Environmental Fate, Ecotoxicity, and Remediation of Heterocyclic Pharmaceuticals as Emerging Contaminants: A Review of Long-Term Risks and Impacts
Abstract
:1. Introduction
2. Overview of Heterocyclic Pharmaceuticals
2.1. Nitrogen-Containing Heterocycles
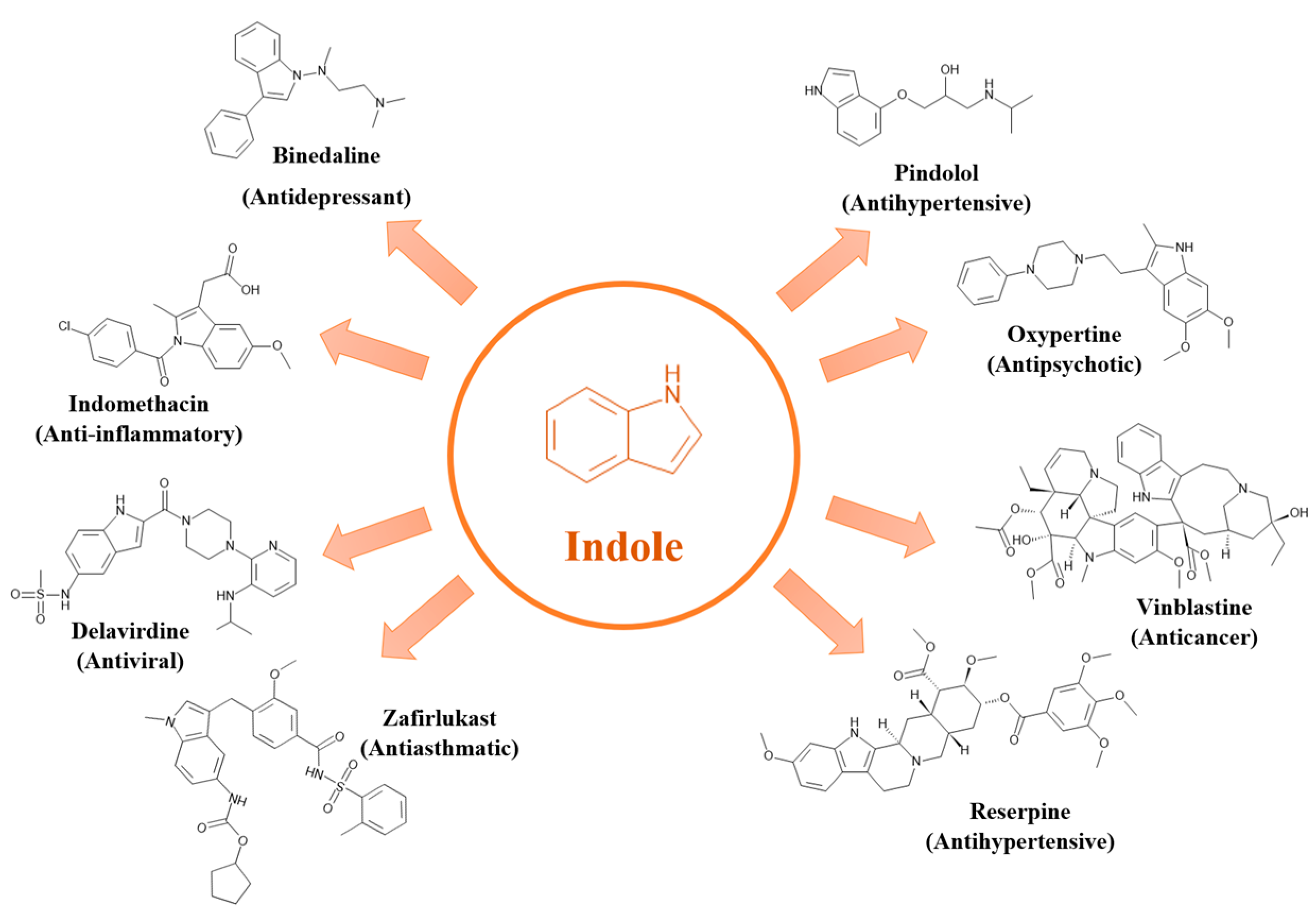
2.1.1. Indole
2.1.2. Benzimidazole and Imidazole
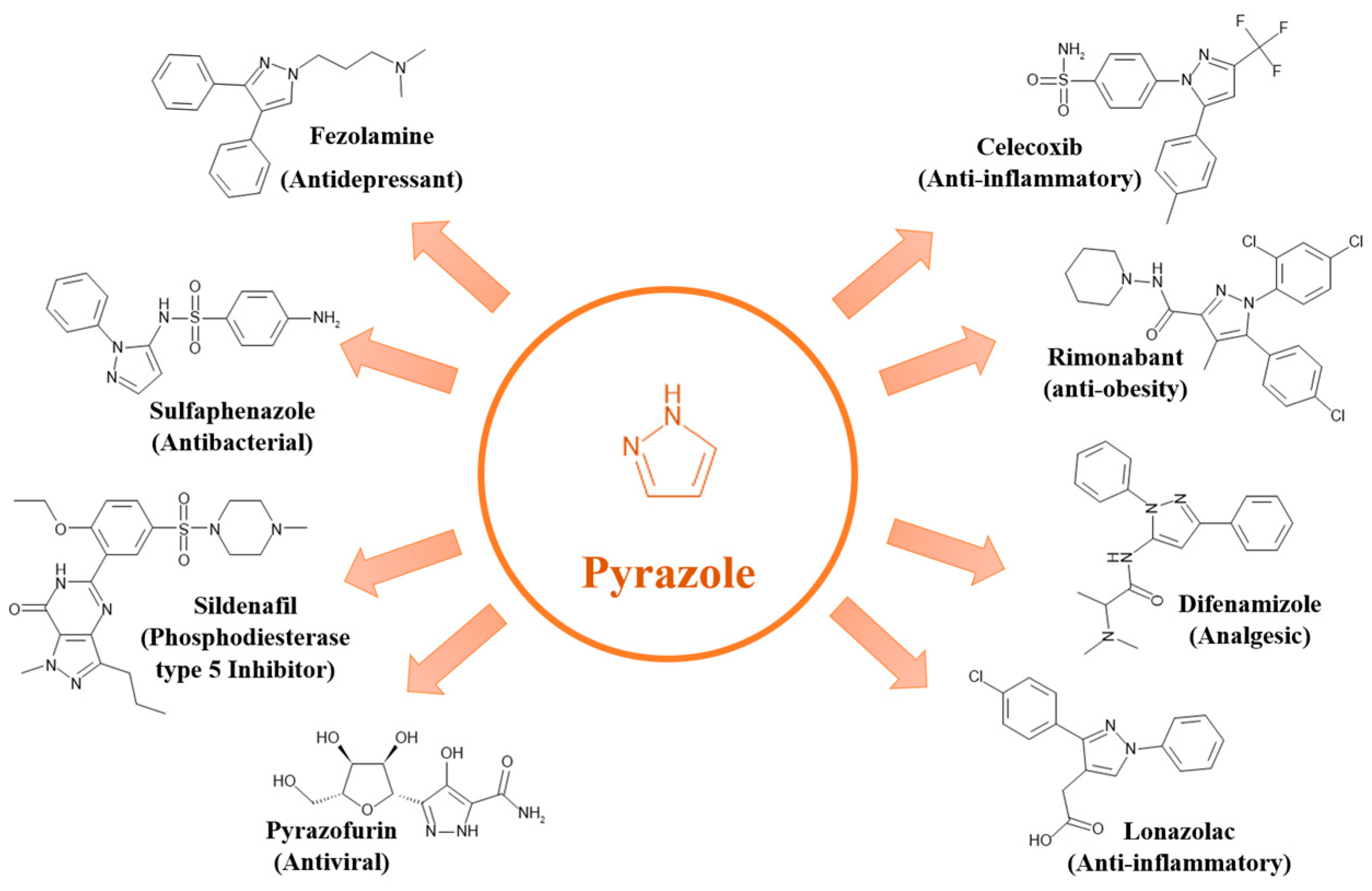
2.1.3. Pyrazole
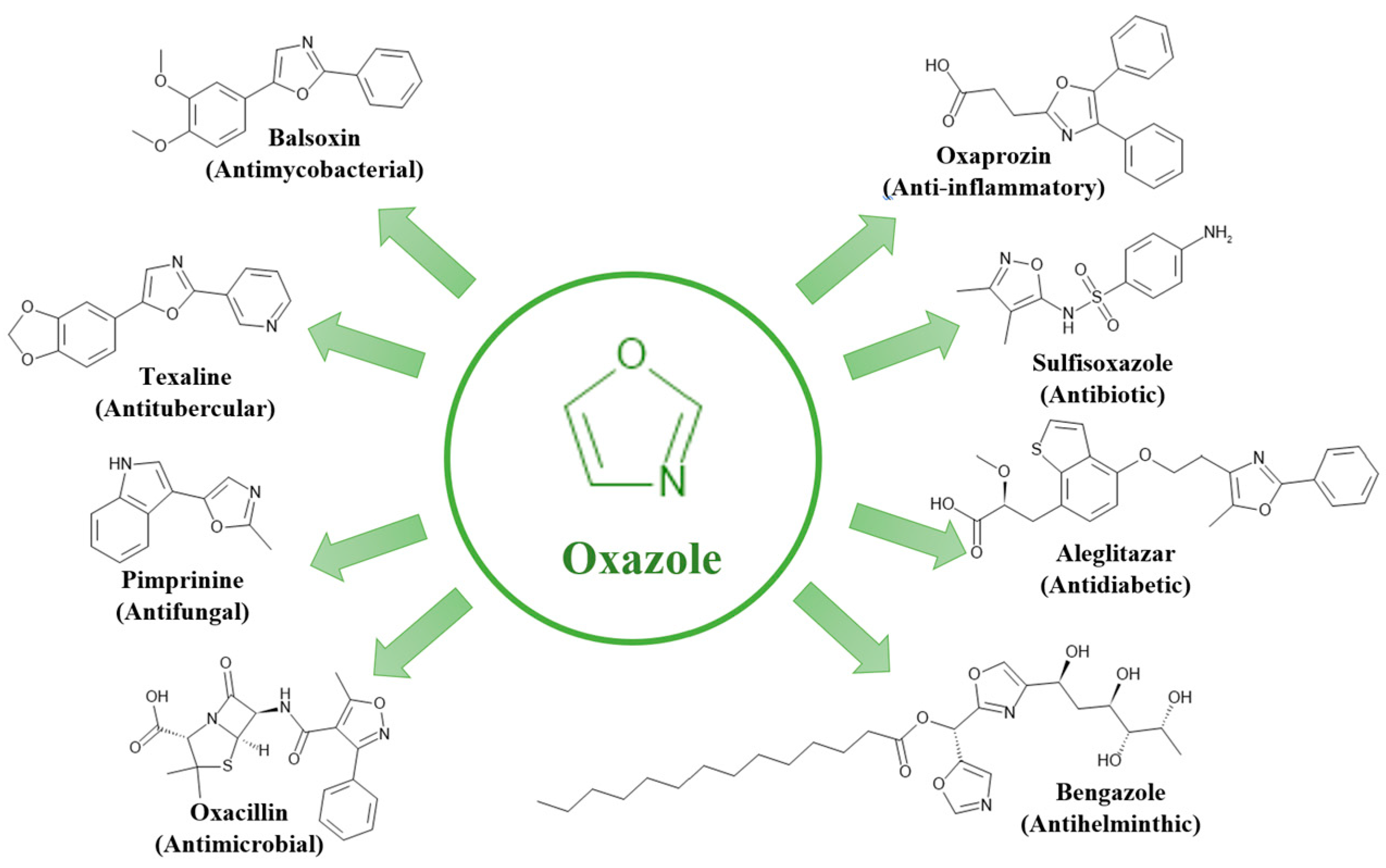
2.2. Oxygen and Nitrogen-Containing Heterocycles
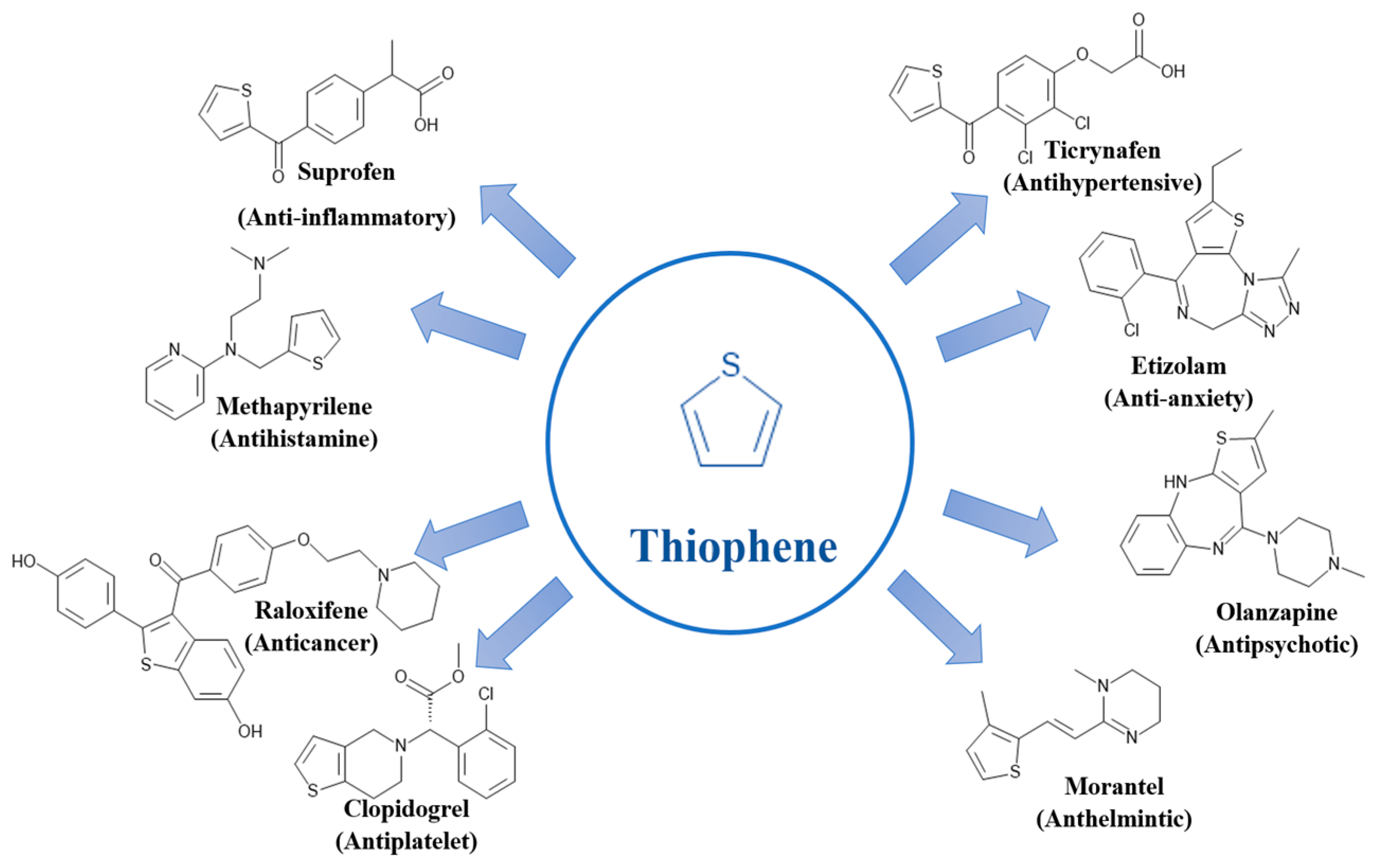
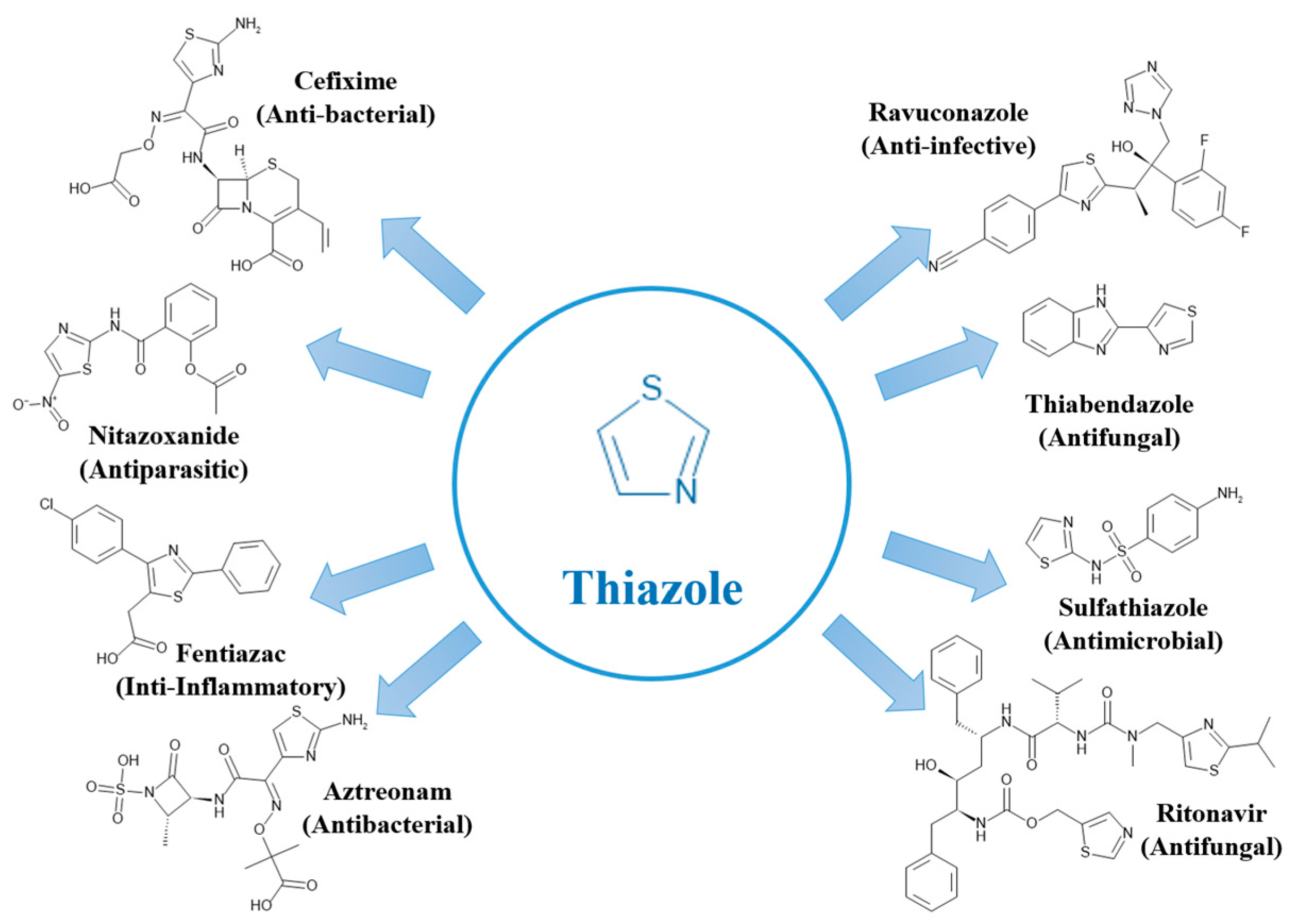
2.3. Sulfur-Containing Heterocycles
2.4. Nitrogen and Sulfur-Containing Heterocycles
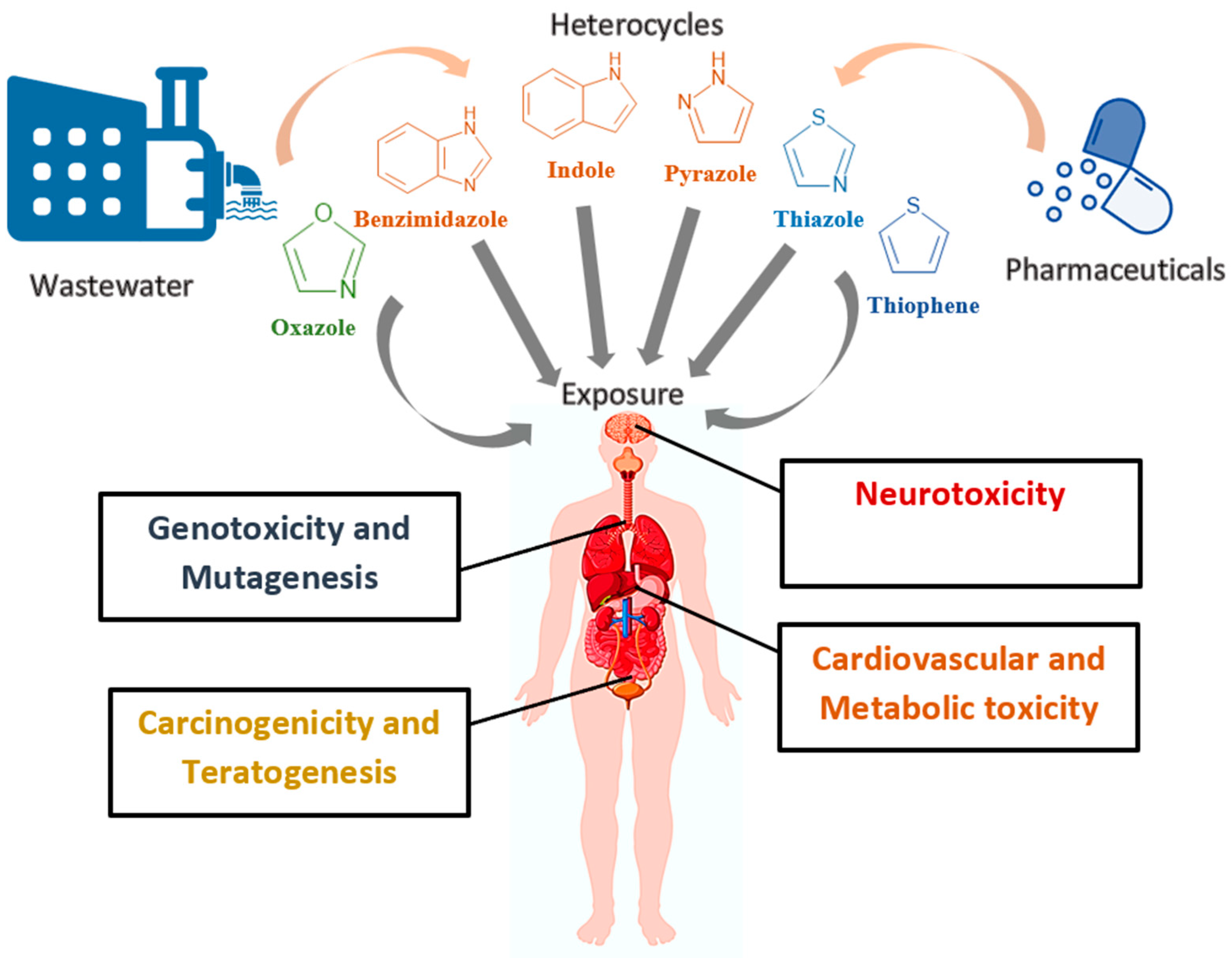
3. Sources and Environmental Fate of Heterocyclic Pharmaceuticals
4. Toxicity, Long-Term Risks, and Impacts on Ecosystems of Heterocyclic Pharmaceuticals
5. Remediation Technologies and Treatment Solutions for Heterocyclic Pharmaceuticals
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A.; Khan, H.K.; Rehman, M.Y.A.; Malik, R.N.; Maculewicz, J.; et al. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. J. Environ. Manag. 2020, 271, 1587–1594. [Google Scholar]
- Broughton, H.B.; Watson, I.A. Selection of heterocycles for drug design. J. Mol. Graph. Model. 2004, 23, 51–58. [Google Scholar] [CrossRef]
- Lamberth, C.; Dinges, J. Bioactive Heterocyclic Compound Classes: Agrochemicals; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Lu, L.Q.; Chen, J.R.; Xiao, W.J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. [Google Scholar] [CrossRef]
- Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Pharmacological significance of synthetic heterocycles scaffold: A review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- Moreno Ríos, A.L.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Silva Oliveira, L.F. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef]
- Tao, H.; Shi, J.; Guo, W.; Zhang, J.; Ge, H.; Wang, Y. Environmental fate and toxicity of androgens: A critical review. Environ. Res. 2022, 214, 113849. [Google Scholar] [CrossRef]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Li Petri, G.; Holl, R.; Spanò, V.; Barreca, M.; Sardo, I.; Raimondi, M.V. Editorial: Emerging heterocycles as bioactive compounds. Front. Chem. 2023, 11, 1202192. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.U.; Mekheimer, R.A.; Abd-Elmonem, M.; Abo-Elsoud, F.A.; Hayallah, A.M.; Mostafa, S.M.; Abdellattif, M.H.; Abourehab, M.A.S.; Farghaly, T.A.; Elkamhawy, A. Recent developments in the synthesis of hybrid heterocycles, a promising approach to develop multi-target antibacterial agents. J. Mol. Struct. 2023, 1286, 135616. [Google Scholar] [CrossRef]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest Manag. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef]
- Va, T. Application of Synthetic Low Molecular Weight Heterocyclic Compounds Derivatives of Pyrimidine, Pyrazole and Oxazole in Agricultural Biotechnology as a New Plant Growth Regulating Substances. Int. J. Med. Biotechnol. Genet. 2017, S2-002, 10–32. [Google Scholar] [CrossRef]
- Babu, A.; Sunil, K.; Sajith, A.M.; Reddy, E.K.; Santra, S.; Zyryanov, G.V.; Venkatesh, T.; Bhadrachari, S.; Nibin Joy, M. NMI-SO2Cl2-Mediated Amide Bond Formation: Facile Synthesis of Some Dihydrotriazolopyrimidine Amide Derivatives as Potential Anti-Inflammatory and Anti-Tubercular Agents. Pharmaceuticals 2024, 17, 548. [Google Scholar] [CrossRef]
- Brendel, S.; Polleichtner, C.; Behnke, A.; Jessel, S.; Hassold, E.; Jennemann, C.; Einhenkel-Arle, D.; Seidel, A. Four selected high molecular weight heterocyclic aromatic hydrocarbons: Ecotoxicological hazard assessment, environmental relevance and regulatory needs under REACH. Ecotoxicol. Environ. Saf. 2018, 163, 340–348. [Google Scholar] [CrossRef] [PubMed]
- González-Andrés, P.; Fernández-Peña, L.; Díez-Poza, C.; Villalobos, C.; Nuñez, L.; Barbero, A. Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches. Mar. Drugs 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Padoley, K.V.; Mudliar, S.N.; Pandey, R.A. Heterocyclic nitrogenous pollutants in the environment and their treatment options—An overview. Bioresour. Technol. 2008, 99, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Mukherji, S. Environmental contamination by heterocyclic Polynuclear aromatic hydrocarbons and their microbial degradation. Bioresour. Technol. 2021, 341, 125860. [Google Scholar] [CrossRef] [PubMed]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in then aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jin, X.; Liu, N.; Luo, Y.; Yan, Z.; Chen, M.; Liu, Y.; Xie, H.; Giesy, J.P.; Wu, F.; et al. Triadimefon in aquatic environments: Occurrence, fate, toxicity, and ecological risk. Environ. Sci. Eur. 2022, 34, 12. [Google Scholar] [CrossRef]
- Cordoba, A.; Saldias, C.; Urzúa, M.; Montalti, M.; Guernelli, M.; Focarete, M.L.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Asfaha, Y.G.; Tekile, A.K.; Zewge, F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean. Eng. Technol. 2021, 4, 100261. [Google Scholar] [CrossRef]
- Wang, J.J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, R.; Konradt-Moraes, L.C.; Vieira, M.F.; Fagundes-Klen, M.R.; Vieira, A.M.S. Performance of a coagulation-ultrafiltration hybrid process for water supply treatment. Chem. Eng. J. 2011, 166, 483–489. [Google Scholar] [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manag. 2015, 158, 146–157. [Google Scholar] [CrossRef]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open Med. Chem. J. 2022, 16. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef] [PubMed]
- Khandale, N.; Ghodke, M.S. Exploring Potential of Indole Derivatives: A Brief Review. Int. J. Pharm. Pharm. Sci. 2023, 15, 1–14. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Ahmed, A. Synthesis of biologically active sulfonamide-based indole analogs: A review. Futur. J. Pharm. Sci. 2023, 9, 46. [Google Scholar] [CrossRef]
- Hao, C.; Lissemore, L.; Nguyen, B.; Kleywegt, S.; Yang, P.; Solomon, K. Determination of pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry. Anal. Bioanal. Chem. 2006, 384, 505–513. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: A review. BMC Chem. 2019, 13, 103021. [Google Scholar] [CrossRef]
- Gaba, M.; Mohan, C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 2016, 25, 173–210. [Google Scholar] [CrossRef]
- Brishty, S.R.; Hossain, M.J.; Khandaker, M.U.; Faruque, M.R.I.; Osman, H.; Rahman, S.M.A. A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 2021, 12, 762807. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Hashem, H.E.; El Bakri, Y. An overview on novel synthetic approaches and medicinal applications of benzimidazole compounds: An overview on novel synthetic approaches and medicinal applications. Arab. J. Chem. 2021, 14, 103418. [Google Scholar] [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015, 86, 799–845. [Google Scholar] [CrossRef]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; da Silva Castro, P.F.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs from Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Saini, D.; Jain, S.; Jain, N. Pyrazole scaffold: A remarkable tool in the development of anticancer agents. Eur. J. Med. Chem. 2013, 70, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Adardour, M.; Ait Lahcen, M.; Oubahmane, M.; Ettahiri, W.; Hdoufane, I.; Bouamama, H.; Alanazi, M.M.; Cherqaoui, D.; Taleb, M.; Garcia, E.Z.; et al. Design, Synthesis, Molecular Modeling and Biological Evaluation of Novel Pyrazole Benzimidazolone Derivatives as Potent Antioxidants. Pharmaceuticals 2023, 16, 1648. [Google Scholar] [CrossRef] [PubMed]
- El-Helw, E.A.E.; Gado, M.M.; El-Ziaty, A.K. Synthesis and anti-rotavirus activity of some nitrogen heterocycles integrated with pyrazole scaffold. J. Iran. Chem. Soc. 2020, 17, 1479–1492. [Google Scholar] [CrossRef]
- Kabi, A.K.; Sravani, S.; Gujjarappa, R.; Garg, A.; Vodnala, N.; Tyagi, U.; Kaldhi, D.; Singh, V.; Gupta, S.; Malakar, C.C. Overview on Biological Activities of Pyrazole Derivatives. In Materials Horizons: From Nature to Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 229–306. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Atmaram, U.A.; Roopan, S.M. Biological activity of oxadiazole and thiadiazole derivatives. Appl. Microbiol. Biotechnol. 2022, 106, 3489–3505. [Google Scholar] [CrossRef]
- Joshi, S.; Mehra, M.; Singh, R.; Kakar, S. Review on Chemistry of Oxazole derivatives: Current to Future Therapeutic Prospective. Egypt. J. Basic Appl. Sci. 2023, 10, 218–239. [Google Scholar] [CrossRef]
- Safarzaei, M.; Maghsoodlou, M.T.; Mollashahi, E.; Hazeri, N.; Lashkari, M. Synthesis of 3-aminoisoxazolmethylnaphthols via one-pot three-component reaction under solvent-free conditions. Res. Chem. Intermed. 2018, 44, 7449–7458. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef]
- Ingall, A.H. Thiopyrans and Fused Thiopyrans. Compr. Heterocycl. Chem. 1984, 3–7, 885–942. [Google Scholar] [CrossRef]
- Laxmikeshav, K.; Kumari, P.; Shankaraiah, N. Expedition of sulfur-containing heterocyclic derivatives as cytotoxic agents in medicinal chemistry: A decade update. Med. Res. Rev. 2022, 42, 513–575. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.X.; Wang, Y.T.; Zhang, S.N.; Li, Y.; Chen, X.B.; Wang, S.Q.; Liu, H.M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Chhabria, M.T.; Patel, S.; Modi, P.; Brahmkshatriya, P.S. Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [CrossRef] [PubMed]
- Khidre, R.E.; Radini, I.A.M. Design, synthesis and docking studies of novel thiazole derivatives incorporating pyridine moiety and assessment as antimicrobial agents. Sci. Rep. 2021, 11, 7846. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M.; Raslan, M.A.; Abbas, A.A.; Mohamed, B.E.; Abdellattif, M.H.; Nafie, M.S.; Hassan, M.K. Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis With Molecular Docking Approaches. Front. Chem. 2021, 9, 694870. [Google Scholar] [CrossRef]
- Hussain, R.; Rehman, W.; Khan, S.; Maalik, A.; Hefnawy, M.; Alanazi, A.S.; Khan, Y.; Rasheed, L. Imidazopyridine-Based Thiazole Derivatives as Potential Antidiabetic Agents: Synthesis, In Vitro Bioactivity, and In Silico Molecular Modeling Approach. Pharmaceuticals 2023, 16, 1288. [Google Scholar] [CrossRef]
- Majumdar, A.; Pal, A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy 2020, 22, 11–42. [Google Scholar] [CrossRef]
- Masanabo, N.; Orimolade, B.; Idris, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Feleni, U. Advances in polymer-based detection of environmental ibuprofen in wastewater. Environ. Sci. Pollut. Res. 2023, 30, 14062–14090. [Google Scholar] [CrossRef]
- Ghosh, P.; Mukherji, S. Fate, detection technologies and toxicity of heterocyclic PAHs in the aquatic and soil environments. Sci. Total Environ. 2023, 892, 164499. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El, A.; Khezami, L. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Paut Kusturica, M.; Tomas, A.; Sabo, A. Disposal of unused drugs: Knowledge and behavior among people around the world. Rev. Environ. Contam. Toxicol. 2017, 240, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Karungamye, P.; Rugaika, A.; Mtei, K.; Machunda, R. The pharmaceutical disposal practices and environmental contamination: A review in East African countries. HydroResearch 2022, 5, 99–107. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; Song, N.; Liu, S.; Wang, Y.; Ye, F.; Ju, Y.; Jiao, S.; Shi, L. New insight into fate and transport of organic compounds from pollution sources to aquatic environment using non-targeted screening: A wastewater treatment plant case study. Sci. Total Environ. 2023, 863, 161031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, C.; Geladakis, G.; Kommata, V.; Batargias, C.; Lagoumintzis, G. Insights in Pharmaceutical Pollution: The Prospective Role of eDNA Metabarcoding. Toxics 2023, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2022, 184, 108693. [Google Scholar] [CrossRef]
- Bellamri, M.; Walmsley, S.J.; Turesky, R.J. Metabolism and biomarkers of heterocyclic aromatic amines in humans. Genes Environ. 2021, 43, 29. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Geng, Y.; Xie, Y.; Li, W.; Ji, J.; Chen, F.; Liao, X.; Hu, X.; Ma, L. Heterocyclic Amines in Meat and Meat Products: Occurrence, Formation, Mitigation, Health Risks and Intervention. Food Rev. Int. 2024, 40, 1503–1519. [Google Scholar] [CrossRef]
- Cao, W.; Yuan, J.; Geng, S.; Zou, J.; Dou, J.; Fan, F. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study. Processes 2023, 11, 52. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Çelik, G.; Stolte, S.; Markiewicz, M. NSO-heterocyclic PAHs—Controlled exposure study reveals high acute aquatic toxicity. J. Hazard. Mater. 2023, 460, 132428. [Google Scholar] [CrossRef]
- Barbuceanu, S.F.; Rosca, E.V.; Apostol, T.V.; Socea, L.I.; Draghici, C.; Farcasanu, I.C.; Ruta, L.L.; Nitulescu, G.M.; Iscrulescu, L.; Pahontu, E.M.; et al. New Heterocyclic Compounds from Oxazol-5(4H)-one and 1,2,4-Triazin-6(5H)-one Classes: Synthesis, Characterization and Toxicity Evaluation. Molecules 2023, 28, 4834. [Google Scholar] [CrossRef] [PubMed]
- Matavos-Aramyan, S. Addressing the microplastic crisis: A multifaceted approach to removal and regulation. Environ. Adv. 2024, 17, 100579. [Google Scholar] [CrossRef]
- Paut Kusturica, M.; Jevtic, M.; Ristovski, J.T. Minimizing the environmental impact of unused pharmaceuticals: Review focused on prevention. Front. Environ. Sci. 2022, 10, 1077974. [Google Scholar] [CrossRef]
- Samadi, A.; Pour, A.K.; Jamieson, R. Development of remediation technologies for organic contaminants informed by QSAR/QSPR models. Environ. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Majee, S.; Shilpa; Sarav, M.; Banik, B.K.; Ray, D. Recent Advances in the Green Synthesis of Active N-Heterocycles and Their Biological Activities. Pharmaceuticals 2023, 16, 873. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s tool Box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; El Aatik, A.; Aliste, M.; Navarro, G.; Fenoll, J.; Navarro, S. Removal of Contaminants of Emerging Concern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water. Air. Soil Pollut. 2023, 234, 55. [Google Scholar] [CrossRef]
- Friedmann, D. A General Overview of Heterogeneous Photocatalysis as a Remediation Technology for Wastewaters Containing Pharmaceutical Compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Huang, Y.; Lu, G. Toxicity evolution and control for the UV/H2O2 degradation of nitrogen-containing heterocyclic compounds: SDZ and PMM. Chemosphere 2023, 338, 139541. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kong, J.; Liu, H.; Chen, P.; Su, Y.; Liu, G.; Lv, W. Removal of indomethacin using UV–vis/peroxydisulfate: Kinetics, toxicity, and transformation pathways. Chem. Eng. J. 2018, 331, 809–817. [Google Scholar] [CrossRef]
- Jia, H.; Xu, H.; Shi, M.; Lu, K.; Tao, Y.; Xia, M.; Wang, F. Construction of ACNF/Polypyrrole/MIL-100-Fe composites with exceptional removal performance for ceftriaxone and indomethacin inspired by “Ecological Infiltration System”. J. Colloid Interface Sci. 2023, 650, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Petrinic, I.; Luxbacher, T.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Memb. Sci. 2009, 330, 90–103. [Google Scholar] [CrossRef]
- Kujawska, A.; Kiełkowska, U.; Atisha, A.; Yanful, E.; Kujawski, W. Comparative analysis of separation methods used for the elimination of pharmaceuticals and personal care products (PPCPs) from water—A critical review. Sep. Purif. Technol. 2022, 290, 120797. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Guo, Y.; Zhang, Y.; Yu, P.; Ye, Z.; Qian, Y.; Yoshimura, C.; Wang, T.; Zhang, L. Photochemical fate of β-blocker pindolol in riverine and its downstream coastal waters. Sci. Total Environ. 2024, 927, 172236. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.; Pandiyaraj, K.N.; Kandavelu, V.; Vasu, D.; Saravanan, D. Efficiency evaluation of the photocatalytic degradation of telmisartan anti-hypertensive drug with Fenton, photo-Fenton and recyclable TiO2 heterogeneous catalyst. React. Kinet. Mech. Catal. 2020, 130, 1141–1154. [Google Scholar] [CrossRef]
- Ljubas, D.; Čizmić, M.; Vrbat, K.; Stipaničev, D.; Repec, S.; Ćurković, L.; Babić, S. Albendazole Degradation Possibilities by UV-Based Advanced Oxidation Processes. Int. J. Photoenergy 2018, 2018, 6181747. [Google Scholar] [CrossRef]
- Fukuda, S.; Sakurai, Y.; Izawa, S. Detoxification of the post-harvest antifungal pesticide thiabendazole by cold atmospheric plasma. J. Biosci. Bioeng. 2023, 136, 123–128. [Google Scholar] [CrossRef]
- Zizzamia, A.R.; Tesoro, C.; Bianco, G.; Bufo, S.A.; Ciriello, R.; Brienza, M.; Scrano, L.; Lelario, F. Efficient photooxidation processes for the removal of sildenafil from aqueous environments: A comparative study. Case Stud. Chem. Environ. Eng. 2024, 9, 100708. [Google Scholar] [CrossRef]
- Arcila-Saenz, J.; Hincapié-Mejía, G.; Londoño-Cañas, Y.A.; Peñuela, G.A. Role of the hydrolytic-acidogenic phase on the removal of bisphenol A and sildenafil during anaerobic treatment. Environ. Monit. Assess. 2023, 195, 1552. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Pardo, R.; Sánchez, M.I.; Muñoz, B.E. Photochemical, thermal, biological and long-term degradation of celecoxib in river water. Degradation products and adsorption to sediment. J. Hazard. Mater. 2018, 342, 252–259. [Google Scholar] [CrossRef]
- Reddy, K.; Renuka, N.; Malla, M.A.; Moodley, B.; Bux, F.; Kumari, S. Enhanced removal efficiency of Tetradesmus obliquus for nevirapine removal via co-substrate supplementation: Removal mechanisms, relative gene expression and metabolomics. Environ. Sci. Water Res. Technol. 2024, 10, 3263–3278. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of iron oxide nanoparticles for the degradation of methylene blue dye, sulfisoxazole antibiotic and removal of bacteria from real water. Heliyon 2022, 8, e10536. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, S.; Zhou, J.; Wu, C.; Li, X.; Yao, M.; Xia, S.; Rittmann, B.E. Biological conversion of sulfisoxazole in an autotrophic hydrogen-based membrane biofilm reactor. J. Water Process Eng. 2023, 51, 103396. [Google Scholar] [CrossRef]
- Giraldo, A.L.; Erazo-Erazo, E.D.; Flórez-Acosta, O.A.; Serna-Galvis, E.A.; Torres-Palma, R.A. Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: Evaluation of degradation routes, organic by-products and effects of water matrix components. Chem. Eng. J. 2015, 279, 103–114. [Google Scholar] [CrossRef]
- Regulska, E.; Karpińska, J. Photocatalytic degradation of olanzapine in aqueous and river waters suspension of titanium dioxide. Appl. Catal. B Environ. 2012, 117–118, 96–104. [Google Scholar] [CrossRef]
- Fedeila, M.; Hachaïchi-Sadouk, Z.; Bautista, L.F.; Simarro, R. Biodegradation of clopidogrel bisulfate by Pseudomonas aeruginosa and Pseudomonas putida strains isolated from Algerian wastewater. J. Contam. Hydrol. 2023, 256, 104198. [Google Scholar] [CrossRef]
- Mansour, D.; Alblawi, E.; Alsukaibi, A.K.D.; Humaidi, J.; Tahraoui, H.; Shatat, M.; Teka, S.; Maisara, S.; Bellakhal, N.; Binous, H.; et al. Modeling and Optimization of Electrochemical Advanced Oxidation of Clopidogrel Using the Doehlert Experimental Design Combined with an Improved Grey Wolf Algorithm. Water 2024, 16, 1964. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [Google Scholar] [CrossRef]
- Aziz, K.; Aziz, F.; Mamouni, R.; Aziz, L.; Anfar, Z.; Azrrar, A.; Kjidaa, B.; Saffaj, N.; Laknifli, A. High thiabendazole fungicide uptake using Cellana tramoserica shells modified by copper: Characterization, adsorption mechanism, and optimization using CCD-RSM approach. Environ. Sci. Pollut. Res. 2022, 29, 86020–86035. [Google Scholar] [CrossRef] [PubMed]
- Hojamberdiev, M.; Vargas, R.; Madriz, L.; Yubuta, K.; Kadirova, Z.C.; Shaislamov, U.; Sannegowda, L.K.; Jędruchniewicz, K.; Typek, R.; Teshima, K.; et al. Unveiling the origin of the efficient photocatalytic degradation of nitazoxanide over bismuth (oxy)iodide crystalline phases. Environ. Sci. Nano 2023, 11, 336–350. [Google Scholar] [CrossRef]

| Pharmaceutical Compound | Heterocycle Type | Method Used | Operating Conditions | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Indomethacin (Anti-inflammatory) | Indole | UV–vis/peroxydisulfate | pH = 7, [IM] = 20 µM and [PDS] = 20 µM | 100% in 24 min | [92] |
| Indomethacin (Anti-inflammatory) | Indole | Adsorption using ACNF/Polypyrrole/MIL-100-Fe composites | [IM] = 1–25 mg/L, pH = 5.0, T = 298 K, adsorbent mass = 0.5 g/L | 99.1% in 256 min | [93] |
| Pindolol (Antihypertensive) | Indole | Membrane filtration (Desal HL thin film composite membrane) | [DOM] = 2 μg/dm3, Membrane from GE Osmonics, 98% Magnesium sulfate | 74% | [94,95] |
| Pindolol (Antihypertensive) | Indole | Photodegradation (photolysis) | River water with riverine [DOM] = 20 mg/L, [NO3] = 1 mM | 68% in 42 min | [96] |
| Telmisartan (Antihypertensive) | Benzimidazole | Photocatalytic Degradation (TiO2) | UV light, pH 7.0, [TN] = 5.5 × 10−5 mol/L, TiO2 mass = 20 mg | 100% in 180 min | [97] |
| Albendazole (Antibacterial) | Benzimidazole | UV-C + H2O2 process | [ALB] = 1 mg/L, [O3] = 1.5 mg/L, UV-C = 185/254 nm radiation peaks and incident photon flux Np = 1.033 × 10−6 Einstein/s | >99% in 120 min | [98] |
| Thiabendazole (Anti-fungal) | Benzimidazole | Cold Atmospheric Plasma | Voltage: 8 kV, Distance: 1 cm, Airflow rate: 0.5 slm | 100% detoxification in 20 min | [99] |
| Sildenafil (Phosphodiesterase type 5 Inhibitor) | Pyrazole | Sunlight/PMS | Synthetic wastewater (pH ±8), [PMS] = 800 μM, [SIL] = 3 mg/L | 100% in 130 min | [100] |
| Sildenafil (Phosphodiesterase type 5 Inhibitor) | Pyrazole | Anaerobic biological Treatment | Inoculum: Hydrolytic/acidogenic (H/A) and methanogenic (MET), [SIL] = 50 μg/L | 43% (H/A inoculum), 41% (MET inoculum) | [101] |
| Celecoxib (Anti-inflammatory) | Pyrazole | Photochemical, UV Lamp | River water, pH = 7.8, [CLC] = 2.00 μg/L, irradiated UV = 254 nm | 100% in 1 week | [102] |
| Nevirapine (Antiretroviral) | Pyrimidine | Bioremediation by microalgae | Cultivation modes: mixotrophic; pH 7.5; 25 °C, Tetradesmus obliquus, mixotrophic cultivation | 80.13% in 8 days | [103] |
| Sulfisoxazole (Antibiotic) | Oxazole | Photodegradation, Fe3O4 Nanoparticles | Fe₃O₄ mass 1 g/L, [SSX] 10 ppm, irradiated with 150 W UV lamp | 60% in 120 min | [104] |
| Sulfisoxazole (Antibiotic) | Oxazole | Hydrogen-based membrane biofilm reactor | [SSX] = 5 mg/L, HRT: 48 h, 30 °C, Continuous H2 feeding, H2 headspace 20 mL | 88% in 28 days | [105] |
| Oxaprozin (Anti-inflammatory) | Oxazole | Electrochemical Anodic Oxidation (Ti/IrO2 anode) | [OXA] = 203 μmol/L, Current density: 30.25 mA/cm2, [sodium chloride] = 0.225 mol/L | 100% in 4 min | [106] |
| Olanzapine (Antipsychotic) | Thiophene | Photocatalytic degradation (TiO2) | [OLA] = 5 × 10−5 mol/L, Solar simulated light (250 and 500 W/m2), catalyst mass 1.56 g/L | 100% in 120 min | [107] |
| Clopidogrel (Antiplatelet) | Thiophene | Aerobic biodegradation | [CPG] = 25 mg/L, pH: 8.5, Temperature: 30 °C, Mixed bacterial culture (Pseudomonas aeruginosa 1 M, Pseudomonas putida 5 M) | 99.08% in 96 h | [108] |
| Clopidogrel (Antiplatelet) | Thiophene | Electrochemical advanced oxidation (Electro-Fenton Process) | [CPG] = 0.02 mM, [Fe2+] = 0.7 mM, [Na2SO4] = 50 mM, pH = 3, I = 0.55 A and V = 0.3 L | 70.4% in 8 h | [109] |
| Cefixime (Antibacterial) | Thiazole | Photocatalytic Degradation (Bi12TiO20) | [CFX] = 10 mg/L, pH = 6, catalyst dosage = 1.5 g/L | 94.93% in 180 min | [110] |
| Thiabendazole (Antifungal) | Thiazole | Adsorption using Cellana tramoserica shells modified by copper (CT-Cu) | pH 5, [THB] = 50 ppm, Adsorbent mass 20 mg | 91% in 120 min | [111] |
| Nitazoxanide (Antiparasitic) | Thiazole | Photocatalytic Degradation (BiOI/Bi4O5I2 heterostructure) | Photocatalyst mass 0.4 mg/L, [NTZ] = 10 mg/L, 150 W mercury lamp (500–550 nm, 7.31–7.53 mW cm−2) | 100% in 60 min | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baaloudj, O.; Scrano, L.; Bufo, S.A.; Modley, L.-A.S.; Lelario, F.; Zizzamia, A.R.; Emanuele, L.; Brienza, M. Environmental Fate, Ecotoxicity, and Remediation of Heterocyclic Pharmaceuticals as Emerging Contaminants: A Review of Long-Term Risks and Impacts. Organics 2025, 6, 1. https://doi.org/10.3390/org6010001
Baaloudj O, Scrano L, Bufo SA, Modley L-AS, Lelario F, Zizzamia AR, Emanuele L, Brienza M. Environmental Fate, Ecotoxicity, and Remediation of Heterocyclic Pharmaceuticals as Emerging Contaminants: A Review of Long-Term Risks and Impacts. Organics. 2025; 6(1):1. https://doi.org/10.3390/org6010001
Chicago/Turabian StyleBaaloudj, Oussama, Laura Scrano, Sabino Aurelio Bufo, Lee-Ann Sade Modley, Filomena Lelario, Angelica Rebecca Zizzamia, Lucia Emanuele, and Monica Brienza. 2025. "Environmental Fate, Ecotoxicity, and Remediation of Heterocyclic Pharmaceuticals as Emerging Contaminants: A Review of Long-Term Risks and Impacts" Organics 6, no. 1: 1. https://doi.org/10.3390/org6010001
APA StyleBaaloudj, O., Scrano, L., Bufo, S. A., Modley, L.-A. S., Lelario, F., Zizzamia, A. R., Emanuele, L., & Brienza, M. (2025). Environmental Fate, Ecotoxicity, and Remediation of Heterocyclic Pharmaceuticals as Emerging Contaminants: A Review of Long-Term Risks and Impacts. Organics, 6(1), 1. https://doi.org/10.3390/org6010001













