Solid-State Electrochemical Energy Storage Based on Soluble Melanin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Melanin Derivatives
2.2. Supercapacitor Assembly and Characterization
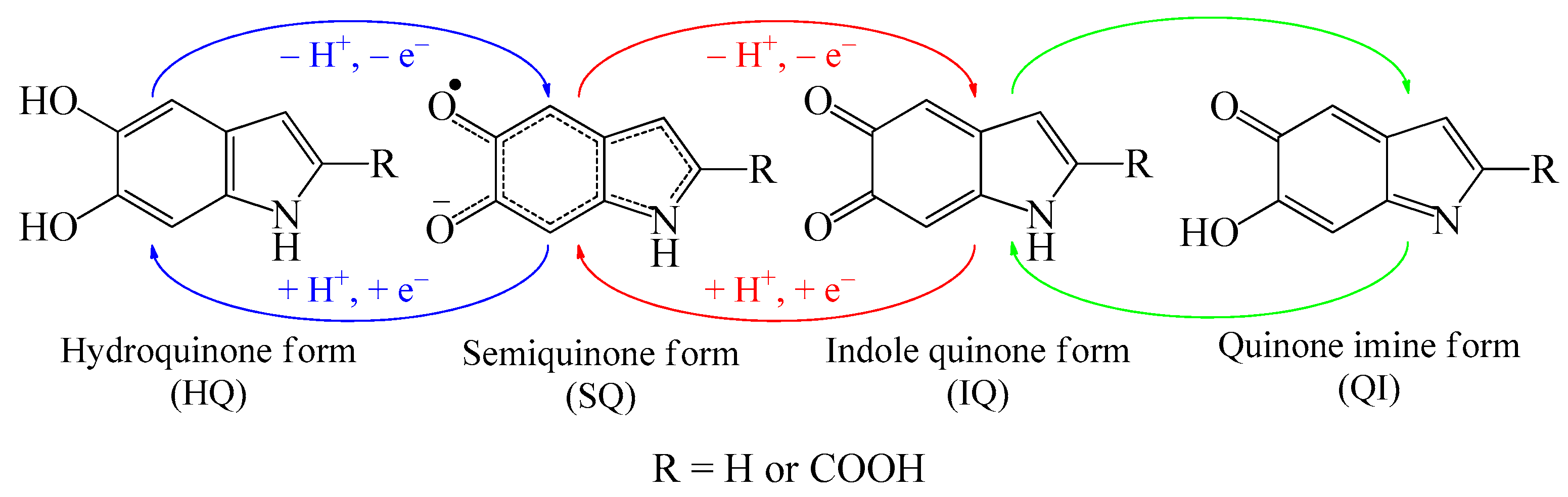
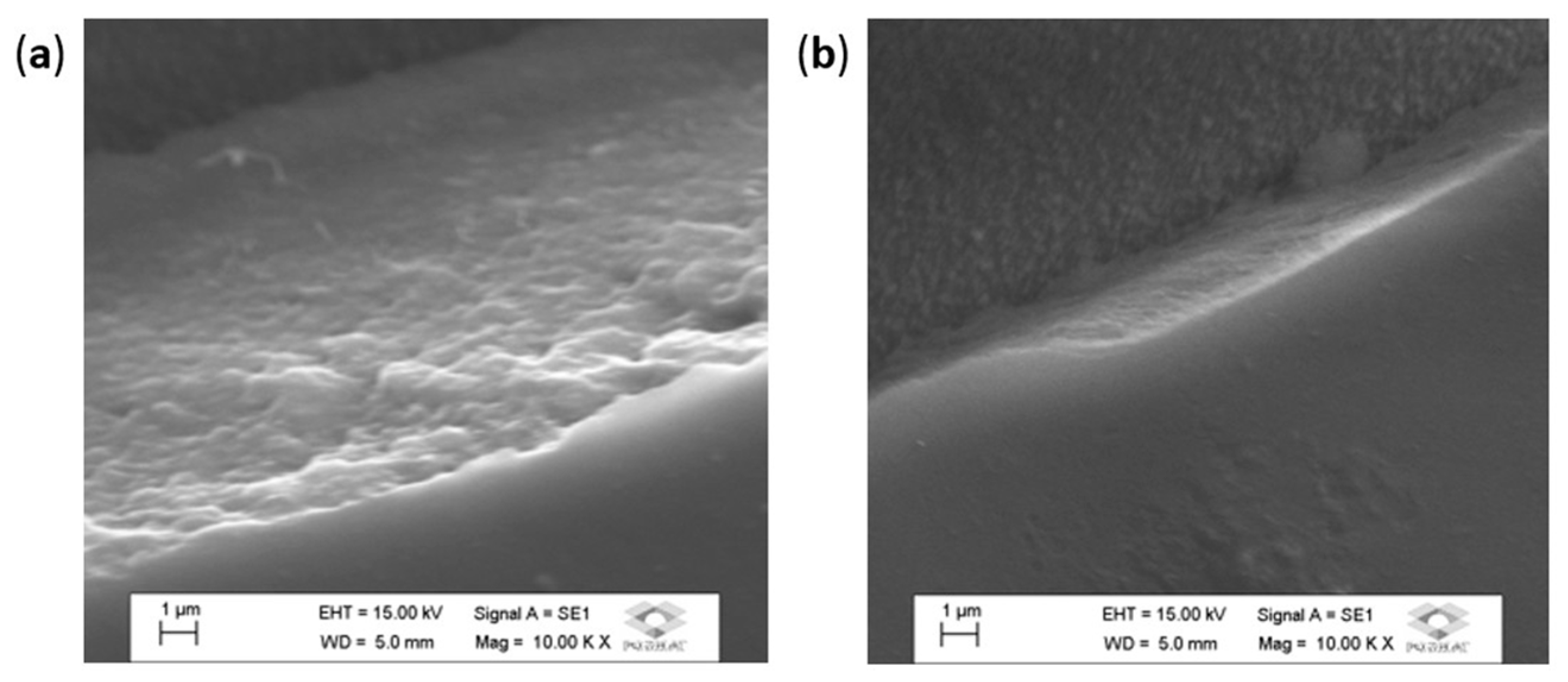
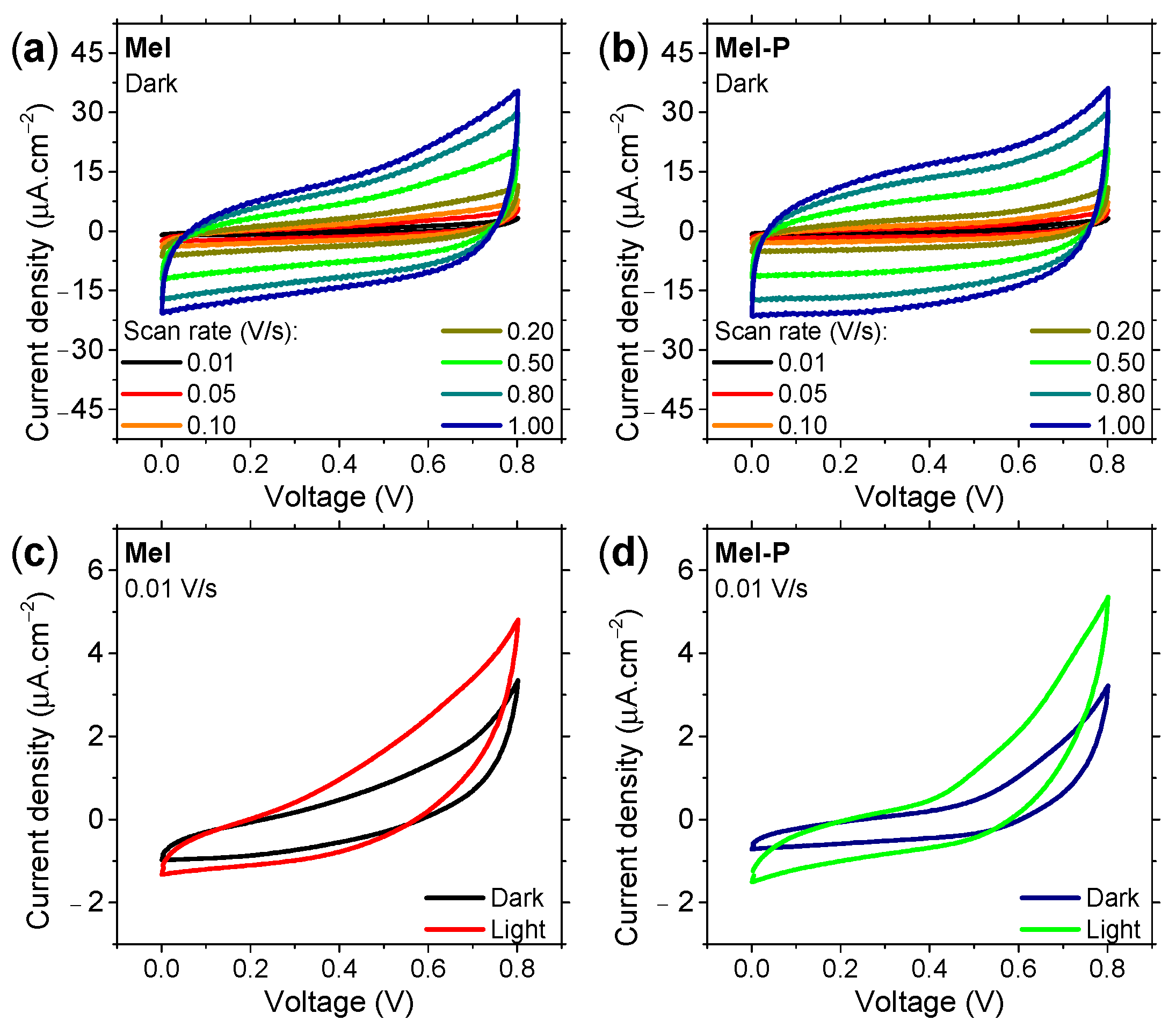
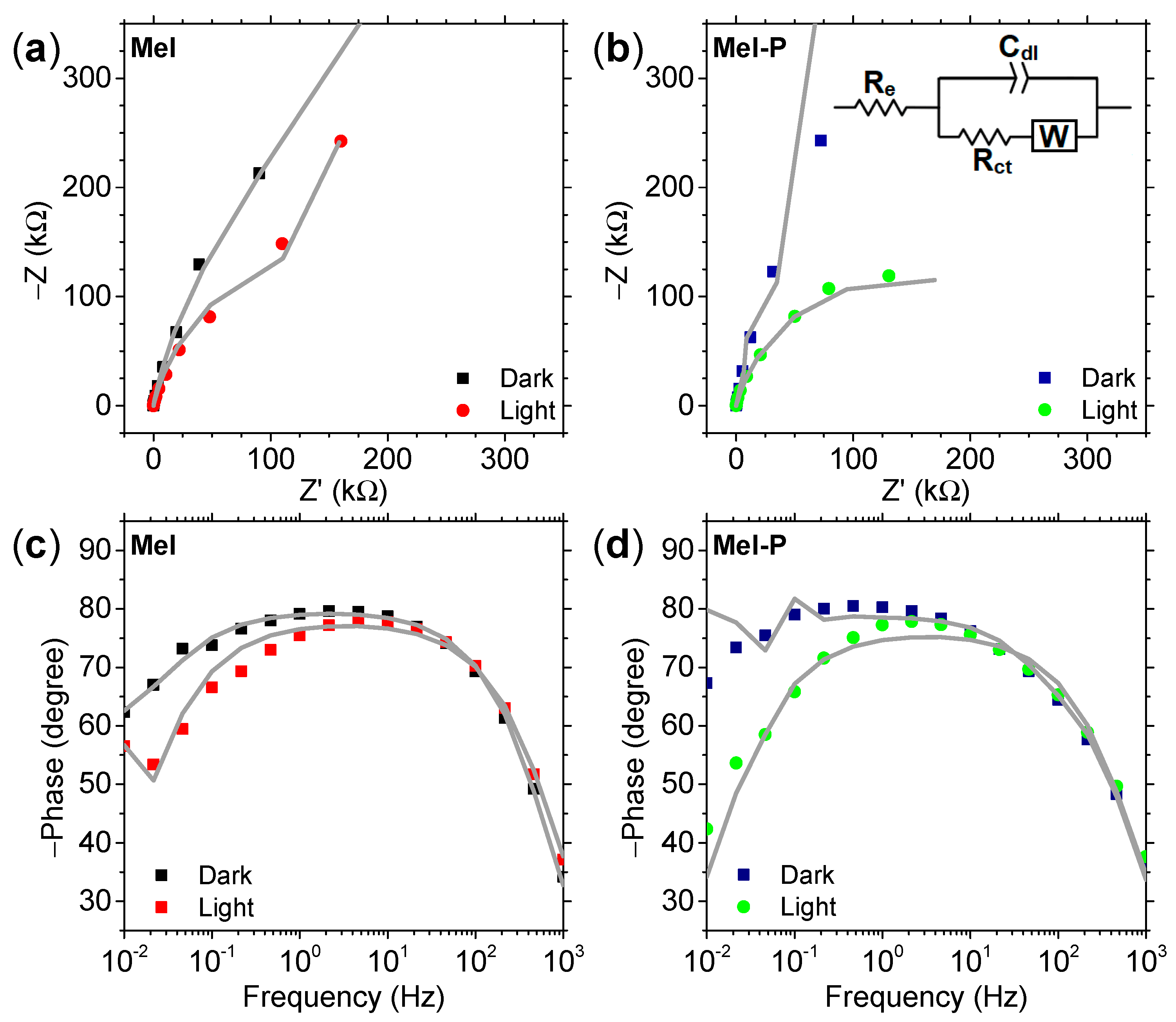
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, S. National Academy of Engineering, Grand Challenges for Engineering: Imperatives, Prospects, and Priorities: Summary of a Forum; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Chen, X.; Villa, N.S.; Zhuang, Y.; Chen, L.; Wang, T.; Li, Z.; Kong, T. Stretchable Supercapacitors as Emergent Energy Storage Units for Health Monitoring Bioelectronics. Adv. Energy Mater. 2020, 10, 1902769. [Google Scholar] [CrossRef]
- Ohayon, D.; Inal, S. Organic Bioelectronics: From Functional Materials to Next-Generation Devices and Power Sources. Adv. Mater. 2020, 32, 2001439. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Ye, T.; Peng, H.; Zhang, Y. Stretchable Energy Storage Devices Based on Carbon Materials. Small 2021, 2005015. [Google Scholar] [CrossRef] [PubMed]
- Vallem, V.; Sargolzaeiaval, Y.; Ozturk, M.; Lai, Y.C.; Dickey, M.D. Energy Harvesting and Storage with Soft and Stretchable Materials. Adv. Mater. 2021, 33, 2004832. [Google Scholar] [CrossRef]
- Tong, X.; Tian, Z.; Sun, J.; Tung, V.; Kaner, R.B.; Shao, Y. Self-healing flexible/stretchable energy storage devices. Mater. Today 2021, 44, 78–104. [Google Scholar] [CrossRef]
- Kim, Y.J.; Wu, W.; Chun, S.; Whitacre, J.F.; Bettinger, C.J. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc. Natl. Acad. Sci. USA 2013, 110, 20912–20917. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.O.; Wu, W.; Chun, S.E.; Whitacre, J.F.; Bettinger, C.J. Catechol-mediated reversible binding of multivalent cations in eumelanin half-cells. Adv. Mater. 2014, 26, 6572–6579. [Google Scholar] [CrossRef]
- Kumar, P.; Di Mauro, E.; Zhang, S.; Pezzella, A.; Soavi, F.; Santato, C.; Cicoira, F. Melanin-based flexible supercapacitors. J. Mater. Chem. C 2016, 4, 9516–9525. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, W.; Zhang, Y.; Wang, H.; Liu, S.; Hao, E.; Zhao, S.; Yang, H. Squid inks-derived nanocarbons with unique “shell@pearls” structure for high performance supercapacitors. J. Power Sources 2017, 354, 116–123. [Google Scholar] [CrossRef]
- Xu, R.; Gouda, A.; Caso, M.F.; Soavi, F.; Santato, C. Melanin: A Greener Route to Enhance Energy Storage under Solar Light. ACS Omega 2019, 4, 12244–12251. [Google Scholar] [CrossRef] [Green Version]
- Ajjan, F.N.; Mecerreyes, D.; Inganäs, O. Enhancing Energy Storage Devices with Biomacromolecules in Hybrid Electrodes. Biotechnol. J. 2019, 14, 1900062. [Google Scholar] [CrossRef]
- Yang, L.; Gu, B.; Chen, Z.; Yue, Y.; Wang, W.; Zhang, H.; Liu, X.; Ren, S.; Yang, W.; Li, Y. Synthetic Biopigment Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 30360–30367. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M.J. Melanin biopolymers: Tailoring chemical complexity for materials design. Angew. Chem. Int. Ed. 2020, 59, 11196–11205. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [Green Version]
- Piacenti-Silva, M.; Matos, A.A.; Paulin, J.V.; Alavarce, R.A.d.S.; de Oliveira, R.C.; Graeff, C.F. Biocompatibility investigations of synthetic melanin and melanin analogue for application in bioelectronics. Polym. Int. 2016, 65, 1347–1354. [Google Scholar] [CrossRef]
- Di Mauro, E.; Xu, R.; Soliveri, G.; Santato, C. Natural melanin pigments and their interfaces with metal ions and oxides: Emerging concepts and technologies. MRS Commun. 2017, 7, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Bronze-Uhle, E.S.; Paulin, J.V.; Piacenti-Silva, M.; Battocchio, C.; Rocco, M.L.M.; Graeff, C.F.D.O. Melanin synthesis under oxygen pressure. Polym. Int. 2016, 65, 1339–1346. [Google Scholar] [CrossRef]
- Jastrzebska, M.M.; Isotalo, H.; Paloheimo, J.; Stubb, H. Electrical conductivity of synthetic DOPA-melanin polymer for different hydration states and temperatures. J. Biomater. Sci. Polym. Ed. 1995, 7, 577–586. [Google Scholar] [CrossRef]
- Mostert, B.; Powell, B.J.; Gentle, I.R.; Meredith, P. On the origin of electrical conductivity in the bio-electronic material melanin. Appl. Phys. Lett. 2012, 100, 093701. [Google Scholar] [CrossRef]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [Green Version]
- Wünsche, J.; Deng, Y.; Kumar, P.; Di Mauro, E.; Josberger, E.; Sayago, J.; Pezzella, A.; Soavi, F.; Cicoira, F.; Rolandi, M.; et al. Protonic and Electronic Transport in Hydrated Thin Films of the Pigment Eumelanin. Chem. Mater. 2015, 27, 436–442. [Google Scholar] [CrossRef]
- Sheliakina, M.; Mostert, A.B.; Meredith, P. Decoupling Ionic and Electronic Currents in Melanin. Adv. Funct. Mater. 2018, 28, 1805514. [Google Scholar] [CrossRef] [Green Version]
- Reali, M.; Saini, P.; Santato, C. Electronic and protonic transport in bio-sourced materials: A new perspective on semiconductivity. Mater. Adv. 2021, 2, 15–31. [Google Scholar] [CrossRef]
- Paulin, J.V.; Mcgettrick, J.D.; Graeff, C.F.O.; Mostert, A.B. Melanin system composition analyzed by XPS depth profiling. Surf. Interface 2021, 24, 101053. [Google Scholar] [CrossRef]
- Akbulut, S.; Yilmaz, M.; Raina, S.; Hsu, S.H.; Kang, W.P. Solid-state supercapacitor cell based on 3D nanostructured MnO2/CNT microelectrode array on graphite and H3PO4/PVA electrolyte. Diam. Relat. Mater. 2017, 74, 222–228. [Google Scholar] [CrossRef]
- Bothma, J.P.; de Boor, J.; Divakar, U.; Schwenn, P.E.; Meredith, P. Device-Quality Electrically Conducting Melanin Thin Films. Adv. Mater. 2008, 20, 3539–3542. [Google Scholar] [CrossRef]
- Abbas, M.; D’Amico, F.; Morresi, L.; Pinto, N.; Ficcadenti, M.; Natali, R.; Ottaviano, L.; Passacantando, M.; Cuccioloni, M.; Angeletti, M.; et al. Structural, electrical, electronic and optical properties of melanin films. Eur. Phys. J. E 2009, 28, 285–291. [Google Scholar] [CrossRef]
- Wünsche, J.; Cicoira, F.; Graeff, C.F.O.; Santato, C. Eumelanin thin films: Solution-processing, growth, and charge transport properties. J. Mater. Chem. B 2013, 1, 3836–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piacenti-Silva, M.; Fernandes, J.C.; de Figueiredo, N.B.; Congiu, M.; Mulato, M.; Graeff, C.F.d.O. Melanin as an active layer in biosensors. AIP Adv. 2014, 4, 037120. [Google Scholar] [CrossRef]
- Albano, L.G.S.; Di Mauro, E.; Kumar, P.; Cicoira, F.; Graeff, C.F.O.; Santato, C. Novel insights on the physicochemical properties of eumelanins and their DMSO derivatives. Polym. Int. 2016, 65, 1315–1322. [Google Scholar] [CrossRef]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; D’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chemie 2013, 52, 12684–12687. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhões, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Huggins, R.A. Simple method to determine electronic and ionic components of the conductivity in mixed conductors a review. Ionics 2002, 8, 300–313. [Google Scholar] [CrossRef]
- Sarna, T.; Sealy, R.C. Free radicals from eumelanins: Quantum yields and wavelength dependence. Arch. Biochem. Biophys. 1984, 232, 574–578. [Google Scholar] [CrossRef]
- Mostert, A.B.; Rienecker, S.B.; Noble, C.; Hanson, G.R.; Meredith, P. The photoreactive free radical in eumelanin. Sci. Adv. 2018, 4, eaaq1293. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Dong, M.; Feng, E.; Peng, H.; Ma, G.; Zhao, G.; Lei, Z. High performance solid state supercapacitor based on a 2-mercaptopyridine redox-mediated gel polymer. RSC Adv. 2015, 5, 22419–22425. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Li, Y.; Wang, H.; Zhang, Q.; Kaner, R.B. Flexible quasi-solid-state planar micro-supercapacitor based on cellular graphene films. Mater. Horiz. 2017, 4, 1145–1150. [Google Scholar] [CrossRef]
- Kundu, A.; Fisher, T.S. Symmetric All-Solid-State Supercapacitor Operating at 1.5 v Using a Redox-Active Gel Electrolyte. ACS Appl. Energy Mater. 2018, 1, 5800–5809. [Google Scholar] [CrossRef]
- Song, J.; Ma, G.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Jiang, Y.; et al. High-conductivity, flexible and transparent PEDOT: PSS electrodes for high performance semi-transparent supercapacitors. Polymers 2020, 12, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Ji, Z.; Feng, Y.; Wang, P.; Huang, Y. Flexible and stretchable polyaniline supercapacitor with a high rate capability. Polym. Int. 2021, 70, 437–442. [Google Scholar] [CrossRef]
- Dezidério, S.N.; Brunello, C.A.; da Silva, M.I.N.; Cotta, M.A.; Graeff, C.F.O. Thin films of synthetic melanin. J. Non. Cryst. Solids 2004, 338–340, 634–638. [Google Scholar] [CrossRef]
- Piacenti-Silva, M.; Bronze-Uhle, E.S.; Paulin, J.V.; Graeff, C.F.O. Temperature-enhanced synthesis of DMSO-Melanin. J. Mol. Struct. 2014, 1056–1057, 135–140. [Google Scholar] [CrossRef]
- Paulin, J.V.; Veiga, A.G.; Garcia-Basabe, Y.; Rocco, M.L.M.; Graeff, C.F. Structural and optical properties of soluble melanin analogues with enhanced photoluminescence quantum efficiency. Polym. Int. 2018, 67, 550–556. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulin, J.V.; Fernandes, S.L.; Graeff, C.F.O. Solid-State Electrochemical Energy Storage Based on Soluble Melanin. Electrochem 2021, 2, 264-273. https://doi.org/10.3390/electrochem2020019
Paulin JV, Fernandes SL, Graeff CFO. Solid-State Electrochemical Energy Storage Based on Soluble Melanin. Electrochem. 2021; 2(2):264-273. https://doi.org/10.3390/electrochem2020019
Chicago/Turabian StylePaulin, João V., Silvia L. Fernandes, and Carlos F. O. Graeff. 2021. "Solid-State Electrochemical Energy Storage Based on Soluble Melanin" Electrochem 2, no. 2: 264-273. https://doi.org/10.3390/electrochem2020019





