Anarcadium occidentale L.) on in vitro susceptibility of the ova and larvae of Haemonchus contortus. Afr. J. Biotechnol. 2011, 10, 9700–9705. [Google Scholar]
- Telascrêa, M.; Leão, A.L.; Ferreira, M.Z.; Pupo, H.F.d.F.; Cherian, B.M.; Narine, S. Use of a cashew nut shell liquid resin as a potential replacement for phenolic resins in the preparation of panels—A review. Mol. Cryst. Liq. Cryst. 2014, 604, 222–232. [Google Scholar] [CrossRef]
- Parkin, D.; Mesher, D.; Sasieni, P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br. J. Cancer 2011, 105, S66–S69. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, Z.; Behzadifar, M.; Behzadifar, M.; Fitzmaurice, C.; Abate, D. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. Glob. Burd. Cancer 2021, 5, 1747–1769. [Google Scholar]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Glanz, K.; Mayer, J.A. Reducing ultraviolet radiation exposure to prevent skin cancer: Methodology and measurement. Am. J. Prev. Med. 2005, 29, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.K.; Samant, M.; Srivastava, R. Natural sunscreen agents: A review. Sch. Acad. J. Pharm. 2013, 2, 458–463. [Google Scholar]
- Food and Drug Administration. Sunscreen: How to Help Protect Your Skin from the Sun. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun#spf (accessed on 6 May 2022).
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Adverse reactions to sunscreen agents: Epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis 2014, 25, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, C.; Schwack, W. Photoprotection in changing times–UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [Green Version]
- Sudjaroen, Y. Antioxidant, antibacterial, and cytotoxicity activities of cashew (Anacardium occidentale) nut shell waste. Int. J. Green Pharm. 2018, 12, S229–S234. [Google Scholar]
- Fact MR. Sun Protection Products Market. Available online: https://www.factmr.com/report/140/sun-protection-products-market (accessed on 3 June 2022).
- V.G. Demand for Sun Protection Products to Remain Strong, Despite COVID-19 Impact. Available online: https://www.premiumbeautynews.com/en/demand-for-sun-protection-products,16786#:~:text=The%20global%20market%20of%20sun,capita%20spending%20on%20cosmeceutical%20products (accessed on 3 June 2022).
- Gandhi, T.; Patel, M.; Dholakiya, B. Studies on effect of various solvents on extraction of cashew nut shell liquid (CNSL) and isolation of major phenolic constituents from extracted CNSL. J. Nat. Prod. Plant Resour. 2012, 2, 135–142. [Google Scholar]
- Gandhi, T.S.; Dholakiya, B.Z.; Patel, M.R. Extraction protocol for isolation of CNSL by using protic and aprotic solvents from cashew nut and study of their physico-chemical parameter. Pol. J. Chem. Technol. 2013, 15, 24–27. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Uslu, N.; Özcan, M.M. Effect of microwave heating on phenolic compounds and fatty acid composition of cashew (Anacardium occidentale) nut and oil. J. Saudi Soc. Agric. Sci. 2019, 18, 344–347. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Martínez Aguilera, M. Determining Optimal Cashew Nut Shell Liquid Extraction Method. 2019. Available online: https://www.academia.edu/40796194/Determining_Optimal_Cashew_nut_shell_liquid_ExtractioN_meThod_Final_report_project (accessed on 13 May 2022).
- Hsu, C.Y. Antioxidant activity of extract from Polygonum aviculare L. Biol. Res. 2006, 39, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.A.L.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn. Mag. 2015, 11, 96. [Google Scholar] [PubMed] [Green Version]
- Dutra, E.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. Ciênc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Mansur, J.d.S.; Breder, M.; Mansur, M.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Şengün, D. Extraction of Phenolic Compounds from Hazelnut Shell Waste; İzmir Institute of Technology: Izmir, Turkey, 2018. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Mubofu, E.; Mgaya, J. Chemical valorization of cashew nut shell waste. Top. Curr. Chem. 2018, 376, 8. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, M.S.; Atarod, M.; Sajjadi, M.; Isaabadi, Z. An Introduction to Green Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Gómez-Caravaca, A.M.; Verardo, V.; Caboni, M.F. Chromatographic techniques for the determination of alkyl-phenols, tocopherols and other minor polar compounds in raw and roasted cold pressed cashew nut oils. J. Chromatogr. A 2010, 1217, 7411–7417. [Google Scholar] [CrossRef]
- Yuliana, M.; Tran-Thi, N.Y.; Ju, Y.-H. Effect of extraction methods on characteristic and composition of Indonesian cashew nut shell liquid. Ind. Crops Prod. 2012, 35, 230–236. [Google Scholar] [CrossRef]
- Nyirenda, J.; Zombe, K.; Kalaba, G.; Siabbamba, C.; Mukela, I. Exhaustive valorization of cashew nut shell waste as a potential bioresource material. Sci. Rep. 2021, 11, 11986. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Khalili, M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from Iran. Trop. J. Pharm. Res. 2019, 18, 1443–1448. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1041. [Google Scholar]
- Maisarah, A.; Nurul Amira, B.; Asmah, R.; Fauziah, O. Antioxidant analysis of different parts of Carica papaya. Int. Food Res. J. 2013, 20, 1043–1048. [Google Scholar]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, E.M.; Romeiro, L.A.S.; Rodrigues, A.P.; Alves, P.S.; da Silva, V.C.; Logrado, L.P.L.; dos Santos, M.L.; Murta, M.M.; da Costa Leitão, Á.A.; Garcia, S. Novel ultraviolet absorbers derived from cashew nut shell liquid: Spectrophotometric, in silico and in vitro assays. Drug Anal. Res. 2020, 4, 40–49. [Google Scholar] [CrossRef]
- Balgude, D.; Sabnis, A.S. CNSL: An environment friendly alternative for the modern coating industry. J. Coat. Technol. Res. 2014, 11, 169–183. [Google Scholar] [CrossRef]

| λ | EE × I (Normalized) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1 |
| Solvents | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) | SPF |
|---|---|---|---|---|
| Chloroform | 34.8 ± 0.4 a,c | 95.4 ± 3.7 | 27.2 ± 2.2 | 22.1 ± 1.1 a |
| Hexane | 30.4 ± 0.7 c | 74.5 ± 2.7 a | 33.3 ± 0.7 d | 16.4 ± 0.8 b |
| Acetone | 33.3 ± 0.4 d,c | 101.2 ± 2.5 b | 20.6 ± 2.5 | 21.5 ± 1.1 c,a |
| Ethanol | 49.3 ± 3.2 | 83.3 ± 3.1 | 26.1 ±1.9 | 17.4 ± 0.9 d |
| Methanol | 47.8 ± 0.9 b | 99.5 ± 0.10 b,c | 23.2 ± 0.5 e | 19.3 ± 1.0 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zombe, K.; Nyirenda, J.; Lumai, A.; Phiri, H. Impact of Solvent Type on Total Phenol and Flavonoid Content and Sun Protection Factor of Crude Cashew Nutshell Liquid. Sustain. Chem. 2022, 3, 334-344. https://doi.org/10.3390/suschem3030021
Zombe K, Nyirenda J, Lumai A, Phiri H. Impact of Solvent Type on Total Phenol and Flavonoid Content and Sun Protection Factor of Crude Cashew Nutshell Liquid. Sustainable Chemistry. 2022; 3(3):334-344. https://doi.org/10.3390/suschem3030021
Chicago/Turabian StyleZombe, Kadango, James Nyirenda, Agape Lumai, and Hellen Phiri. 2022. "Impact of Solvent Type on Total Phenol and Flavonoid Content and Sun Protection Factor of Crude Cashew Nutshell Liquid" Sustainable Chemistry 3, no. 3: 334-344. https://doi.org/10.3390/suschem3030021