An Approach to the Simultaneous Determination of a Panel of Five Biomarkers for the Early Detection of Brain Cancer Using the Stochastic Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Instruments
2.4. Design of 3D Microsensors Based on MWCNT
2.5. Biological Samples
3. Results and Discussion
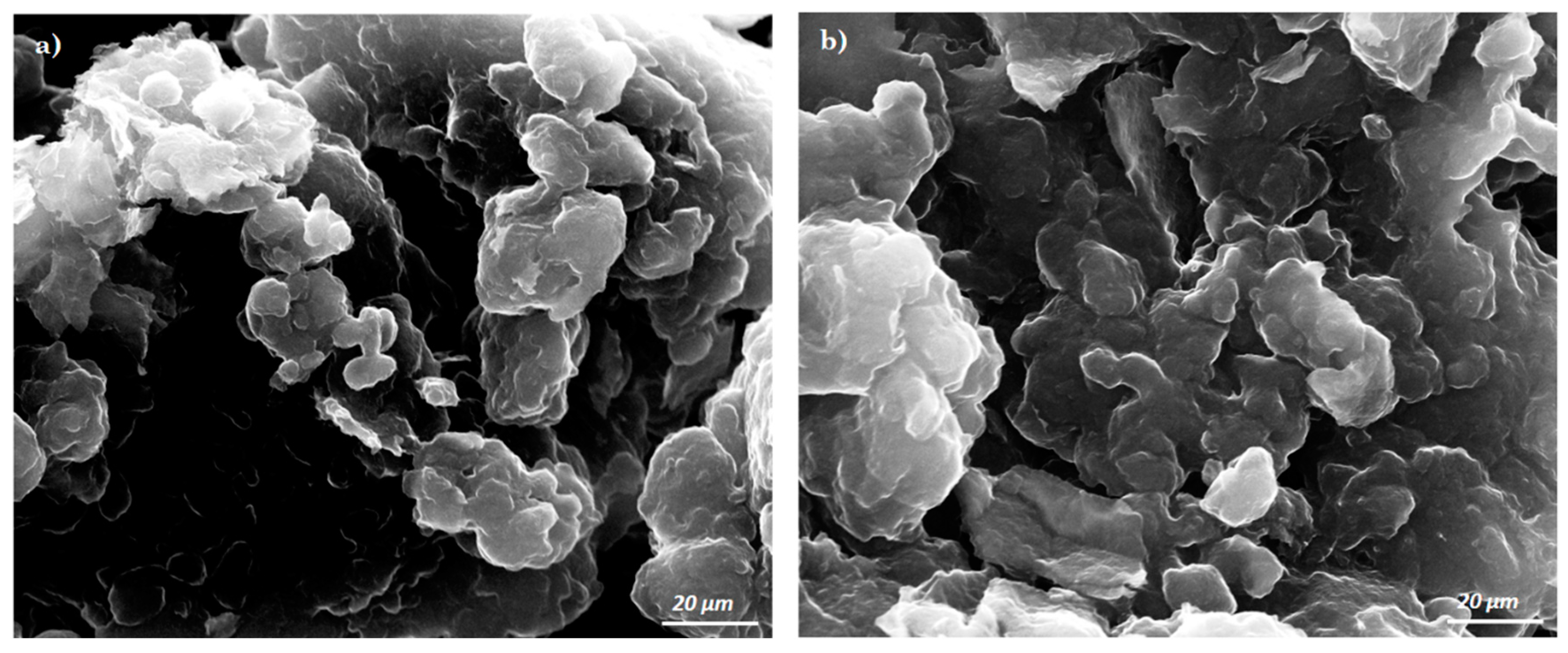
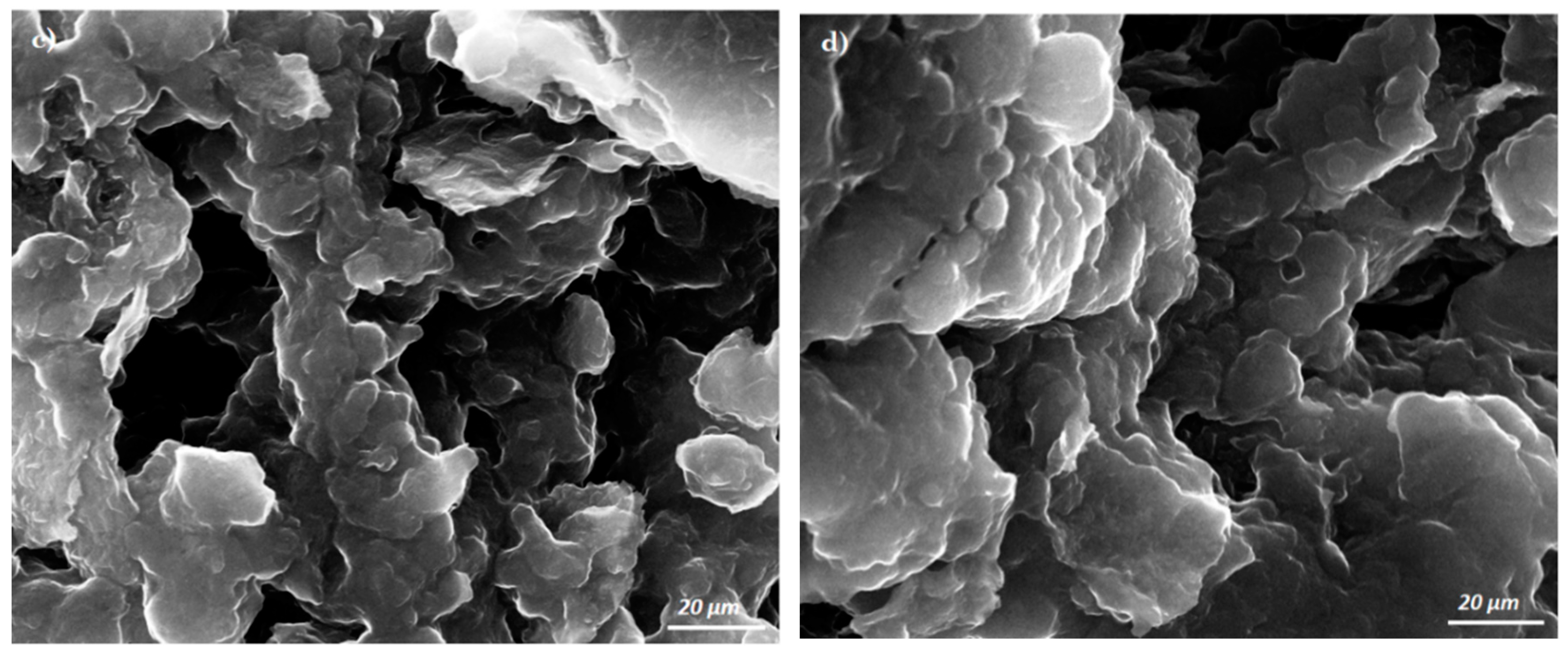
3.1. Morphological Characterization of the Pastes
3.2. Response Characteristics of Stochastic Microsensors
3.3. Selectivity
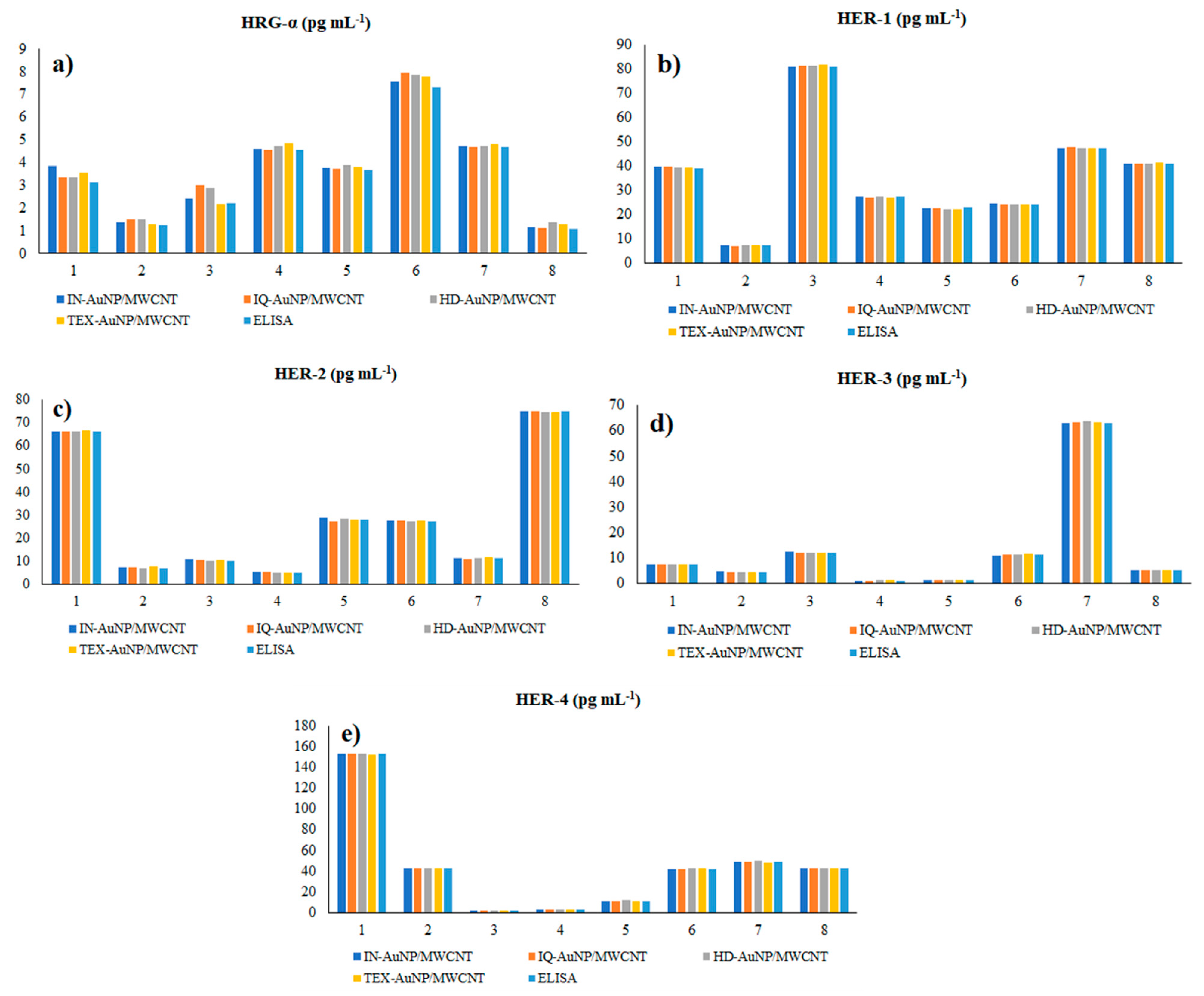
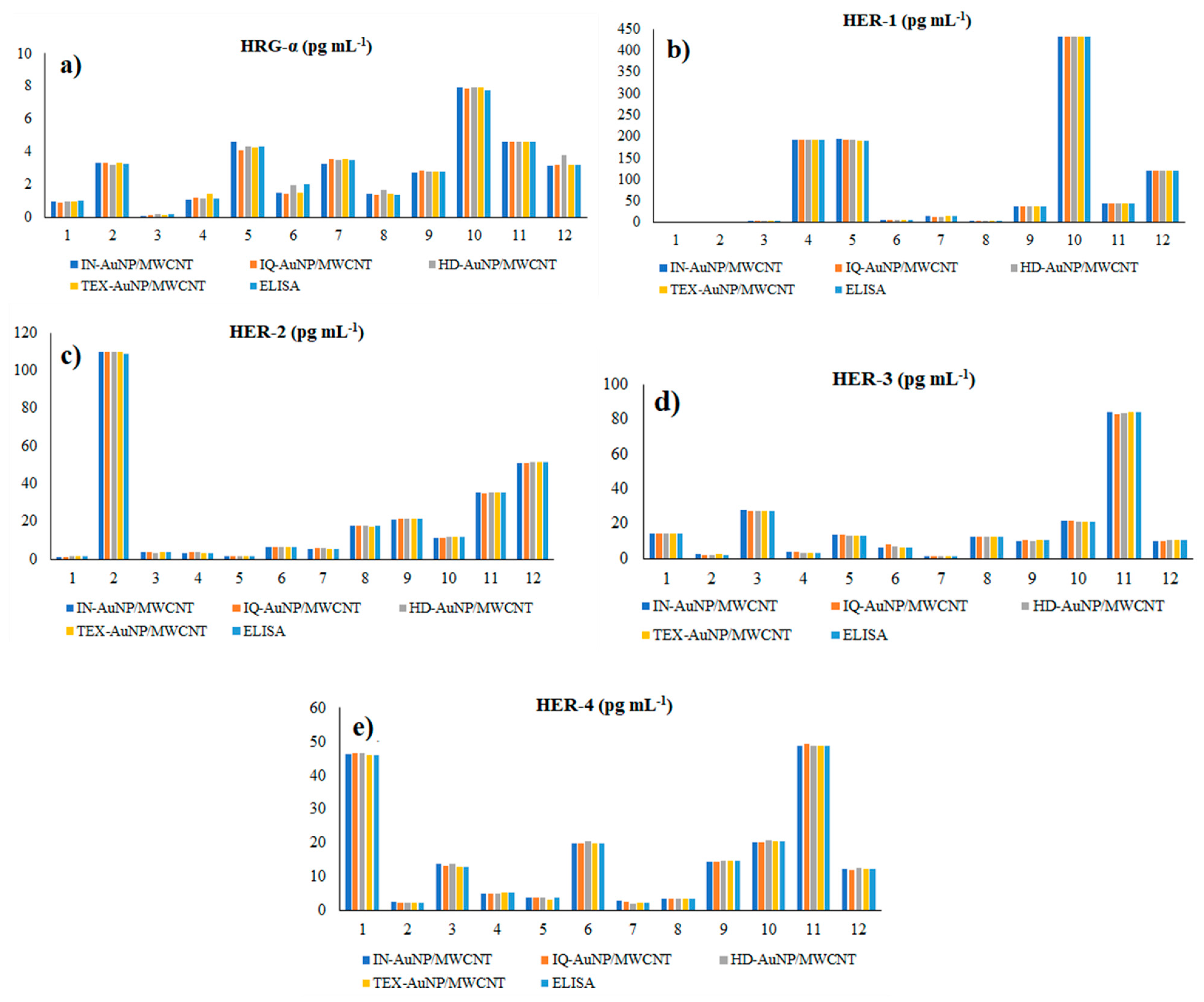
3.4. Molecular Recognition and Quantification of HRG-α, HER-1, HER-2, HER-3, and HER-4 in Biological Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falls, D.L.; Rosen, K.M.; Corfas, G.; Lane, W.S.; Fischbach, G.D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the new ligand family. Cell 1993, 72, 801–815. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Tan, Y.N.; Singh, S.P. Next Generation Point-of-Care Biomedical Sensors Technologies for Cancer Diagnosis; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Smoll, N.R.; Schaller, K.; Gautschi, O.P. Long-term survival of patients with glioblastoma multiforme (GBM). J. Clin. Neurosci. 2013, 20, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.S.; Cioates Negut, C.; Badulescu, M.; Stefan-van Staden, R.I. Sensitive Detection of Heregulin-α from Biological Samples Using a Disposable Stochastic Sensor Based on Plasma Deposition of GNPs–AgPs’ Nanofilms on Silk. Life 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Cioates Negut, C.; Gheorghe, S.S.; Ciorita, A. 3D Stochastic microsensors for molecular recognition and determination of heregulin-α in biological samples. Anal. Bioanal. Chem. 2021, 413, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Comnea-Stancu, I.R.; Surdu-Bob, C.C. Molecular screening of blood samples for the simultaneous detection of CEA, HER-1, NSE, CYFRA 21-1 using stochastic sensors. J. Electrochem. Soc. 2017, 164, B267–B273. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I.; Moldoveanu, I.; Stanciu-Gavan, C. Pattern recognition of HER-1 in biological fluids using stochastic sensing. J. Enzyme Inhib. Med. Chem. 2015, 30, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, I.; Stefan-van Staden, R.I.; van Staden, J.F.; Stanciu-Gavan, C.; Savlovschi, C. Platform based on microsensors used for the screening of HER-1 in peritoneal fluid. Rev. Roum. Chim. 2015, 60, 447–451. [Google Scholar]
- Stefan-van Staden, R.I.; Comnea-Stancu, I.R.; Surdu-Bob, C.C.; Badulescu, M. Nanostructured materials detect epidermal growth factor receptor, neuron-specific enolase and carcinoembryonic antigen. Nanoscale 2015, 7, 15689–15694. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, I.; Stefan-van Staden, R.I. Pattern recognition of HER-2 in whole blood samples using stochastic microsensors. ECS J. Solid State Sci. Technol. 2015, 4, S3067–S3070. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.J.; Guan, Y.; Luo, P.; Wang, Y. Recent advances in fabricating monolithic 3D porous graphene and their applications in biosensing and biofuel cells. Biosens. Bioelectron. 2017, 89, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, S.; Gupta, A.A.; Izquierdo, R.; Nabki, F. Suspended carbon nanotubes for humidity sensing. Sensors 2018, 18, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayley, H.; Cremer, P.S. Stochastic sensors inspired by biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, C.; Yang, M.; Rasooly, A. Polycytosine DNA electric-current generated immunosensor for electrochemical detection of human epidermal growth factor receptor 2 (HER2). Anal. Chem. 2018, 90, 4764–4769. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, A.; Noorbakhsh, A.; Sharifi, E. Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens. Bioelectron. 2017, 95, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zapatero-Rodriguez, J.; Saxena, R.; O’ Kennedy, R.; Srivastava, S. Ultrasensitive direct impedimetric immunosensor for detection of serum HER2. Biosens. Bioelectron. 2018, 106, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sypabekova, M.; Amantayeva, A.; Vangelista, L.; Gonzalez-Vila, A.; Caucheteur, C.; Tosi, D. Ultralow limit detection of soluble HER2 biomarker in serum with a fiber-optic ball-tip resonator. ACS Meas. Sci. Au 2022, 2, 309–316. [Google Scholar] [CrossRef]





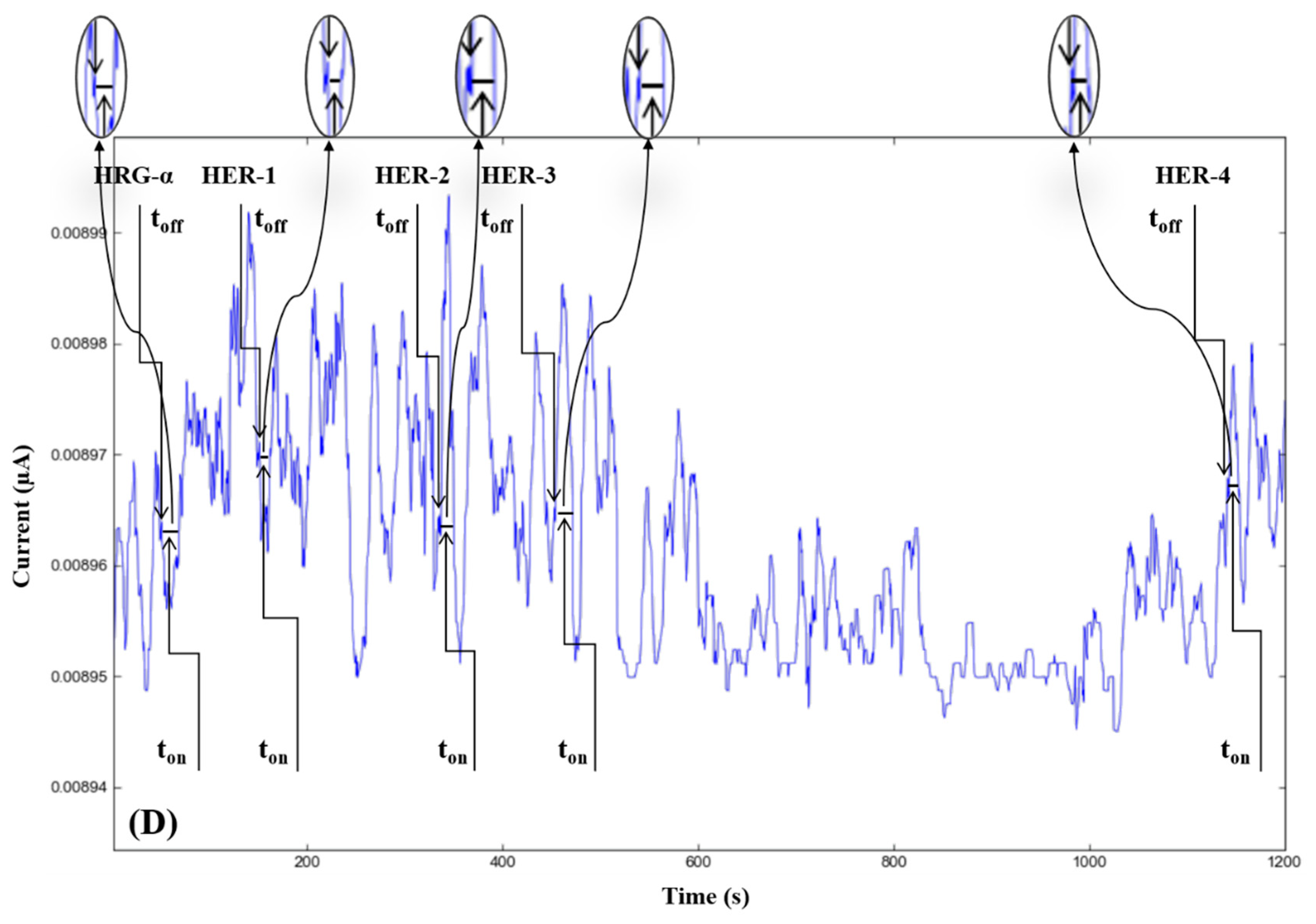
| 3D Microsensor Based on AuNP-MWCNT and | Signature of the Analyte toff (s) | Linear Concentration Range (µg mL−1) | Calibration Equations; the Correlation Coefficient; r * | Sensitivity (s µg mL−1) | LOQ (fg mL−1) |
|---|---|---|---|---|---|
| HRG-α | |||||
| IN | 1.10 | 4.1 × 10−9–2.56 × 10−6 | 1/ton = 0.31 + 2.18 × 106 × C; r = 0.9977 | 2.18 × 106 | 4.1 |
| IQ | 1.00 | 4.1 × 10−9–1.02 × 10−6 | 1/ton = 0.39 + 2.30 × 106 × C; r = 0.9999 | 2.30 × 106 | 4.1 |
| HD | 1.10 | 4.1 × 10−9–2.56 × 10−6 | 1/ton = 0.78 + 2.49 × 107 × C; r = 0.9997 | 2.49 × 107 | 4.1 |
| TEX | 0.80 | 4.1 × 10−9–2.56 × 10−6 | 1/ton = 0.61 + 2.55 × 105 × C; r = 0.9969 | 2.55 × 105 | 4.1 |
| HER-1 | |||||
| IN | 1.60 | 3.9 × 10−9–3.9 × 10−6 | 1/ton = 0.07 + 2.38 × 105 × C; r = 0.9999 | 2.38 × 105 | 3.9 |
| IQ | 1.60 | 3.9 × 10−9–3.9 × 10−5 | 1/ton = 0.51 + 1.90 × 105 × C; r = 0.9963 | 1.90 × 105 | 3.9 |
| HD | 0.95 | 3.9 × 10−9–3.9 × 10−6 | 1/ton = 0.58 + 2.17 × 106 × C; r = 0.9999 | 2.17 × 106 | 3.9 |
| TEX | 1.40 | 3.9 × 10−9–3.9 × 10−1 | 1/ton = 0.42 + 2.15 × 105 × C; r = 0.9989 | 2.15 × 105 | 3.9 |
| HER-2 | |||||
| IN | 1.30 | 3.9 × 10−6–3.9 × 10−1 | 1/ton = 0.09 + 6.17 × 103 × C; r = 0.9998 | 6.17 × 103 | 3.9 × 103 |
| IQ | 1.30 | 3.9 × 10−9–3.9 × 10−2 | 1/ton = 0.39 + 5.43 × 105 × C; r = 0.9960 | 5.43 × 105 | 3.9 |
| HD | 0.80 | 3.9 × 10−9–3.9 | 1/ton = 0.22 + 2.64 × 106 × C; r = 0.9999 | 2.64 × 106 | 3.9 |
| TEX | 1.20 | 3.9 × 10−9–3.9 × 10−1 | 1/ton = 0.34 + 1.98 × 106 × C; r = 0.9995 | 1.98 × 106 | 3.9 |
| HER-3 | |||||
| IN | 0.80 | 1.0 × 10−9–1.0 × 10−2 | 1/ton = 0.34 + 2.83 × 106 × C; r = 0.9999 | 2.83 × 106 | 1.0 |
| IQ | 0.80 | 1.0 × 10−9–1.0 × 10−7 | 1/ton = 0.09 + 2.58 × 106 × C; r = 0.9998 | 2.58 × 106 | 1.0 |
| HD | 1.30 | 1.0 × 10−9–1.0 × 10−7 | 1/ton = 0.12 + 1.13 × 107 × C; r = 0.9999 | 1.13 × 107 | 1.0 |
| TEX | 1.10 | 1.0 × 10−7–1.0 × 10−5 | 1/ton = 0.24 + 2.26 × 105 × C; r = 0.9997 | 2.26 × 105 | 100.0 |
| HER-4 | |||||
| IN | 0.50 | 1.0 × 10−9–1.0 | 1/ton = 0.32 + 1.12 × 106 × C; r = 0.9958 | 1.12 × 106 | 1.0 |
| IQ | 0.40 | 1.0 × 10−9–1.0 × 10−1 | 1/ton = 0.10 + 6.70 × 105 × C; r = 0.9983 | 6.70 × 105 | 1.0 |
| HD | 1.80 | 1.0 × 10−8–1.0 × 10−1 | 1/ton = 0.13 + 2.37 × 106 × C; r = 0.9999 | 2.37 × 106 | 10.0 |
| TEX | 2.20 | 1.0 × 10−9–1.0 × 10−2 | 1/ton = 0.57 + 1.10 × 105 × C; r = 0.9999 | 1.10 × 105 | 1.0 |
| 3D Microsensors Based on AuNP-MWCNT and | toff (s), Signature | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HRG-α | HER-1 | HER-2 | HER-3 | HER-4 | IL-2 | IL-4 | IDH-1 | IDH-2 | |
| IN | 1.10 | 1.60 | 1.30 | 0.80 | 0.50 | 1.90 | 2.10 | 2.70 | 3.50 |
| IQ | 1.00 | 1.60 | 1.30 | 0.80 | 0.40 | 0.20 | 2.40 | 2.90 | 3.10 |
| HD | 1.10 | 0.95 | 0.80 | 1.30 | 1.80 | 0.30 | 2.20 | 2.50 | 3.00 |
| TEX | 0.80 | 1.40 | 1.20 | 1.10 | 2.20 | 0.40 | 1.70 | 3.00 | 2.80 |
| Biological Samples | %, Recovery | 3D Microsensors Based on AuNP-MWCNT and | |||
|---|---|---|---|---|---|
| IN | IQ | HD | TEX | ||
| Brain tumor tissue | HRG-α | 97.75 ± 0.01 | 97.17 ± 0.03 | 97.27 ± 0.01 | 95.97 ± 0.01 |
| HER-1 | 98.47 ± 0.02 | 97.15 ± 0.02 | 98.00 ± 0.03 | 97.14 ± 0.03 | |
| HER-2 | 97.27 ± 0.05 | 98.00 ± 0.04 | 97.43 ± 0.02 | 97.47 ± 0.02 | |
| HER-3 | 98.14 ± 0.03 | 97.49 ± 0.02 | 97.00 ± 0.01 | 97.29 ± 0.01 | |
| HER-4 | 97.05 ± 0.01 | 97.12 ± 0.03 | 97.14 ± 0.02 | 97.05 ± 0.05 | |
| Whole blood | HRG-α | 99.81 ± 0.02 | 99.78 ± 0.01 | 99.95 ± 0.01 | 98.42 ± 0.03 |
| HER-1 | 99.03 ± 0.03 | 99.57 ± 0.02 | 99.47 ± 0.02 | 98.12 ± 0.05 | |
| HER-2 | 99.08 ± 0.01 | 99.15 ± 0.01 | 99.32 ± 0.01 | 99.00 ± 0.02 | |
| HER-3 | 99.15 ± 0.03 | 99.27 ± 0.03 | 99.20 ± 0.03 | 98.20 ± 0.08 | |
| HER-4 | 99.98 ± 0.01 | 99.90 ± 0.01 | 99.15 ± 0.02 | 98.03 ± 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioates Negut, C.; Stefan-van Staden, R.-I.; Sfirloaga, P. An Approach to the Simultaneous Determination of a Panel of Five Biomarkers for the Early Detection of Brain Cancer Using the Stochastic Method. Chemistry 2022, 4, 1382-1394. https://doi.org/10.3390/chemistry4040090
Cioates Negut C, Stefan-van Staden R-I, Sfirloaga P. An Approach to the Simultaneous Determination of a Panel of Five Biomarkers for the Early Detection of Brain Cancer Using the Stochastic Method. Chemistry. 2022; 4(4):1382-1394. https://doi.org/10.3390/chemistry4040090
Chicago/Turabian StyleCioates Negut, Catalina, Raluca-Ioana Stefan-van Staden, and Paula Sfirloaga. 2022. "An Approach to the Simultaneous Determination of a Panel of Five Biomarkers for the Early Detection of Brain Cancer Using the Stochastic Method" Chemistry 4, no. 4: 1382-1394. https://doi.org/10.3390/chemistry4040090







