Abstract
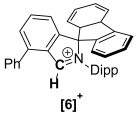
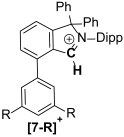
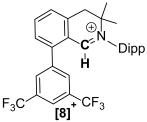
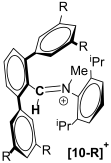
The synthesis of a small library of fused Cyclic Aryl Amino Carbon (f-CArAC) carbene precursors in the form of 1,1,2,4-tetraaryl-1H-isoindol-2-ium triflate (6), (7-R) (R = tBu, CF3) or 3,3-dimethyl-2,8-bis-arene-substituted-3,4-dihydro-isoquinolin-2-ium hydrogen-dichloride (8) and 2,4,8-tri(substituted)-isoquinolin-2-ium tosylate salts (12) has been achieved. All of them feature an arene incorporated on the annulated benzene ring of the corresponding heterocycle, introduced at the early stages of their synthesis via the Suzuki cross-coupling reaction between 2,6-dibromo-benzaldehyde and the desired aryl boronic acid. The terphenyl-2′carbaldehyde by-products of this Suzuki reaction are useful starting points for the preparation of two new iminium iodide salts (10-R) (R = H, CF3) as potential precursors to access ACyclic Amino Carbon (ACAC) carbenes. Compounds (6) and (7-tBu) react readily with hydroxide either in THF or in a biphasic Et2O/aqueous OH− solution to produce the substituted isoindolinols (13) and (14), respectively. The thermal dehydration of the former generates the corresponding f-CArAC carbene in situ, which is trapped by Cu(I)Cl furnishing, a rare example of a two-coordinate Cu(I) complex (15) supported by this new ligand scaffold.
1. Introduction
Nitrogen containing heterocycles are ubiquitous compounds with a multitude of applications in polymers [,], pharmaceuticals [,], dyes [,,], organocatalysis [,,,,] and more recently photochemistry [,,,], to name a few. From the standpoint of organometallic chemistry, they feature as important moieties in various ligands, out of which N-heterocyclic-ylidenes (NHCs) [,], incorporating at least another heteroatom in their scaffold [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], are an important class with some representative examples shown in Figure 1(1A). Τheir diverse substitution patterns and architectural motifs enable the manipulation of their intrinsic electronic properties (e.g., HOMO-LUMO gap) [,], as well as their donating (σ-donation/π-acceptance) [,,,,,,,,,,,,,,,,,] and steric parameters [,,,,] especially when coordinated to metals, usually, as L type ligands [,,,,,,,,,].
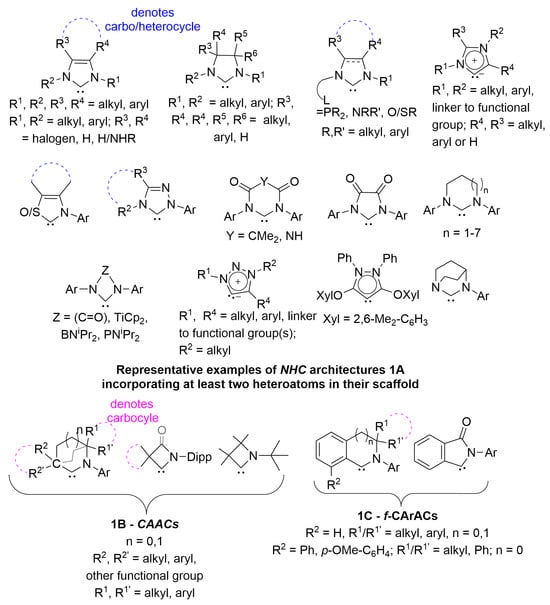
Figure 1.
Representative NHC architectures (1A) (top); Reported scaffolds of CAACs (1B) (below) and f-CArACs (1C); the grey ethylene bridge denotes bicylic CAACs where no R1′, R2′ are present.
Substituting the N-R1 group in the case of saturated imidazolin-2-ylidenes (i.e., second scaffold from left at the top of Figure 1(1A)) by a CRR’ moiety gives rise to Cyclic Alkyl Amino Carbenes (CAACs) (Figure 1(1B))—an important subclass of isolable singlet state carbenes [,,,,,,,,,,,,,,,] with a reduced HOMO-LUMO energy gap. This increases their σ-donating properties, but crucially also renders the orthogonal empty p-orbital of the carbene carbon (i.e., the LUMO) energetically accessible for π-backdonation. As a result, they can promote reactivity reminiscent of transition metals [] and allow the stabilisation and isolation of low coordinate compounds of both transition metals [,,,,,,,,,,,] and main group elements [,,,,,,,,,,,,,,] when used as supporting ligands. This crucial increased π-acidity of CAACs, becomes even more pronounced via benzannulation of their 5 or 6-member scaffold (Figure 1(1C), fused Cyclic Aryl Alkyl Amino Carbenes; f-CArAC) [,,,], which due to conjugation further lowers the energy of this LUMO empty p-orbital. Variations in this latter scaffold featuring more than one nitrogen atom have also been reported [,,].
Our attention was drawn to the two known f-CArAC (1C) salt precursors incorporating an auxiliary arene in the fused benzene ring [], for which no known well-defined complexes have been reported. Therefore, we decided to pursue such ligand motifs to try and address this paucity. In this paper we report on the synthesis, isolation and characterisation of a small library of 1,1,2,4-tetraaryl-1H-isoindol-2-ium (n = 0) and 2,3,3,8-tetra(substituted)-3,4-dihydro-isoquinolin-2-ium (n = 1) carbene salt precursors ([f-CArAC-H]+X− (X = OTf−, [HCl2]−) incorporating such auxiliary arenes. We present aspects of their reactivity ultimately leading to the isolation and structural characterisation of the first f-CArAC: → Cu(I) complex featuring such a motif. As part of these studies, we also demonstrate the use of synthetic intermediates en-route to the afore-mentioned [f-CArAC-H]+X−salts to access iminium salts ([ACAC-H]+) and a 2,4,8-tri(substituted)-isoquinolin-2-ium salts, both of which are of interest as useful carbene precursors.
2. Materials and Methods
2.1. Reagents
All reactions were carried out using standard Schlenk and glovebox techniques unless otherwise stated. For air-sensitive reactions the solvents used were dried by refluxing under an inert atmosphere (N2) either over molten K (THF, n-hexane, toluene) or Na wire/benzophenone-ketyl (Et2O, n-pentane) for a minimum of three days and stored in Young’s or ROTAFLO ampules over activated 4 Å molecular sieves (the activation was performed using a heat-gun (ca 200 °C) under a dynamic vacuum until a stable vacuum ≤ 3 × 10−2 mbar was maintained). All other solvents were used as received and H2O was deionized. Column chromatography was performed using silica gel 0.035–0.07 mm, 60 Å purchased from Thermos Scientific, while TLC plates were from Macherey–Nagel (Alugram Xtra SIL G UV254). HCl 2.5 M solution in CPME (cyclopentyl–methyl-ether) was purchased from Acros in an AcroSeal bottle and was transferred upon arrival in a Young’s ampule and stored under N2 at 5 °C. Triflic anhydride (Tf2O) was purchased from Fluorochem and was transferred in a Young’s ampule upon arrival and stored at 5 °C under N2. Pd(PPh3)4 was purchased from Fluorochem and kept under N2 at 5 °C in a Schlenk flask. n-BuLi (2.5M solution in n-hexane) was purchased from Acros in an AcroSeal bottle and was stored in a glovebox freezer (−30 °C) under N2 and titrated prior to use. Benzophenone was sublimed, recrystallized from PE 40—60, dried and stored in an N2 filled glovebox at RT. Toluene-d8 (C7D8) was freeze-pump-thawed twice, before being refluxed in a Young’s amoule over molten K (120 °C) for a minimum of three days, followed by vac-to-vac transfer under static vacuum over activated 4 Å molecular sieves. All other chemicals and solvents were purchased from commercial sources and were used without further purification unless otherwise stated. The 2,6-dibromo-benzaldehyde was prepared according to the literature using EtOCHO instead of DMF. [] Detailed syntheses of all compounds reported herein are provided in the ESI of this manuscript.
2.2. Spectroscopic and Analytic Techniques
1H, 13C{1H}, 19F NMR as well as correlation spectra were acquired on a Bruker Avance 400 (1H) NMR spectrometer. NMR spectra were referenced using the residual protio signals of the deuterated solvent (1H) or the signals of the solvent (13C{1H}), while in the case of 19F they were referenced externally relative to C6F6; all NMR spectra are reported using the δ scale (ppm). Where unambiguous assignment of resonances is possible, this is provided as per the numbering scheme of each compound in the ESI. IR spectra were recorded on a Shimadzu IRAffinity-1 spectrometer equipped with a QATR 10 single reflection ATR probe. HiRes mass-spectra were measured in ESI+ mode, using either a Brucker Maxis Impact QTOF spectrometer or an Agilent 6550 LC/Q-TOF equipped with an iFunnel sample introduction probe. All these data is provided free of charge in the ESI of this manuscript.
2.3. Single Crystal X-Ray Crystallography
Data for 5-H (CCDC: 2490407), 7-tBu (CCDC: 2490402), 10-CF3 (CCDC: 2490403), 12 (CCDC: 2490408), 14 (CCDC: 2490405), 15 (CCDC: 2490406) and 16 (CCDC: 2490404) were collected at the NKUA X-ray core facility on a dual source (IμS Diamond Cu/Κα and Mo/Κα) Bruker D8-Venture SC-XRD instrument equipped with a Photon-III area detector at 100K using an Oxford Cryosystems 100 cryostream. In the case of 5-H, 7-tBu, 12, 14, 16 and 17 data were collected using Cu/Κα to a resolution of 0.83 Å, while for 5-H to a resolution of 0.79 Å. For compounds 10-CF3 and 15 data were collected using Mo/Kα to a resolution of 0.70 Å.
Data collection was achieved using φ and ω scans to fill the Ewald sphere using a 4-circle kappa goniometer controlled by the APEX5 software package (version 2023.3.2 64-bit) which also handled subsequent processing. A multi-scan absorption correction (SADABS 2016/2) was applied in all cases.
Crystals were mounted on Mitigen cryo-crystallography loops from either dried vacuum-pump oil stored in a N2 filled glovebox over 4 Å molecular sieves (compound (15)) or silicon oil in air.
For the datasets of the compounds listed above, data solution (ShelXT, []) and subsequent model refinement (ShelXL, []) were achieved using the graphic interface of the Olex2-1.5 software package []. For the graphics of the molecular structures ORTEP-III was used [].
All atoms were refined anisotropically and hydrogen atoms were added using the riding model, unless otherwise stated.
Special Refinement Details:
(7-tBu): The model was refined as an inversion twin with a BASF of 0.05. The model shows occupational disorder on one of the tert-butyl groups which was modelled using the PART command. The asymmetric unit contains a molecule of toluene situated at a special position showing occupational disorder over two positions as well as half a molecule of Et2O again at a special position also displaying the same disorder. These disorders were modelled using the PART command, while in the case of the disordered Et2O use of RIGU and ISOR restraints as well as an EADP constrain were necessary for a stable and conversing refinement.
(10-CF3): One of the CF3 substituents displays occupational disorder over two positions of two of its fluorine atoms. This was successfully modelled using the PART command with the occupancy of each site-let to refine freely and converge to a value of ca 60:40. A SADI restraint was used for the C-F distances of the minor part and its respective fluorine atoms were refined isotropically using an ISOR restraint.
(12): Three tert-butyl substituents show occupational disorder over two positions of their methyls (all three methyls for one of them and two of their methyls for the remaining). This was successfully modelled using the PART command with the occupancy of each site-let to refine freely and converge to values of ca 62:38, 72:28 and 86:14. Three of the carbons (C71A, C64A and C63A) belonging to the methyls in these minor parts, were refined isotropically using an ISOR restraint.
All other SC-XRD collection details and final refinement parameters are given in Tables S1 and S2 in the ESI.
3. Results and Discussion
3.1. Synthesis of f-CArAC Salt Precursors
The synthesis of the [f-CArAC-H]+X− salts and their precursors is summarised in Scheme 1:
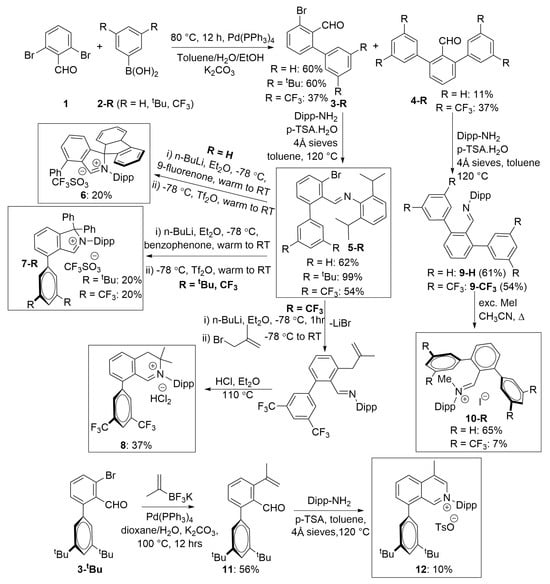
Scheme 1.
Synthetic routes to [f-CArAC-H]+X− salt precursors (isolated yields).
The introduction of the auxiliary arene via the Suzuki coupling between 2,6-dibromo-benzaldehyde 1 and the appropriate arene boronic acid 2-R, is the first stage of the synthesis, and depending on 2-R, a mixture of the corresponding mono and di-substituted benzaldehydes 3-R and 4-R, in varying ratios, is formed (Table 1). Attempts to selectively prepare aldehydes 3-R, starting from 2-iodo-6-bromo-benzaldehyde, did not mitigate the formation of mixtures and were further hampered by the capricious synthesis [,] of this aldehyde, making it unsuitable for scale-up preparations. Introduction of other arenes such as 2,4,6-Me3-C6H2 and 3,5-F2-C6H3, albeit successful under these conditions, produced mixtures of 3-R and 4-R which were unfortunately inseparable by common laboratory methods. Attempts to solve this problem using Pd2(dba)3 in the presence of a Buchwald phosphine in a 1:1 ratio as the pre-catalyst did not fare any better and thus, they were not pursued any further.

Table 1.
Relative ratio of mono- (3-R) and di-arene (4-R) substituted benzaldehydes based on the relative integration of their CHO protons (400 MHz) in the crude reaction mixture.
Condensation of aldehydes 3-R with Dipp-NH2 (Dipp = 2,6-iPr2-C6H3) using ca 5% p-TSA.H2O as a catalyst in the presence of 4 Å molecular sieves in toluene at 120 °C under N2, furnishes imines 5-R in reasonable to excellent yields after column chromatography. Imine 5-H, has been previously reported to be used in the next steps of the synthesis of f-CArAC’s without further purification; in our case this proved problematic, but its purification at this stage is straightforward and resolved the issue. Other anilines (p-toluidine, 3,5-xylyl-amine and aniline) can also be introduced using these conditions but were not amenable to further purification due to their facile hydrolysis.
Starting from imines 5-R, the 1,1,2,4-tetraaryl-1H-isoindol-2-ium triflate [f-CArAC-H]+OTf− salt precursors 6 and 7-R can be accessed in low but re-producible yields via a one-pot procedure. This method has also allowed us to install a planar and non-flexible fluorene moiety in the spiro position of the 5-member heterocycle in the case of 6 by using 9-fluorenone instead of benzophenone. Imines 5-R can also act as versatile starting points for the preparation of 6-member f-CArAC salt precursors, as exemplified by 5-CF3 leading to the isolation of the 3,4-dihydro-isoquinolin-2-ium compound 8 as its HCl2− salt.
Unfortunately, attempts to further derivatise imines 5-R by using them as Suzuki coupling partners with CH2=CMe(BF3K) were unsuccessful, leading to intractable mixtures with some of the identified products resulting from C=N-Dipp bond hydrolysis or de-halogenation. Therefore, we opted to use aldehyde 3-tBu as a model for the Suzuki coupling with CH2=CMe(BF3K); indeed, this strategy proved successful yielding aldehyde 11 in a moderate isolated yield of 56%. The condensation reaction between 11 and Dipp-NH2, under the same conditions for the preparations of imines 5-R, proved sluggish and therefore an increase in catalyst loading to 10% of p-TSA.H2O was used. Surprisingly, this resulted in the one-step formation of the isoquinolin-2-ium tosylate heterocycle 12. A plausible mechanism for the formation of 12 is shown in Scheme 2 involving an intermediary imine and an allylic stabilised terminal carbon-cation to rationalise the formation of the 6-membered ring. When the reaction was repeated under the same conditions, but using a stoichiometric amount of p-TSA.H2O, compound 12 was not formed, and formation of [Dipp-NH3]OTs was observed instead.
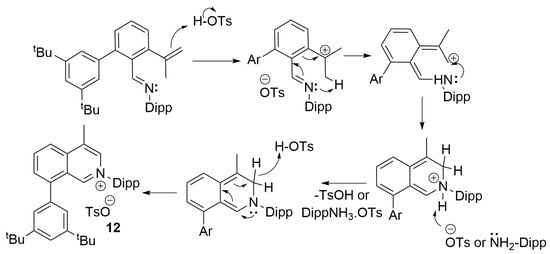
Scheme 2.
Plausible mechanism leading to salt 12.
We have also demonstrated the utility of the two by-products 4-H and 4-CF3 as precursors to access iminium salts 10-R, which we are currently investigating as precursors to access Acyclic Carbon Amino Carbenes [,,,,]. Although the formation of their precursor imines 9-H/CF3 proceeds at reasonable yields, the following quarternisation step in the case of 10-CF3, is low yielding despite prolonged heating and elevated temperatures, most likely due to the electron-withdrawing CF3 substituents.
3.2. Characterisation of f-CArAC Salt Precursors
The afore-mentioned compounds and their precursors have been spectroscopically and analytically characterised. Their Hi-Res ESI+ mass spectra, show the parent [M]+ ion ([f-CArAC-H]+) or the [M+H]+ (precursor imines) or [M+Na]+ (precursor aldehydes) in good agreement with the theoretical values and isotopic envelopes. The NMR (1H, 13C{1H} and 19F) spectra of the reported [f-CArAC-H]X and iminium salts are consistent with an average Cs symmetry in solution. Table 2 and Table 3 summarise the 1H and 13C{1H} NMR chemical shifts in their characteristic protons and corresponding carbons.

Table 2.
Characteristic 1H (400 MHz) and 13C{1H} (100.6 MHz) NMR chemical shifts for the cations in 6, 7-R and 8.

Table 3.
Characteristic (400 MHz) and 13C{1H} (100.6 MHz) chemical shifts for the cations in 10-R and 12.
It is worth pointing out that the organic cation in 12 possesses two sites (marked in red and blue) where potential deprotonation to generate a carbene can take place as reflected by their 1H-NMR chemical shift protons (see Figure S114 for their unambiguous assignment). The structure of the cation in 12 (vide infra) is redolent of a recently reported benzo[h]isoquinolinium salt prepared by Bertrand et al. [], which upon deprotonation furnishes the corresponding pyridin-1-ylidene (i.e., equivalent to removal by base of the red hydrogen in 12+) that is in equilibrium with its pyridine-3-ylidene isomer (i.e., equivalent to deprotonation of the blue hydrogen in 12+), as evidenced by trapping experiments. We are currently exploring the deprotonation of 12 [] especially since the increased steric hindrance imposed by the proximal bulky auxiliary arene could produce the corresponding isoquinolin-3-ylidene selectively.
The identity of compounds 7-tBu, 10-CF3 and 12 was also unambiguously confirmed by SC-XRD studies, and the molecular structures of their cations are shown in Figure 2.
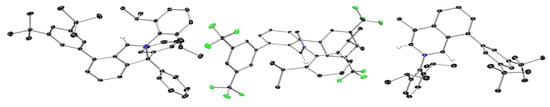
Figure 2.
From left to right: ORTEP-III diagrams of the molecular structures of the cations in 7-tBu, 10-CF3 and 12, respectively, displaying 50% ADP’s; counter-anions, co-crystallisation solvent (C7H8 and Et2O in the case of 7-tBu, CH3CN in the case of 10-CF3 and H2O in the case of 12) and most H atoms have been omitted for clarity (colour code: black: carbon; blue: nitrogen; green: fluorine; wheat: hydrogen).
The solid-state structures agree with their observed NMR spectra and are retained in solution. Their molecular structures exhibit no intra- or inter-molecular interactions between the counter anions and any of the hydrogen in the organic cations.
3.3. Attempts at Generating f-CArAC Free Carbenes and Synthesis of a Cu(I) Complex
Having successfully isolated the small library of [f-CArAC-H]+X− salts 6, 7-R (R = tBu, CF3) and 8 (Scheme 1), we proceeded with their deprotonation to generate and hopefully isolate the corresponding free carbenes. Unfortunately, our efforts using various bases (e.g., AN″ (A = Li+, Na+, K+; N″ = [N(SiMe3)3]−, LiNiPr2, KOtBu, LiTMP (TMP = 2,2.6,6-tetramethyl-piperidide) in THF or Et2O proved invariably unsuccessful, resulting in intractable mixtures even when the subsequent work-up was performed at low temperatures not exceeding −20 °C.
Jana and coworkers (in the case of CAAC’s) [] and more recently Bertrand et al. (for f-CArAC’s) [] have demonstrated that pyrrolidin-2-ols and isoindolin-1-ols (i.e., the products of H-OH addition at the corresponding carbene carbon respectively—Figure 1(1C)), can behave as carbene precursors upon heating with concomitant loss of water. Therefore, in view of our limited success with isolating free carbenes, we decided to explore this route in the presence of Cu(I)Cl to trap any in situ generated carbene. Salts 6 and 7-tBu were chosen as model compounds to test this hypothesis and gauge the effect of the auxiliary’s arene steric hindrance. The corresponding isoindolinols 13 and 14 can be easily synthesised via the reaction of 6 or 7-tBu, respectively, with either anhydrous NaOH in THF or KOH in a biphasic Et2O/KOH(aq) (0.2 M) system (Scheme 3).
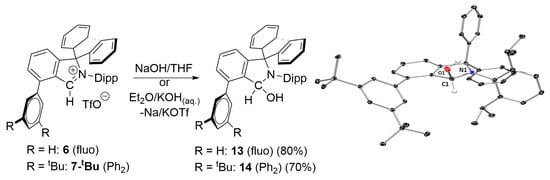
Scheme 3.
Synthesis of isoindolinols 13 and 14 and ORTEP-III diagram of the molecular structure of 14 (50% ADP’s); C1-N1: 1.430(2) Å.
Isoindolinols 13 and 14 have been characterised spectroscopically, analytically and, in the case of the latter, structurally as well. Their 1H-NMR and 13C{1H} spectra display a loss of the Cs symmetry observed in their parent compounds, characteristically evidenced by the succinct appearance of 4 doublets and two septets for the isopropyl substituents of the Dipp arene. Furthermore, the isoindol-2-ium protons (Table 2) for 6 (s, 9.45 ppm) and 7-tBu (s, 9.19) have shifted up-field to 6.70 ppm for 13 and 6.81 for 14, appearing as doublets due to coupling with the OH protons, and resonating at 2.18 ppm in 13 and 2.38 ppm in 14, with a 3JHH coupling of ca 4 Hz. Finally, the C1-N1 (Scheme 3) bond distance of 1.430(2) Å in the solid-state molecular structure of 14 is characteristic of a carbon nitrogen single bond.
Heating a solution of 13 in toluene in the presence of a small excess of Cu(I)Cl and 3Å molecular sieves at 100 °C in a Young’s ampoule overnight, causes a gradual colour change from pale yellow to deep red-purple. After filtration, removal of volatiles and washing with n-hexane to remove coloured impurities, a yellow residue is obtained, which after re-crystallisation from THF/n-hexane produced the desired Cu(I) complex 15 (Scheme 4) in 13–22% (depending on scale) isolated yields crystals suitable for an SC-XRD study (Figure 3).
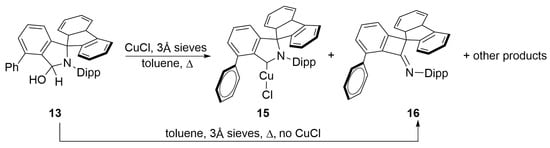
Scheme 4.
Concurrent formation of Cu(I) complex 15 and the ring contraction by-product 16.
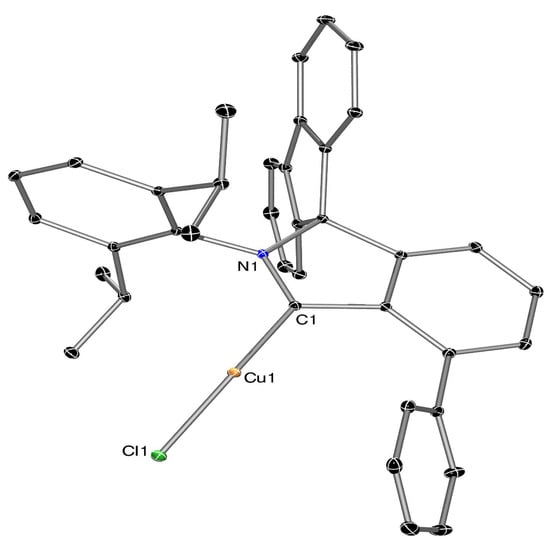
Figure 3.
ORTEP-III diagram of the molecular structure of Cu(I) complex 15 showing 50% ADP’s with H atoms omitted for clarity. Selected bond distances (Å) and angles (°): Cu1-Cl1: 2.1144(3), Cu1-C1: 1.8800(12), N1-C1: 1.3190(16), C1-Cu1-Cl1: 171.20(4).
The geometry around the two-coordinate Cu(I) metal centre in 15 is almost linear with the carbene carbon copper bond distance of 1.8800(12) Å equal within esd’s to the two previously structurally characterised f-CArAC: →Cu(I)-X complexes (X = Cl (1.879(3) Å []), Br (1.884(3) Å []; f-CArAC: = 2-[Dipp]-3,3-diphenyl-2,3-dihydro-1H-isoindol-1-ylidene, i.e., the analogous ligand bearing no auxiliary phenyl and incorporating two phenyls instead of a fluorene moiety), but longer than the derivatives of the latter where the halogen has been exchanged by another anionic ligand ([C5Me5]–: 1.845(2) Å; [C5H5]−: 1.8466(10); acac−: 1.848(2) Å; [acac(Ph)2]−: 1.8499(14) Å; pyrazolide: 1.8639(16) Å; acac = acetyl-acetonate) []. Based on the afore-mentioned metrics, the auxiliary arene in 15 does not seem to significantly affect the bonding in Cu(I) complexes supported by f-CArAC: donor ligands. Furthermore, the solid-state structure did not reveal any interaction between the Cu(I) metal centre and the auxiliary phenyl either intra- or inter-molecularly even in the packed unit cell in the case of the latter. Nevertheless, it is interesting to note that the phenyl ring projects towards the Cu(I) metal centre, with possible implications to its reactivity.
Complex 15 has also been spectroscopically characterised, with its 1H and 13C{1H} NMR spectra being consistent with the determined solid-state structure that is retained in solution, and the carbene carbon (i.e., C1 in Figure 4) resonating at 232.3 ppm (δ(C6D6)) in the 13C{1H} NMR spectrum, in agreement with previous literature examples [,,].
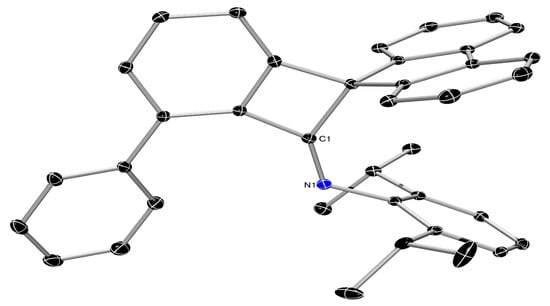
Figure 4.
ORTEP-III diagram of the molecular structure of 16 showing 50% ADP’s with H atoms omitted for clarity.
The isolation of complex 15, is a good indication of the in situ formation of the f-CArAC: carbene via the thermally induced dehydration of the corresponding isoindolinol 13. In view of the low isolated yield of 15, we decided to follow the reaction in C7D8, under otherwise similar conditions. After 2 h of heating the 1H-NMR spectrum of an aliquot from the reaction mixture revealed that a small amount of 15 had already formed (ca 5% based on relevant integration of Dipp resonances), along with unreacted 13 and a new species 16 at a 3:1 approximate ratio, respectively, based on their relative integration (Figure S145 in ESI). Almost complete consumption of 13 was observed after ca 22 h, with the two identifiable species (Figure S146 in the deep red-purple solution being the Cu(I) complex 15 and the new species 16 (Scheme 4). After work-up, the identity of 16 was unambiguously confirmed via a SC-XRD study (Figure 4) on crystals grown from a saturated Et2O solution of the insoluble in n-pentane solids (which also contain complex 15).
Imine 16 (N1-C1 bond distance of 1.2586(19) Å) is the result of ring contraction with a plausible mechanism for its formation shown in Scheme 5. This involves the generation of the free carbene 13C: [], followed by the formation of a substituted vinylidene-λ2-azane that subsequently undergoes the observed ring contraction in a similar manner to that recently posited by Bertrand and coworkers []. Indeed, when 13 is heated in the absence of Cu(I)Cl, formation of 16 is observed in >90% spectroscopic yield (Figure S142 in the ESI). Together these results advocate for the formation of 13C: as a fleeting species in solution. We are currently investigating alternative reaction conditions to suppress the formation of 16 by increasing the rate of trapping of 13C: by a metal centre.
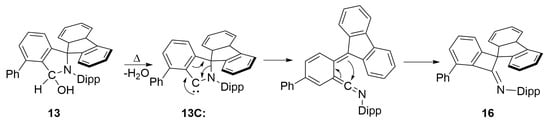
Scheme 5.
Plausible mechanism explaining the formation of 16.
When isoindolin-1-ol 14 (Scheme 3) was heated in a similar manner as above, either in the presence or absence of Cu(I)Cl, then the same colour changes were observed, but no well-defined products could be isolated. This suggests that steric hindrance plays a crucial role, and we are currently working on introducing less bulky arenes on the N-heteroatom.
4. Conclusions
In summary we report on the successful synthesis and isolation of a small library of new fused Cyclic Aryl Amino Carbene salt precursors, functionalized with an auxiliary arene situated on the annulated benzene ring. We have used Suzuki cross-couplings, using commercially available 2,6-dibromo-benzaldehyde, as the key step to introduce various arenes and consequently access various scaffolds, including two new Acyclic Aryl Amino Carbene salt precursors and an unexpected isoquinolin-2-ium salt. Although, access to the corresponding carbenes has been elusive, their in-situ generation from the thermal dehydration of isoindolin-1-ols is further documented by, (a) a trapping experiment resulting in the isolation of a new Cu(I) complex and (b) the isolation of a ring contraction product. We are currently expanding on the insights offered by these results and our efforts will be reported in a forthcoming publication.
Supplementary Materials
Schemes S1-S16: Synthesis of compounds reported here-in; Figures S1–S146: Spectroscopic (NMR, IR) data of compounds reported herein; Tables S1&S2: Collection and model refinement details of SC-XRD studies for compounds 5-H, 7-tBu, 10-CF3, 12, 14, 15 and 16. The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6040051/s1, Crystallographic information files (.cif) of compounds 5-H, 7-tBu, 10-CF3, 12, 14, 15 and 16 have been deposited at the CCDC; these data is free of charge. References [,,] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, N.T.; methodology, N.T. and P.C.I.; synthesis, P.C.I., N.T.; SC-XRD collection, solution and model refinement, N.T.; formal analysis, P.C.I. and N.T., Hi-Res Mass-spec, S.-P.K.; writing—original draft preparation, P.C.I. and N.T.; writing—review and editing, P.C.I. and N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded within the framework of the National Recovery and Resilience Plan Greece 2.0, funded by the European Union–NextGenerationEU, as implemented by the Hellenic Foundation for Research and Innovation (H.F.R.I.), grant number 15293 (acronym CEPTr).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
Access to the SC-XRD instrumentation in the National and Kapodistrian University of Athens Core Facility is greatly acknowledged. The authors are also grateful to C. Mantzourani and N. Stini for Hi-Res mass spectra acquisition and A. A. Danopoulos for helpful discussions, access to glovebox facilities and a generous loan of anhydrous Cu(I)Cl.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hill, S.A.; Steinfort, R.; Hartmann, L. Progress, Challenges and Future Directions of Heterocycles as Building Blocks in Iterative Methodologies towards Sequence-Defined Oligomers and Polymers. Polym. Chem. 2021, 12, 4439–4450. [Google Scholar] [CrossRef]
- Murugan, R. Heterocycles in Polymers. Prog. Heterocycl. Chem. 2023, 35, 93–118. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909–1951. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Qadir, T.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on The Medicinal And Industrial Applications of N-Containing Heterocycles. Open Med. Chem. J. 2022, 16. [Google Scholar] [CrossRef]
- Cai, S.; Hu, X.; Han, J.; Zhang, Z.; Li, X.; Wang, C.; Su, J. Efficient Organic Dyes Containing Dibenzo Heterocycles as Conjugated Linker Part for Dye-Sensitized Solar Cells. Tetrahedron 2013, 69, 1970–1977. [Google Scholar] [CrossRef]
- Abbotto, A.; Beverina, L.; Bozio, R.; Facchetti, A.; Ferrante, C.; Pagani, G.A.; Pedron, D.; Signorini, R. Novel Heterocycle-Based Two-Photon Absorbing Dyes. Org. Lett. 2002, 4, 1495–1498. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The Advent and Development of Organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Han, B.; He, X.H.; Liu, Y.Q.; He, G.; Peng, C.; Li, J.L. Asymmetric Organocatalysis: An Enabling Technology for Medicinal Chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Barik, S.; Biju, A.T. N-Heterocyclic Carbene (NHC) Organocatalysis: From Fundamentals to Frontiers. Chem. Soc. Rev. 2024, 54, 1102–1124. [Google Scholar] [CrossRef]
- Ha, H.-J. Recent Advances in Synthesizing and Utilizing Nitrogen-Containing Heterocycles. Front. Chem. 2023, 11, 1279418. [Google Scholar] [CrossRef]
- Galathri, E.M.; Kuczmera, T.J.; Nachtsheim, B.J.; Kokotos, C.G. Organocatalytic Friedel-Crafts Arylation of Aldehydes with Indoles Utilizing N-Heterocyclic Iod(Az)Olium Salts as Halogen-Bonding Catalysts. Green Chem. 2023, 26, 825–831. [Google Scholar] [CrossRef]
- Spyropoulou, C.K.; Skolia, E.; Flesariu, D.F.; Zissimou, G.A.; Gkizis, P.L.; Triandafillidi, I.; Athanasiou, M.; Itskos, G.; Koutentis, P.A.; Kokotos, C.G. 3H-Phenothiazin-3-One: A Photocatalyst for the Aerobic Photochemical Oxidation of Sulfides to Sulfoxides. Adv. Synth. Catal. 2023, 365, 2643–2650. [Google Scholar] [CrossRef]
- Zhilyaev, K.A.; Lipilin, D.L.; Kosobokov, M.D.; Samigullina, A.I.; Dilman, A.D. Preparation and Evaluation of Sterically Hindered Acridine Photocatalysts. Adv. Synth. Catal. 2022, 364, 3295–3301. [Google Scholar] [CrossRef]
- Rickertsen, D.R.L.; Crow, J.D.; Das, T.; Ghiviriga, I.; Hirschi, J.S.; Seidel, D. Acridine/Lewis Acid Complexes as Powerful Photocatalysts: A Combined Experimental and Mechanistic Study. ACS Catal. 2024, 14, 14574–14585. [Google Scholar] [CrossRef]
- Sideri, I.K.; Voutyritsa, E.; Kokotos, C.G. Photoorganocatalysis, Small Organic Molecules and Light in the Service of Organic Synthesis: The Awakening of a Sleeping Giant. Org. Biomol. Chem. 2018, 16, 4596–4614. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Grubbs, R.H. A Stable Four-Membered N-Heterocyclic Carbene. J. Am. Chem. Soc. 2004, 126, 10198–10199. [Google Scholar] [CrossRef]
- Beaumier, E.P.; Gordon, C.P.; Harkins, R.P.; Mcgreal, M.E.; Wen, X.; Copéret, C.; Goodpaster, J.D.; Tonks, I.A. Cp2Ti(Κ2-tbuncntbu): A Complex with an Unusual Κ2 Coordination Mode of a Heterocumulene Featuring a Free Carbene. J. Am. Chem. Soc. 2020, 142, 8006–8018. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Donnadieu, B.; Bertrand, G. S2307 Four-Electron, Four-Membered Heterocyclic Cations and Carbenes. Proc. Natl. Acad. Sci. USA 2006, 12, 13585–13588. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Monakhov, K.Y.; Braunstein, P. Anionic N-Heterocyclic Carbene Ligands from Mesoionic Imidazolium Precursors: Remote Backbone Arylimino Substitution Directs Carbene Coordination. Chem. Eur. J. 2013, 19, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Reiß, G.; Ganter, C. The First Structurally Characterized N-Heterocyclic Carbene Complex with a Ligand Derived from Pyrimidine. J. Organomet. Chem. 2010, 695, 474–477. [Google Scholar] [CrossRef]
- Makhloufi, A.; Frank, W.; Ganter, C. Diamino- and Mixed Amino-Amido-N-Heterocyclic Carbenes Based on Triazine Backbones. Organometallics 2012, 31, 2001–2008. [Google Scholar] [CrossRef]
- Karl, L.; Meisner, J.; Ganter, C. Investigations on Novel 1,3-Diazetidine Based Four-Membered N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2023, 26, e202300022. [Google Scholar] [CrossRef]
- Makhloufi, A.; Wahl, M.; Frank, W.; Ganter, C. A New Mixed Amino-Amido N-Heterocyclic Carbene Based on Anthranilic Acid. Organometallics 2013, 32, 854–861. [Google Scholar] [CrossRef]
- Braun, M.; Frank, W.; Reiss, G.J.; Ganter, C. An N-Heterocyclic Carbene Ligand with an Oxalamide Backbone. Organometallics 2010, 29, 4418–4420. [Google Scholar] [CrossRef]
- Brüggemann, P.; Wahl, M.; Schwengers, S.; Buhl, H.; Ganter, C. Access to a Cationic, Electron-Poor N-Heterocyclic Carbene with a Quinazolinium Core by Postsynthetic Modification of Related Neutral Derivatives. Organometallics 2018, 37, 4276–4286. [Google Scholar] [CrossRef]
- Bazinet, P.; Ong, T.G.; O’Brien, J.S.; Lavoie, N.; Bell, E.; Yap, G.P.A.; Korobkov, I.; Richeson, D.S. Design of Sterically Demanding, Electron-Rich Carbene Ligands with the Perimidine Scaffold. Organometallics 2007, 26, 2885–2895. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Winston, S.; Gelbrich, T.; Hursthouse, M.B.; Tooze, R.P. Synthesis and Structural Characterisation of Stable Pyridine- and Phosphine-Functionalised N-Heterocyclic Carbenes. Chem. Commun. 2002, 5, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, A.A.D.; Danopoulos, A.A.; Kleinhenz, S.; Light, M.E.; Hursthouse, M.B.; Eastham, G. Structural Diversity of Pyridine-N-Functionalized Carbene Copper(I) Complexes. Organometallics 2001, 20, 2027–2031. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Goerlich, J.R.; Marshall, W.J. A Stable Thiazol-2-Ylidene and Its Dimer. Liebigs Ann. 1997, 1997, 365–374. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, D.; Ung, G.; Donnadieu, B.; Bertrand, G. Mesoionic Thiazol-5-Ylidenes as Ligands for Transition Metal Complexes. Chem. Commun. 2011, 47, 10614–10616. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Li, X.; Lv, A.; Li, X.; Wang, Z.; Wang, R.; Ma, Y.; Fang, R.; Szostak, R.; et al. Thiazol-2-Ylidenes as N-Heterocyclic Carbene Ligands with Enhanced Electrophilicity for Transition Metal Catalysis. Commun. Chem. 2022, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, N.; César, V.; Lugan, N.; Lavigne, G. An Ambidentate Janus-Type Ligand System Based on Fused Carbene and Imidato Functionalities. Chem.—A Eur. J. 2011, 17, 13151–13155. [Google Scholar] [CrossRef]
- Perry, M.C.; Cui, X.; Powell, M.T.; Hou, D.R.; Reibenspies, J.H.; Burgess, K. Optically Active Iridium Imidazol-2-Ylidene-Oxazoline Complexes: Preparation and Use in Asymmetric Hydrogenation of Arylalkenes. J. Am. Chem. Soc. 2003, 125, 113–123. [Google Scholar] [CrossRef]
- Teng, Q.; Wu, W.; Duong, H.A.; Huynh, H.V. Ring-Expanded N-Heterocyclic Carbenes as Ligands in Iron-Catalysed Cross-Coupling Reactions of Arylmagnesium Reagents and Aryl Chlorides. Chem. Commun. 2018, 54, 6044–6047. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.J.; Wang, W.; Li, S.; Shi, M. Chiral NHC-Metal-Based Asymmetric Catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as Chiral Building Blocks for Imidazolium Salts and N-Heterocyclic Carbene Ligands. Chem. Commun. 2002, 2, 2704–2705. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Monge, D.; Álcarazo, M.; Álvarez, E.; Lassaletta, J.M.; Fernández, R. Synthesis, Structure, and Applications of N-Dialkylamino-N′-Alkylimidazol-2-Ylidenes as a New Type of NHC Ligands. Organometallics 2006, 25, 6039–6046. [Google Scholar] [CrossRef]
- Massey, R.S.; Collett, C.J.; Lindsay, A.G.; Smith, A.D.; O’Donoghue, A.C. Proton Transfer Reactions of Triazol-3-Ylidenes: Kinetic Acidities and Carbon Acid p K a Values for Twenty Triazolium Salts in Aqueous Solution. J. Am. Chem. Soc. 2012, 134, 20421–20432. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Pearson, S. Abnormal N-Heterocyclic Carbenes. Coord. Chem. Rev. 2007, 251, 596–609. [Google Scholar] [CrossRef]
- Regnier, V.; Planet, Y.; Moore, C.E.; Pecaut, J.; Philouze, C.; Martin, D. Stable Di- and Tri-coordinated Carbon(II) Supported by an Electron-Rich Β-Diketiminate Ligand. Angew. Chem. Int. Ed. Engl. 2017, 129, 1051–1055. [Google Scholar] [CrossRef]
- Krahulic, K.E.; Tuononen, H.M.; Parvez, M.; Roesler, R. Isolation of Free Phenylide-like Carbanions with N-Heterocyclic Carbene Frameworks. J. Am. Chem. Soc. 2009, 131, 5858–5865. [Google Scholar] [CrossRef]
- Vermersch, F.; Wang, V.T.; Abdellaoui, M.; Jazzar, R.; Bertrand, G. Ambiphilicity of Ring-Expanded N-Heterocyclic Carbenes. Chem. Sci. 2024, 15, 3707–3710. [Google Scholar] [CrossRef]
- Hobbs, M.G.; Knapp, C.J.; Welsh, P.T.; Borau-Garcia, J.; Ziegler, T.; Roesler, R. Anionic N-Heterocyclic Carbenes with N,N′-Bis(Fluoroaryl) and N,N′-Bis(Perfluoroaryl) Substituents. Chem.—A Eur. J. 2010, 16, 14520–14533. [Google Scholar] [CrossRef]
- Enders, D.; Breuer, K.; Raabe, G.; Runsink, J.; Teles, J.H.; Melder, J.-P.; Ebel, K.; Brode, S. Preparation, Structure, and Reactivity of 1,3,4-Triphenyl-4,5-dihydro-1H-1,2,4-triazol-5-ylidene, a New Stable Carbene. Angew. Chem. Int. Ed. Engl. 1995, 34, 1021–1023. [Google Scholar] [CrossRef]
- Schuster, O.; Yang, L.; Raubenheimer, H.G.; Albrecht, M. Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other Classes of NHC Ligands with Reduced Heteroatom Stabilization. Chem. Rev. 2009, 109, 3445–3478. [Google Scholar] [CrossRef]
- Martin, D.; Lassauque, N.; Donnadieu, B.; Bertrand, G. A Cyclic Diaminocarbene with a Pyramidalized Nitrogen Atom: A Stable N-Heterocyclic Carbene with Enhanced Electrophilicity. Angew. Chem. Int. Ed. Engl. 2012, 51, 6172–6175. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Alcarazo, M.; Krause, H.; Lehmann, C.W. Effective Modulation of the Donor Properties of N-Heterocyclic Carbene Ligands by “Through-Space” Communication within a Planar Chiral Scaffold. J. Am. Chem. Soc. 2007, 129, 12676–12677. [Google Scholar] [CrossRef]
- Alder, R.W.; Butts, C.P.; Orpen, A.G. Stable Aminooxy- and Aminothiocarbenes. J. Am. Chem. Soc. 1998, 120, 11526–11527. [Google Scholar] [CrossRef]
- Tej Raviprolu, V.; Gregory, A.; Banda, I.; McArthur, S.G.; McArthur, S.E.; Goddard III, W.A.; Musgrave III, C.B.; Lavallo, V. Confirmation of Breslow’s Hypothesis: A Carbene Stable in Liquid Water. Sci. Adv. 2025, 11, 9681. [Google Scholar] [CrossRef]
- Alcarazo, M.; Roseblade, S.J.; Cowley, A.R.; Fernández, R.; Brown, J.M.; Lassaletta, J.M. Imidazo [1,5-a]Pyridine: A Versatile Architecture for Stable N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2005, 127, 3290–3291. [Google Scholar] [CrossRef]
- Lee, S.; Gabidullin, B.; Richeson, D. Distinct Palladium(II) Carbene Complexes Supported by Six-Membered 1,3-Disubstituted Permidin-2-Ylidene, Six-Membered N-Heterocyclic Carbenes. ACS Omega 2018, 3, 6587–6594. [Google Scholar] [CrossRef]
- Lee, B.C.; Liu, C.F.; Lin, L.Q.H.; Yap, K.Z.; Song, N.X.; Ko, C.H.M.; Chan, P.H.; Koh, M.J. N-Heterocyclic Carbenes as Privileged Ligands for Nickel-Catalysed Alkene Functionalisation. Chem. Soc. Rev. 2023, 52, 2946–2991. [Google Scholar] [CrossRef]
- Beig, N.; Goyal, V.; Bansal, R.K. Application of N-Heterocyclic Carbene–Cu(I) Complexes as Catalysts in Organic Synthesis: A Review. Beilstein J. Org. Chem. 2023, 19, 1408–1442. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; He, Y.M.; Pan, Y.; Li, F.; Li, D.; Hao, W.; Fan, Q.H. Tunable Unsymmetrical Chiral N-Heterocyclic Carbene Ligands for Highly Diastereo- and Enantioselective Copper-Catalyzed Tandem Borylative Cyclization: Ligand Controlled Diastereoselectivity Reversal. CCS Chem. 2023, 5, 2088–2100. [Google Scholar] [CrossRef]
- Chung, C.K.; Grubbs, R.H. Olefin Metathesis Catalyst: Stabilization Effect of Backbone Substitutions of N-Heterocyclic Carbene. Org. Lett. 2008, 10, 2693–2696. [Google Scholar] [CrossRef]
- Lavallo, V.; Dyker, C.A.; Donnadieu, B.; Bertrand, G. Synthesis and Ligand Properties of Stable Five-Membered-Ring Allenes Containing Only Second-Row Elements. Angew. Chem. Inter. Ed. Engl. 2008, 47, 5411–5414. [Google Scholar] [CrossRef] [PubMed]
- Zinner, S.C.; Herrmann, W.A.; Kühn, F.E. Synthesis and Characterization of Asymmetric NHC Complexes. J. Organomet. Chem. 2008, 693, 1543–1546. [Google Scholar] [CrossRef]
- Breitwieser, K.; Munz, D. Cyclic (Alkyl)(Amino)Carbene (CAAC) Ligands: Electronic Structure and Application as Chemically- and Redox-Non-Innocent Ligands and Chromophores. In Advances in Organometallic Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 78, pp. 79–132. ISBN 9780323990905. [Google Scholar]
- Dorta, R.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and Electronic Properties of N-Heterocyclic Carbenes (NHC): A Detailed Study on Their Interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef]
- Hillier, A.C.; Sommer, W.J.; Yong, B.S.; Petersen, J.L.; Cavallo, L.; Nolan, S.P. A Combined Experimental and Theoretical Study Examining the Binding of N-Heterocyclic Carbenes (NHC) to the Cp*RuCl (Cp* = H5-C5Me5) Moiety: Insight into Stereoelectronic Differences between Unsaturated and Saturated NHC Ligands. Organometallics 2003, 22, 4322–4326. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Kolychev, E.L.; Kronig, S.; Brandhorst, K.; Freytag, M.; Jones, P.G.; Tamm, M. Iridium(I) Complexes with Anionic N-Heterocyclic Carbene Ligands as Catalysts for the Hydrogenation of Alkenes in Nonpolar Media. J. Am. Chem. Soc. 2013, 135, 12448–12459. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.P.; Nasr, A.; Jones, P.G.; Altun, A.; Neese, F.; Bistoni, G.; Tamm, M. London Dispersion Interactions in Pnictogen Cations [ECl2]+ and [E=E]2+ (E=P, As, Sb) Supported by Anionic N-Heterocyclic Carbenes. Chem. Eur. J. 2018, 24, 18922–18932. [Google Scholar] [CrossRef] [PubMed]
- Hadei, N.; Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Electronic Nature of N-Heterocyclic Carbene Ligands: Effect on the Suzuki Reaction. Org. Lett. 2005, 7, 1991–1994. [Google Scholar] [CrossRef]
- Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Group 11 Metal Complexes of N-Heterocyclic Carbene Ligands: Nature of the Metal-Carbene Bond. Organometallics 2004, 23, 755–764. [Google Scholar] [CrossRef]
- Moerdyk, J.P.; Schilter, D.; Bielawski, C.W. N,N’-Diamidocarbenes: Isolable Divalent Carbons with Bona Fide Carbene Reactivity. Acc. Chem. Res. 2016, 49, 1458–1468. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Bielawski, C.W. An N,N′-Diamidocarbene: Studies in C-H Insertion, Reversible Carbonylation, and Transition-Metal Coordination Chemistry. J. Am. Chem. Soc. 2009, 131, 16039–16041. [Google Scholar] [CrossRef]
- Powell, M.T.; Hou, D.R.; Perry, M.C.; Cui, X.; Burgess, K. Chiral Imidazolylidine Ligands for Asymmetric Hydrogenation of Aryl Alkenes. J. Am. Chem. Soc. 2001, 123, 8878–8879. [Google Scholar] [CrossRef]
- Sanderson, M.D.; Kamplain, J.W.; Bielawski, C.W. Quinone-Annulated N-Heterocyclic Carbene-Transition-Metal Complexes: Observation of π-Backbonding Using FT-IR Spectroscopy and Cyclic Voltammetry. J. Am. Chem. Soc. 2006, 128, 16514–16515. [Google Scholar] [CrossRef]
- César, V.; Lugan, N.; Lavigne, G. Electronic Tuning of a Carbene Center via Remote Chemical Induction, and Relevant Effects in Catalysis. Chem. Eur. J. 2010, 16, 11432–11442. [Google Scholar] [CrossRef]
- Sau, S.C.; Hota, P.K.; Mandal, S.K.; Soleilhavoup, M.; Bertrand, G. Stable Abnormal N-Heterocyclic Carbenes and Their Applications. Chem. Soc. Rev. 2020, 49, 1233–1252. [Google Scholar] [CrossRef]
- Vermersch, F.; Yazdani, S.; Junor, G.P.; Grotjahn, D.B.; Jazzar, R.; Bertrand, G. Stable Singlet Carbenes as Organic Superbases. Angew. Chem. Inter. Ed. Engl. 2021, 60, 27253–27257. [Google Scholar] [CrossRef]
- Pranckevicius, C.; Stephan, D.W. Three-Coordinate, Cyclic Bent Allene Iron Complexes. Organometallics 2013, 32, 2693–2697. [Google Scholar] [CrossRef]
- Takasao, G.; Maity, B.; Dutta, S.; Kancherla, R.; Rueping, M.; Cavallo, L. NHC-Cracker: A Platform for the In Silico Engineering of N-Heterocyclic Carbenes for Diverse Chemical Applications. ACS Catal. 2025, 15, 5915–5927. [Google Scholar] [CrossRef]
- Clavier, H.; Nolan, S.P. Percent Buried Volume for Phosphine and N-Heterocyclic Carbene Ligands: Steric Properties in Organometallic Chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca 2. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef]
- Dröge, T.; Glorius, F. The Measure of All Rings—N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 2010, 49, 6940–6952. [Google Scholar] [CrossRef]
- Munz, D. Pushing Electrons—Which Carbene Ligand for Which Application? Organometallics 2018, 37, 275–289. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Synthesis and Activity of Ruthenium Olefin Metathesis Catalysts Coordinated with Thiazol-2-Ylidene Ligands. J. Am. Chem. Soc. 2008, 130, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Charra, V.; de Frémont, P.; Braunstein, P. Multidentate N-Heterocyclic Carbene Complexes of the 3d Metals: Synthesis, Structure, Reactivity and Catalysis. Coord. Chem. Rev. 2017, 341, 53–176. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-Heterocyclic Carbenes: Emerging Powerful Catalysts. Trends Chem. 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC Heterocycle Complexes in Catalysis and Biological Applications: Systematic Review. Mater. Today Proc. 2020, 31, S122–S129. [Google Scholar] [CrossRef]
- Lehtonen, A. Metal Complexes of Redox Non-Innocent Ligand N,N′-Bis(3,5-Di-Tertbutyl-2-Hydroxy-Phenyl)-1,2-Phenylenediamine. Molecules 2024, 29, 1088–1102. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. Engl. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Jazzar, R.; Dewhurst, R.D.; Bourg, J.B.; Donnadieu, B.; Canac, Y.; Bertrand, G. Intramolecular “Hydroiminiumation” of Alkenes: Application to the Synthesis of Conjugate Acids of Cyclic Alkyl Amino Carbenes (CAACs). Angew. Chem. Int. Ed. Engl. 2007, 46, 2899–2902. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C.M.; Junor, G.P.; Tolentino, D.R.; Jazzar, R.; Melaimi, M.; Bertrand, G. Highly Ambiphilic Room Temperature Stable Six-Membered Cyclic (Alkyl)(Amino)Carbenes. J. Am. Chem. Soc. 2018, 140, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Mendivil, E.; Hansmann, M.M.; Weinstein, C.M.; Jazzar, R.; Melaimi, M.; Bertrand, G. Bicyclic (Alkyl)(Amino)Carbenes (BICAACs): Stable Carbenes More Ambiphilic than CAACs. J. Am. Chem. Soc. 2017, 139, 7753–7756. [Google Scholar] [CrossRef] [PubMed]
- Pichon, D.; Soleilhavoup, M.; Morvan, J.; Junor, G.P.; Vives, T.; Crévisy, C.; Lavallo, V.; Campagne, J.M.; Mauduit, M.; Jazzar, R.; et al. The Debut of Chiral Cyclic (Alkyl)(Amino)Carbenes (CAACs) in Enantioselective Catalysis. Chem. Sci. 2019, 10, 7807–7811. [Google Scholar] [CrossRef] [PubMed]
- Kumar Kushvaha, S.; Mishra, A.; Roesky, H.W.; Chandra Mondal, K. Recent Advances in the Domain of Cyclic (Alkyl)(Amino) Carbenes. Chem. Asian. J. 2022, 17, e202101301. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamiyama, S.; Ishii, A.; Nakata, N. 2-[2,6-Diisopropylphenyl]-4-Phenyl-5H-5,9b[1′,2′]-Benzonaphtho[1,2-b]Pyrrol-2-Ium Tetrafluoroborate. Molbank 2023, 2023, M1601. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamiyama, S.; Ota, K.; Nakata, N. Flash Communication: Exocyclic Alkenyl Tuning of Cyclic (Amino)Carbene: Enhancing π-Acceptor Property without Ring Unsaturation. Organometallics 2025, 44, 1510–1514. [Google Scholar] [CrossRef]
- Siwatch, R.K.; Ng, Z.J.G.; Lim, J.J.S.; Zhang, Z.F.; Su, M.D.; So, C.W. Strained Cyclic Alkyl(Amino)Carbene-Stabilized Tetraatomic Silicon(0) Cluster. J. Am. Chem. Soc. 2025, 147, 20251–20256. [Google Scholar] [CrossRef]
- Karl, L.; Deißenbeck, D.; Meisner, J.; Ganter, C. β-Lactam Ylidenes: An Overlooked Class of N-Heterocyclic Carbenes. Chem. Eur. J. 2025, 31, e202501320. [Google Scholar] [CrossRef]
- Serrato, M.R.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(Barrelene)Carbenes: An Original Family of CAACs through a Novel Synthetic Pathway. Chem. Commun. 2022, 58, 7519–7521. [Google Scholar] [CrossRef]
- Majumder, C.; Sharma, A.; Das, B.; Yadav, R.; Kundu, S. Cyclic (Alkenyl)(Amino)Carbene (SMeCAenAC): Introducing a Member to the Cyclic (Alkyl)(Amino)Carbenes Family Featuring a Narrow Energy Gap. J. Am. Chem. Soc. 2025, 147, 6905–6913. [Google Scholar] [CrossRef]
- Volk, J.; Heinz, M.; Guthardt, R.; Yadav, S.; Bruhn, C.; Holthausen, M.C.; Siemeling, U. A Strongly Ambiphilic Ferrocene-Based Cyclic (Alkyl)(Amino)Carbene—Specific Decomposition to an Enamine by a 1,2-Phenyl Shift. Chem. Eur. J. 2024, 30, e202403028. [Google Scholar] [CrossRef] [PubMed]
- Nikovskii, I.A.; Spiridonov, K.A.; Pavlov, A.A.; Nelyubina, Y.V.; Karnaukh, K.M.; Polezhaev, A.V. Synthetic Approaches to New Redox-Active Carbene Ligands. Russ. J Coord. Chem. 2021, 47, 117–126. [Google Scholar] [CrossRef]
- Madron du Vigné, A.; Cramer, N. Systematic Synthesis of Chiral CAACs with Three Independent Stereogenic Centers for Enantioselective Copper-Catalyzed Conjugate Borylation of Michael Acceptors. ACS Catal. 2025, 15, 15102–15111. [Google Scholar] [CrossRef]
- Frey, G.D.; Lavallo, V.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Facile Splitting of Hydrogen and Ammonia by Nucleophilic Activation at a Single Carbon Center. Science (1979) 2007, 316, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mondal, K.C.; Roesky, H.W. Cyclic Alkyl(Amino) Carbene Stabilized Complexes with Low Coordinate Metals of Enduring Nature. Acc. Chem. Res. 2016, 49, 357–369. [Google Scholar] [CrossRef]
- Arnold, N.; Braunschweig, H.; Brenner, P.B.; Celik, M.A.; Dewhurst, R.D.; Haehnel, M.; Kramer, T.; Krummenacher, I.; Marder, T.B. Correlations and Contrasts in Homo- and Heteroleptic Cyclic (Alkyl)(Amino)Carbene-Containing Pt0 Complexes. Chem. Eur. J. 2015, 21, 12357–12362. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, A.; Goswami, B.; Breitwieser, K.; Morgenstern, B.; Gimferrer, M.; Heinemann, F.W.; Momper, D.M.; Kay, C.W.M.; Munz, D. Palladium Terminal Imido Complexes with Nitrene Character. J. Am. Chem. Soc. 2022, 144, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Goodner, S.J.; Grünwald, A.; Heinemann, F.W.; Munz, D. Carbon Dioxide Activation by a Palladium Terminal Imido Complex. Aust. J. Chem. 2019, 72, 900–903. [Google Scholar] [CrossRef]
- Martinez-Vollbert, E.; Anjana, S.S.; Dankert, F.; Morgenstern, B.; Munz, D. Gold(I) Terminal Imides: CH Auration and Dehydrogenation. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Munz, D. How to Tame a Palladium Terminal Oxo. Chem. Sci. 2018, 9, 1155–1167. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef]
- Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)- And (Aryl)-(Amino)Carbene Coinage Metal Complexes and Their Applications. Chem. Rev. 2020, 120, 4141–4168. [Google Scholar] [CrossRef]
- Chu, J.; Munz, D.; Jazzar, R.; Melaimi, M.; Bertrand, G. Synthesis of Hemilabile Cyclic (Alkyl)(Amino)Carbenes (CAACs) and Applications in Organometallic Chemistry. J. Am. Chem. Soc. 2016, 138, 7884–7887. [Google Scholar] [CrossRef]
- Rao, B.; Tang, H.; Zeng, X.; Liu, L.L.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(Aryl)Carbenes (CAArCs) as Strong Σ-Donating and Π-Accepting Ligands for Transition Metals. Angew. Chem. Int. Ed. Engl. 2015, 127, 15128–15132. [Google Scholar] [CrossRef]
- Puerta Lombardi, B.M.; Faas, M.R.; West, D.; Suvinen, R.A.; Tuononen, H.M.; Roesler, R. An Isolable, Chelating Bis[Cyclic (Alkyl)(Amino)Carbene] Stabilizes a Strongly Bent, Dicoordinate Ni(0) Complex. Nat. Commun. 2024, 15, 3417–3424. [Google Scholar] [CrossRef]
- Braunschweig, H.; Krummenacher, I.; Legare, M.A.; Matler, A.; Radacki, K.; Ye, Q. Main-Group Metallomimetics: Transition Metal-like Photolytic CO Substitution at Boron. J. Am. Chem. Soc. 2017, 139, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Böhnke, J.; Braunschweig, H.; Ewing, W.C.; Hörl, C.; Kramer, T.; Krummenacher, I.; Mies, J.; Vargas, A. Diborabutatriene: An Electron-Deficient Cumulene. Angew. Chem. Int. Ed. Engl. 2014, 53, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Légaré, M.-A.; Bélanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen Fixation and Reduction at Boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Braunschweig, H.; Celik, M.A.; Dellermann, T.; Dewhurst, R.D.; Ewing, W.C.; Hammond, K.; Kramer, T.; Krummenacher, I.; Mies, J.; et al. Neutral Zero-Valent s-Block Complexes with Strong Multiple Bonding. Nat. Chem. 2016, 8, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gilliard, R.J.; Cummins, C.C. Arene Extrusion as an Approach to Reductive Elimination at Boron: Implication of Carbene-Ligated Haloborylene as a Transient Reactive Intermediate. Chem. Sci. 2024, 15, 17873–17880. [Google Scholar] [CrossRef]
- Wang, G.; Freeman, L.A.; Dickie, D.A.; Mokrai, R.; Benkő, Z.; Gilliard, R.J. Isolation of Cyclic(Alkyl)(Amino) Carbene–Bismuthinidene Mediated by a Beryllium(0) Complex. Chem. Eur. J. 2019, 25, 4335–4339. [Google Scholar] [CrossRef]
- Wang, G.; Walley, J.E.; Dickie, D.A.; Pan, S.; Frenking, G.; Gilliard, R.J. A Stable, Crystalline Beryllium Radical Cation. J. Am. Chem. Soc. 2020, 142, 4560–4564. [Google Scholar] [CrossRef]
- Freeman, L.A.; Obi, A.D.; Machost, H.R.; Molino, A.; Nichols, A.W.; Dickie, D.A.; Wilson, D.J.D.; Machan, C.W.; Gilliard, R.J. Soluble, Crystalline, and Thermally Stable Alkali CO2−and Carbonite (CO22−) Clusters Supported by Cyclic(Alkyl)(Amino) Carbenes. Chem. Sci. 2021, 12, 3544–3550. [Google Scholar] [CrossRef]
- Hollister, K.K.; Yang, W.; Mondol, R.; Wentz, K.E.; Molino, A.; Kaur, A.; Dickie, D.A.; Frenking, G.; Pan, S.; Wilson, D.J.D.; et al. Isolation of Stable Borepin Radicals and Anions. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202516. [Google Scholar] [CrossRef]
- Kretschmer, R.; Ruiz, D.A.; Moore, C.E.; Rheingold, A.L.; Bertrand, G. One-, Two-, and Three-Electron Reduction of a Cyclic Alkyl(Amino)Carbene- SbCl3 Adduct. Angew. Chem. Int. Ed. Engl. 2014, 53, 8176–8179. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.K.; Melaimi, M.; Gembicky, M.; Munz, D.; Bertrand, G. A Crystalline Doubly Oxidized Carbene. Nature 2023, 623, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, M.; Schwarzmaier, C.; Riesinger, C.; Timoshkin, A.Y.; Melaimi, M.; Bertrand, G.; Scheer, M. Reactivity of Yellow Arsenic towards Cyclic (Alkyl)(Amino) Carbenes (CAACs). Chem. Eur. J. 2023, 29, e202300280. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mondal, K.C.; Roesky, H.W.; Zhu, H.; Stollberg, P.; Herbst-Irmer, R.; Stalke, D.; Andrada, D.M. Acyclic Germylones: Congeners of Allenes with a Central Germanium Atom. J. Am. Chem. Soc. 2013, 135, 12422–12428. [Google Scholar] [CrossRef]
- Brenton Gildner, M.; Hudnall, T.W. Cyclic (Aryl)(Amido)Carbenes: Pushing the π-Acidity of Amidocarbenes through Benzannulation. Chem. Commun. 2019, 55, 12300–12303. [Google Scholar] [CrossRef]
- Maity, S.; Muthig, A.M.T.; Sen, I.; Mrózek, O.; Belyaev, A.; Hupp, B.; Steffen, A. A [2.2]Isoindolinophanyl-Based Carbene (IPC) Ligand: Synthesis, Electronic and Photophysical Properties, and Application in Photocatalysis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202409115. [Google Scholar] [CrossRef]
- Jazzar, R.; Bourg, J.B.; Dewhurst, R.D.; Donnadieu, B.; Bertrand, G. Intramolecular “Hydroiminiumation and -Amidiniumation” of Alkenes: A Convenient, Flexible, and Scalable Route to Cyclic Iminium and Imidazolinium Salts. J. Org. Chem. 2007, 72, 3492–3499. [Google Scholar] [CrossRef]
- Magriz, A.; Gómez-Bujedo, S.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Phthalazin-2-Ylidenes as Cyclic Amino Aryl Carbene Ligands in Rhodium(I) and Iridium(I) Complexes. Organometallics 2010, 29, 5941–5945. [Google Scholar] [CrossRef]
- Jothibasu, R.; Huynh, H.V. Versatile Coordination Chemistry of Indazole-Derived Carbenes. Chem. Commun. 2010, 46, 2986–2988. [Google Scholar] [CrossRef]
- Deck, E.; Reiter, K.; Klopper, W.; Breher, F. A Dinuclear Gold(I) Bis(Carbene) Complex Based on a Ditopic Cyclic (Aryl)(Amino)Carbene Framework. Z. Anorg. Allg. Chem. 2016, 642, 1320–1328. [Google Scholar] [CrossRef]
- Luliński, S.; Serwatowski, J. Regiospecific Metalation of Oligobromobenzenes. J. Org. Chem. 2003, 68, 5384–5387. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta. Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. Computer Program Abstracts ORTEP-3 for Windows-a Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Boga, S.B.; Alhassan, A.B.; Hesk, D. Efficient Synthesis of 2H & 13C Labeled Benzaldehydes via Regio-Selective Formylation. Tetrahedron Lett. 2014, 55, 4442–4444. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Jaradat, K.T.; Al-Jammal, W.K.; McDonald, R. Mild, Efficient, and Highly Regioselective Synthesis of 2,6-Diiodobenzaldehyde Derivatives. Synlett 2020, 31, 953–958. [Google Scholar] [CrossRef]
- Magis, D.; Cabrera-Trujillo, J.J.; Vignolle, J.; Sotiropoulos, J.M.; Taton, D.; Miqueu, K.; Landais, Y. Expedient Synthesis of Thermally Stable Acyclic Amino(Haloaryl)Carbenes: Experimental and Theoretical Evidence of “Push-Pull” Stabilized Carbenes. J. Am. Chem. Soc. 2024, 146, 16802–16813. [Google Scholar] [CrossRef]
- Lavallo, V.; Mafhouz, J.; Canac, Y.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Synthesis, Reactivity, and Ligand Properties of a Stable Alkyl Carbene. J. Am. Chem. Soc. 2004, 126, 8670–8671. [Google Scholar] [CrossRef]
- Sole, S.; Gornitzka, H.; Schoeller, W.W.; Bourissou, D.; Bertrand, G. (Amino)(Aryl)Carbenes: Stable Carbenes Featuring a Spectator Substituent. Science 2001, 292, 1901–1903. [Google Scholar] [CrossRef]
- Slaughter, L.M. Acyclic Aminocarbenes in Catalysis. ACS Catal. 2012, 2, 1802–1816. [Google Scholar] [CrossRef]
- Nakano, R.; Jazzar, R.; Bertrand, G. A Crystalline Monosubstituted Carbene. Nat. Chem. 2018, 10, 1196–1200. [Google Scholar] [CrossRef]
- Lorkowski, J.; Gojiashvili, L.; Yorkgitis, P.; Pichon, D.; Talcik, J.; Gembicky, M.; Roisnel, T.; Baslé, O.; Jazzar, R.; Mauduit, M.; et al. A Crystalline Annelated Pyridin-1-Ylidene and Its Isomerization into a Pyridin-3-Ylidene. J. Am. Chem. Soc. 2025, 147, 14972–14977. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bujedo, S.; Alcarazo, M.; Pichon, C.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Isoquinolin-1-Ylidenes as Electronically Tuneable Ligands. Chem. Commun. 2007, 11, 1180–1182. [Google Scholar] [CrossRef]
- Das, A.; Elvers, B.J.; Nayak, M.K.; Chrysochos, N.; Anga, S.; Kumar, A.; Rao, D.K.; Narayanan, T.N.; Schulzke, C.; Yildiz, C.B.; et al. Realizing 1,1-Dehydration of Secondary Alcohols to Carbenes: Pyrrolidin-2-Ols as a Source of Cyclic (Alkyl)(Amino)Carbenes. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202637. [Google Scholar] [CrossRef] [PubMed]
- Lorkowski, J.; Yorkgitis, P.; Serrato, M.R.; Gembicky, M.; Pietraszuk, C.; Bertrand, G.; Jazzar, R. Genuine Carbene versus Carbene-like Reactivity. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401020. [Google Scholar] [CrossRef]
- Lorkowski, J.; Krahfuß, M.; Kubicki, M.; Radius, U.; Pietraszuk, C. Intramolecular Ring-Expansion Reaction (RER) and Intermolecular Coordination of In Situ Generated Cyclic (Amino)(Aryl)Carbenes (CAArCs). Chem. Eur. J. 2019, 25, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Zeng, X. Ring Contraction by Rearrangement of Sterically Congested Cyclic (Amino)(Aryl)Carbenes. J. Org. Chem. 2024, 89, 7795–7803. [Google Scholar] [CrossRef]
- Gernert, M.; Balles-Wolf, L.; Kerner, F.; Müller, U.; Schmiedel, A.; Holzapfel, M.; Marian, C.M.; Pflaum, J.; Lambert, C.; Steffen, A. Cyclic (Amino)(Aryl)Carbenes Enter the Field of Chromophore Ligands: Expanded π System Leads to Unusually Deep Red Emitting Cu(I) Compounds. J. Am. Chem. Soc. 2020, 142, 8897–8909. [Google Scholar] [CrossRef] [PubMed]
- O'Shea, D.F.; Sharp, T. Benzoxepine formation by the 1,7 electrocyclisation of diene-conjugated carbonyl ylides: Studies on relative rates of cyclisation via intramolecular competition reactions. J. Chem. Soc. Perkin Trans. 1997, 3025–3034. [Google Scholar] [CrossRef]
- Chao, B.; Bai, C.; Yan, H.; Zhao, R.; Liu, D.; Muschin, T.; Bao, A.; Eerdun, C.; Bao, Y.-S. Suzuki–Miyaura Type Regioselective C–H Arylation of Aromatic Aldehydes by a Transient Directing Strategy. Org. Lett. 2023, 25, 6823–6829. [Google Scholar] [CrossRef]
- Tredwell, M.J.; Gulias, M.; Bremeyer, N.G.; Johansson, C.C.C.; Collins, B.S.L.; Gaunt, M.J. Palladium(II)-Catalyzed C-H Bond Arylation of Electron-Deficient Arenes at Room Temperature. Angew. Chem. Int. Ed. 2011, 50, 1076–1079. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).