Effect of pH, Surfactant, and Temperature on Mixed-Phase Structure and Band Gap Properties of BiNbO4 Nanoparticles Prepared Using Different Routes
Abstract
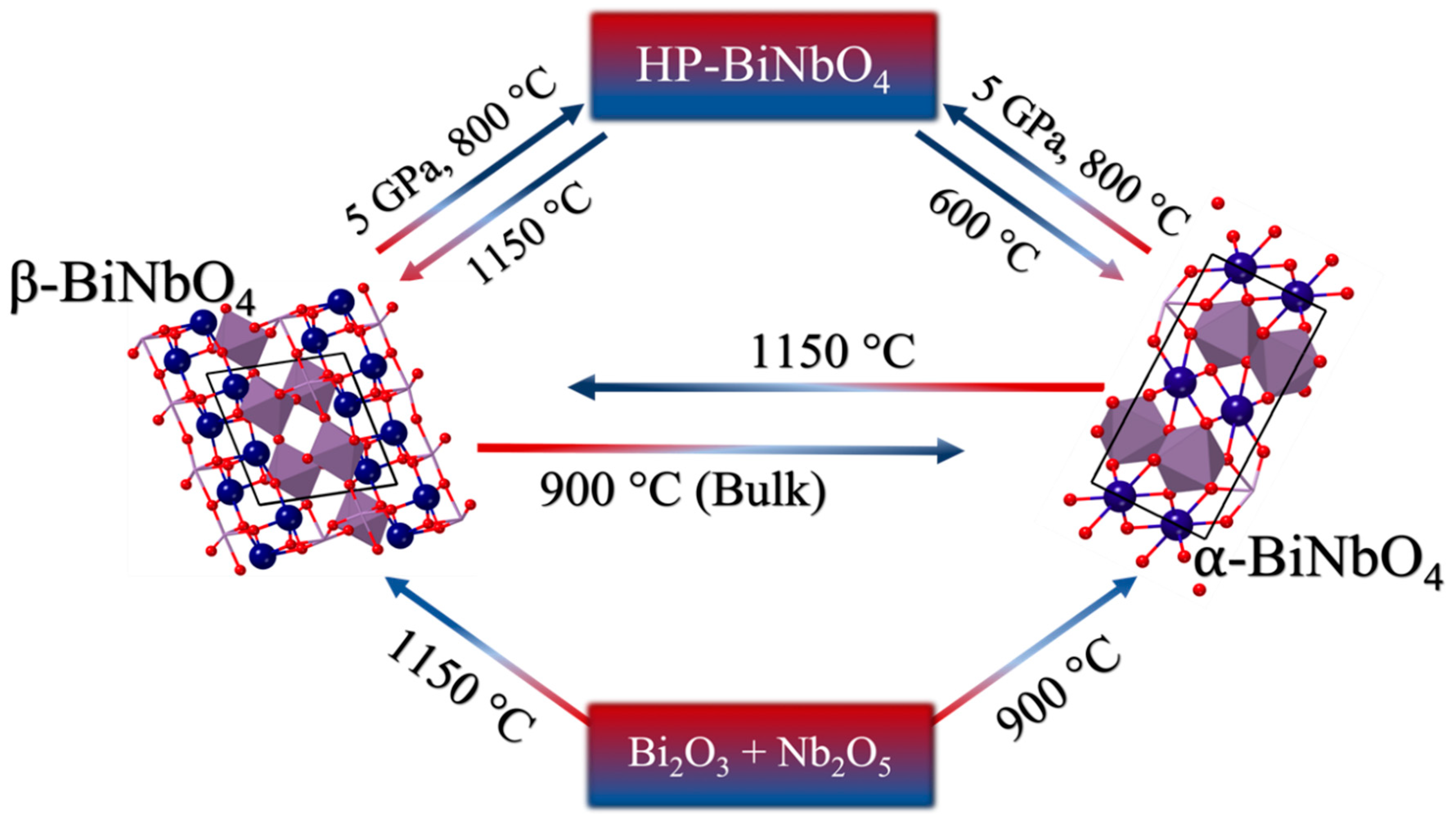
:1. Introduction
2. Experimental
2.1. Materials
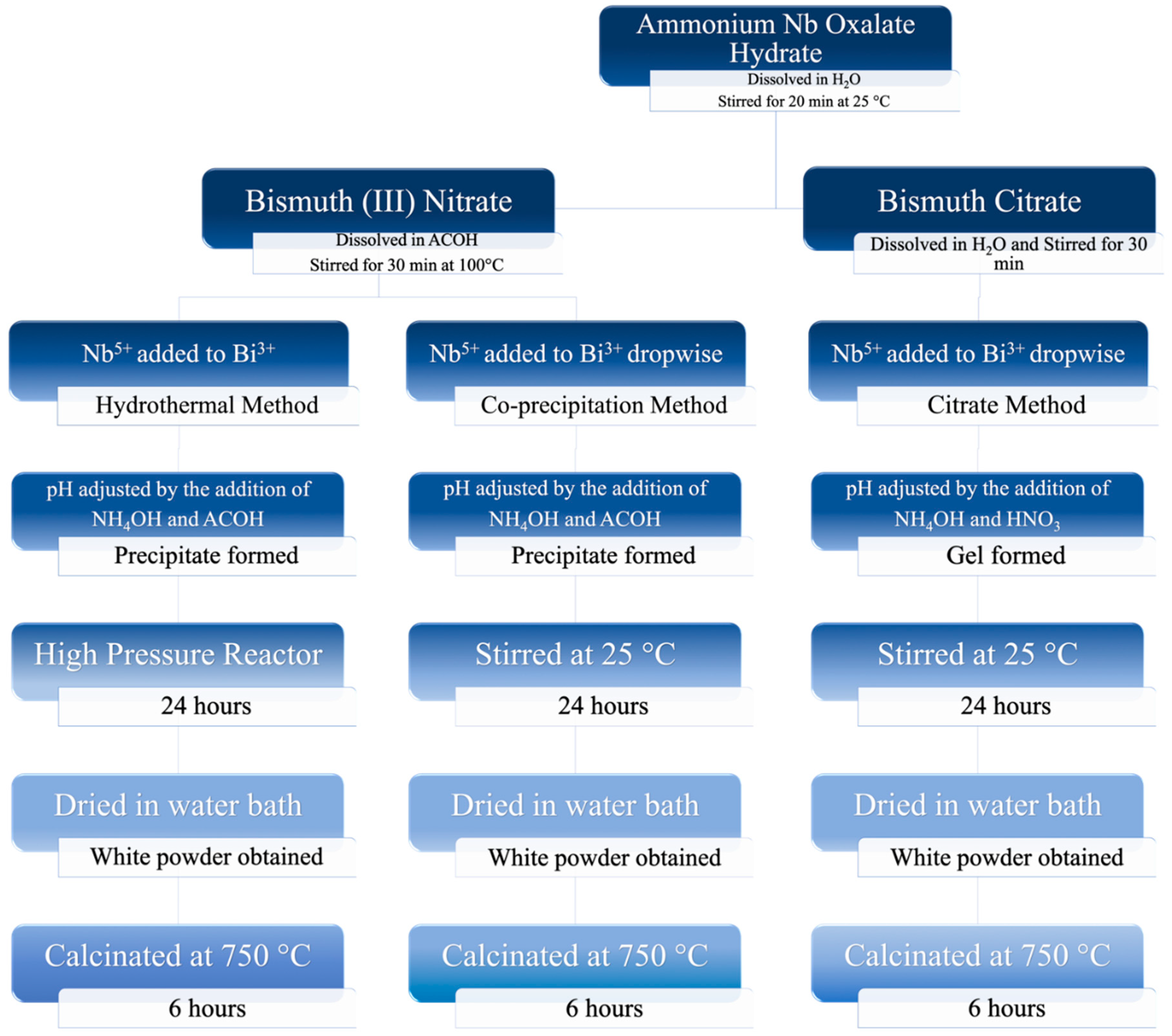
2.2. Preparation of Mixed-Phase BiNbO4 Nanoparticles
2.2.1. Co-precipitation Method
2.2.2. Hydrothermal Method
2.2.3. Citrate Precursor Method
2.3. Characterization of the Mixed-Phase BiNbO4
2.3.1. Powder X-Ray Diffraction (PXRD)
2.3.2. UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS)
2.3.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
2.3.4. Specific Surface Area and Porosity
3. Results and Discussion
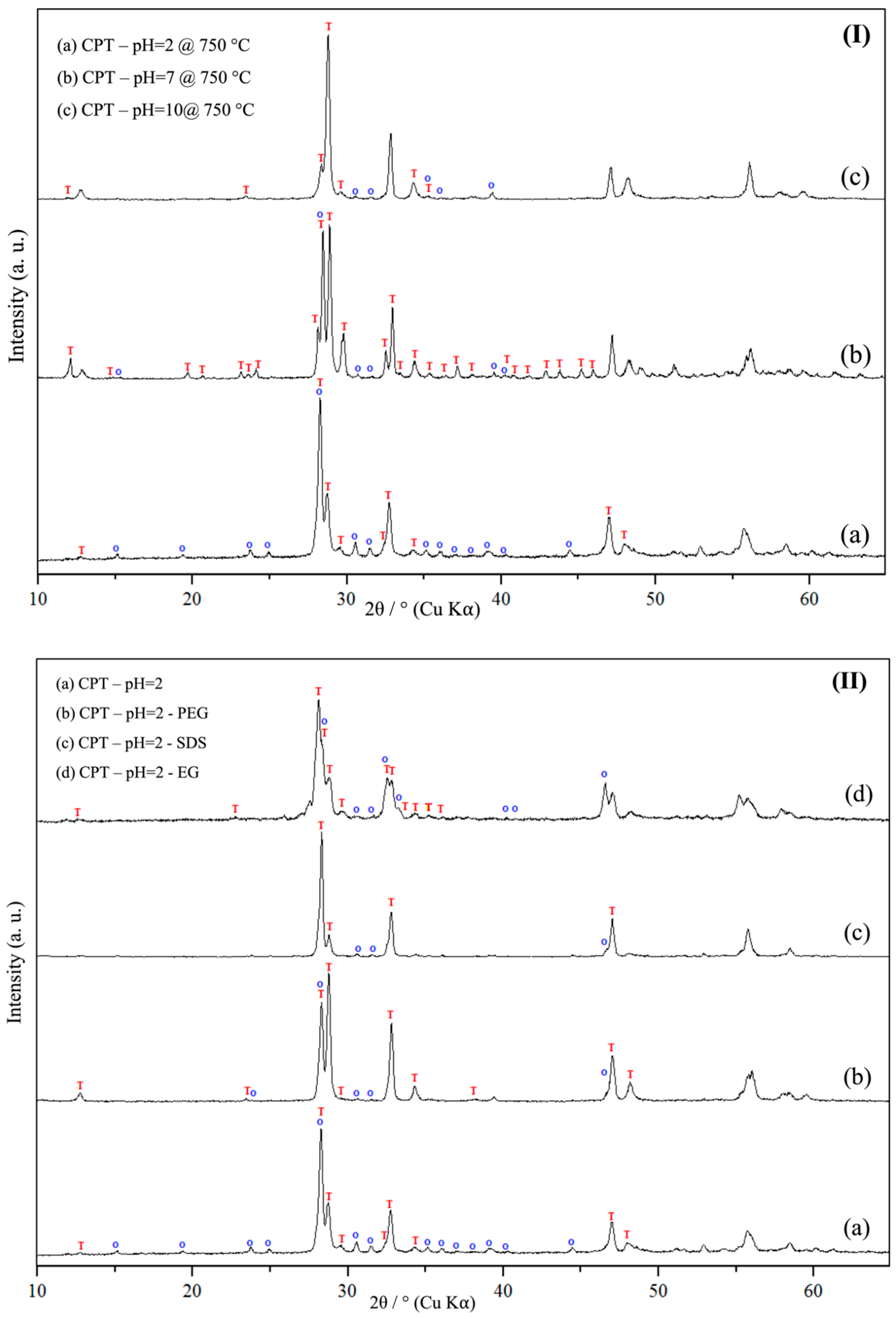
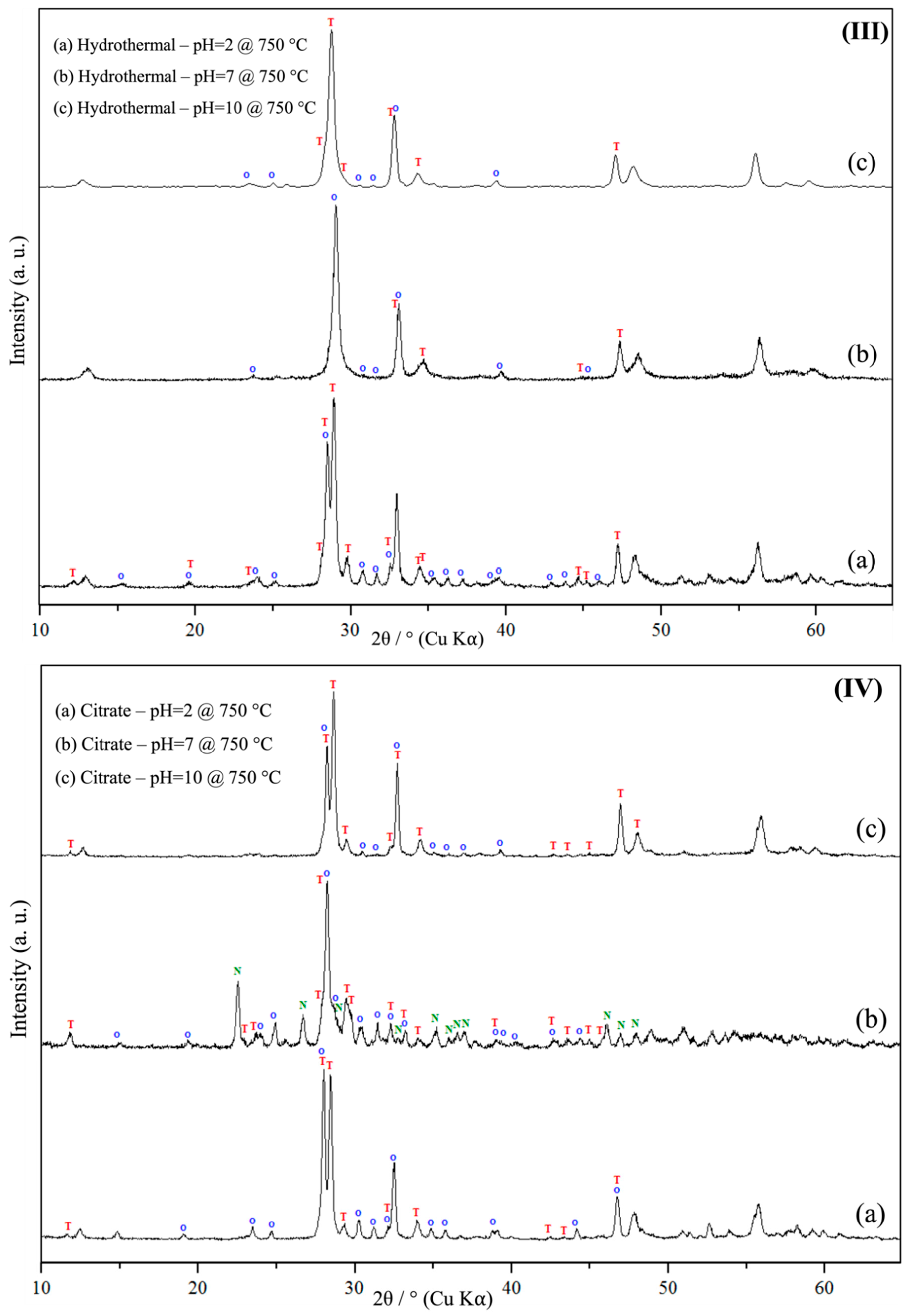
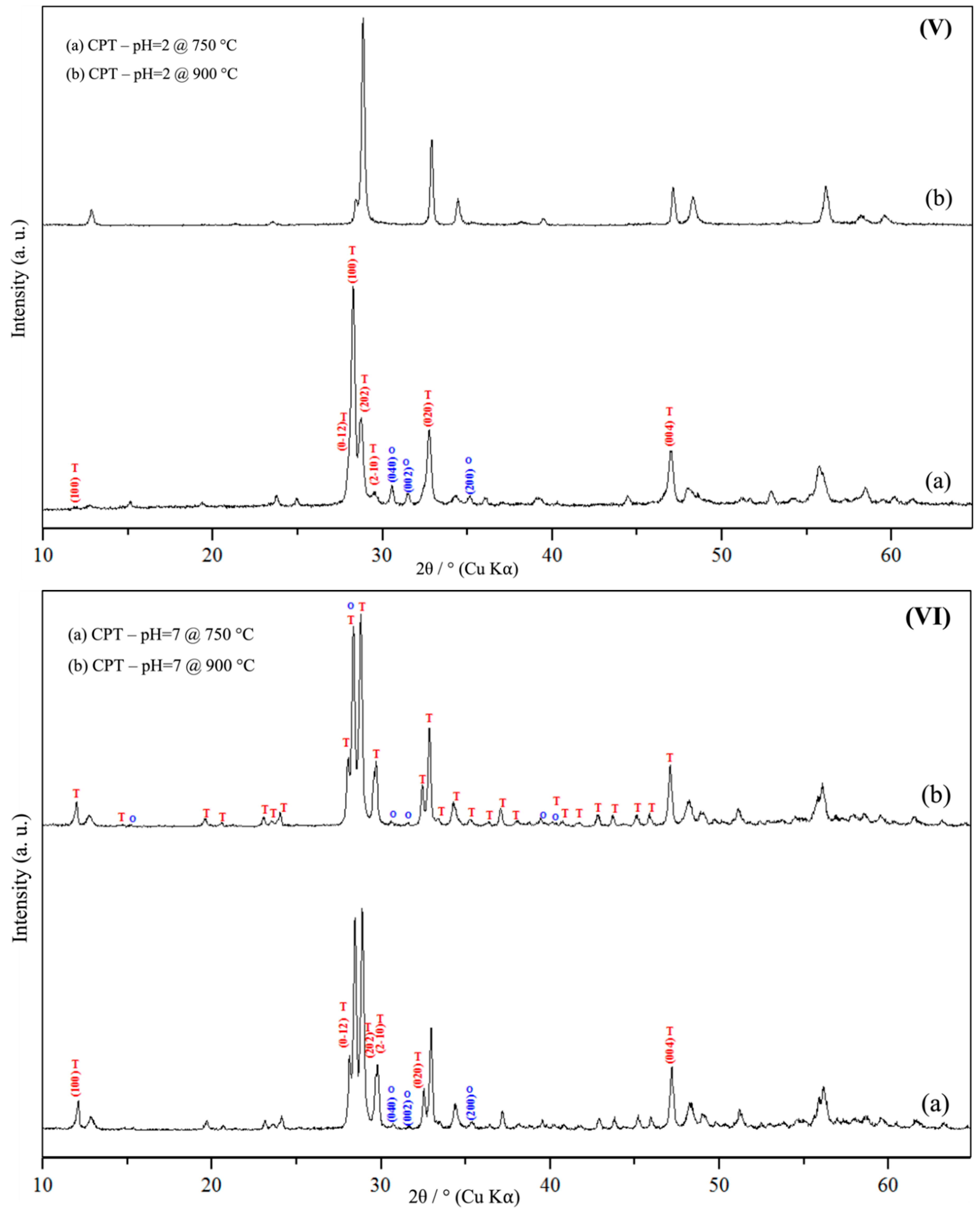
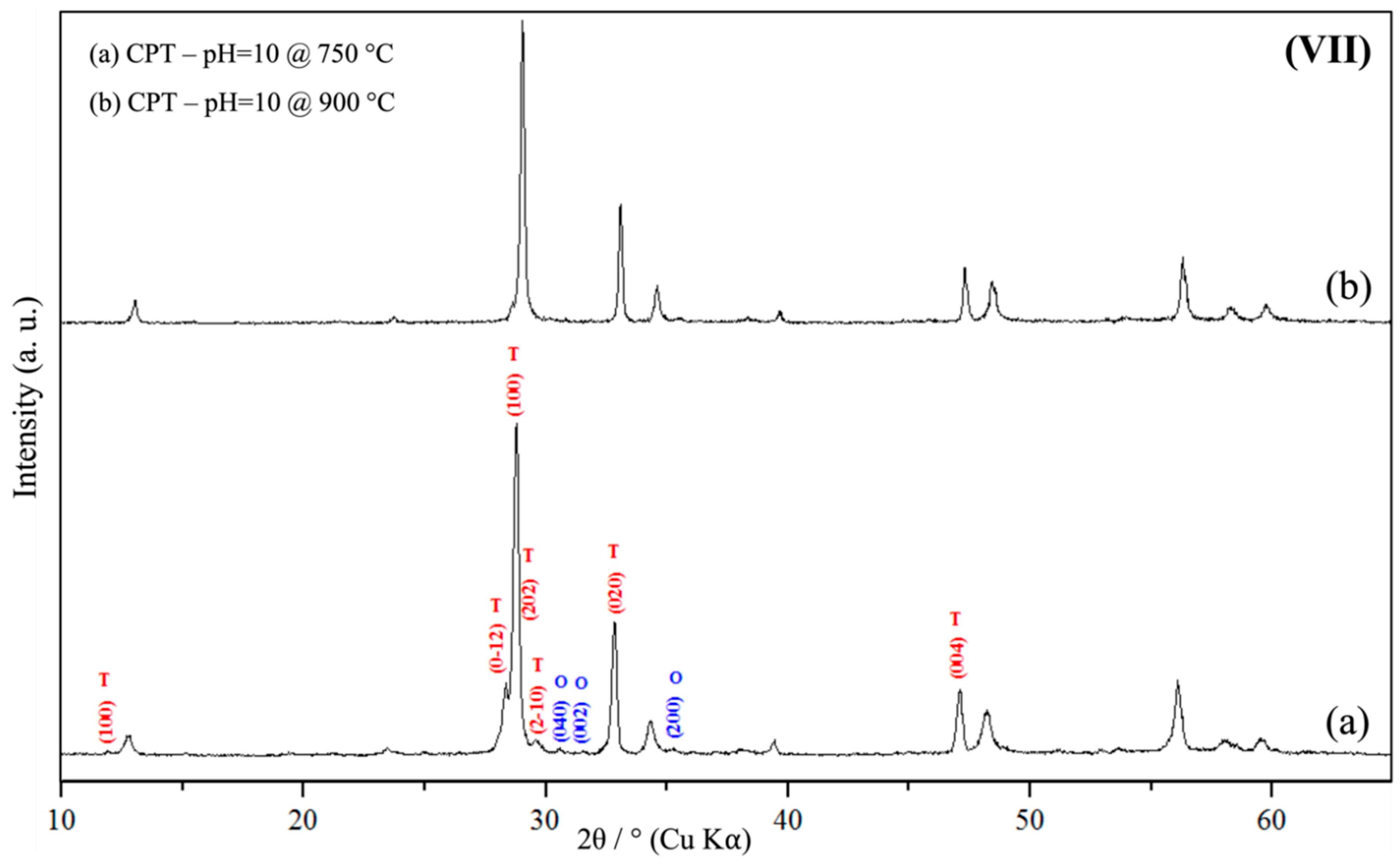
3.1. Powder X-Ray Diffraction Spectroscopy (PXRD)
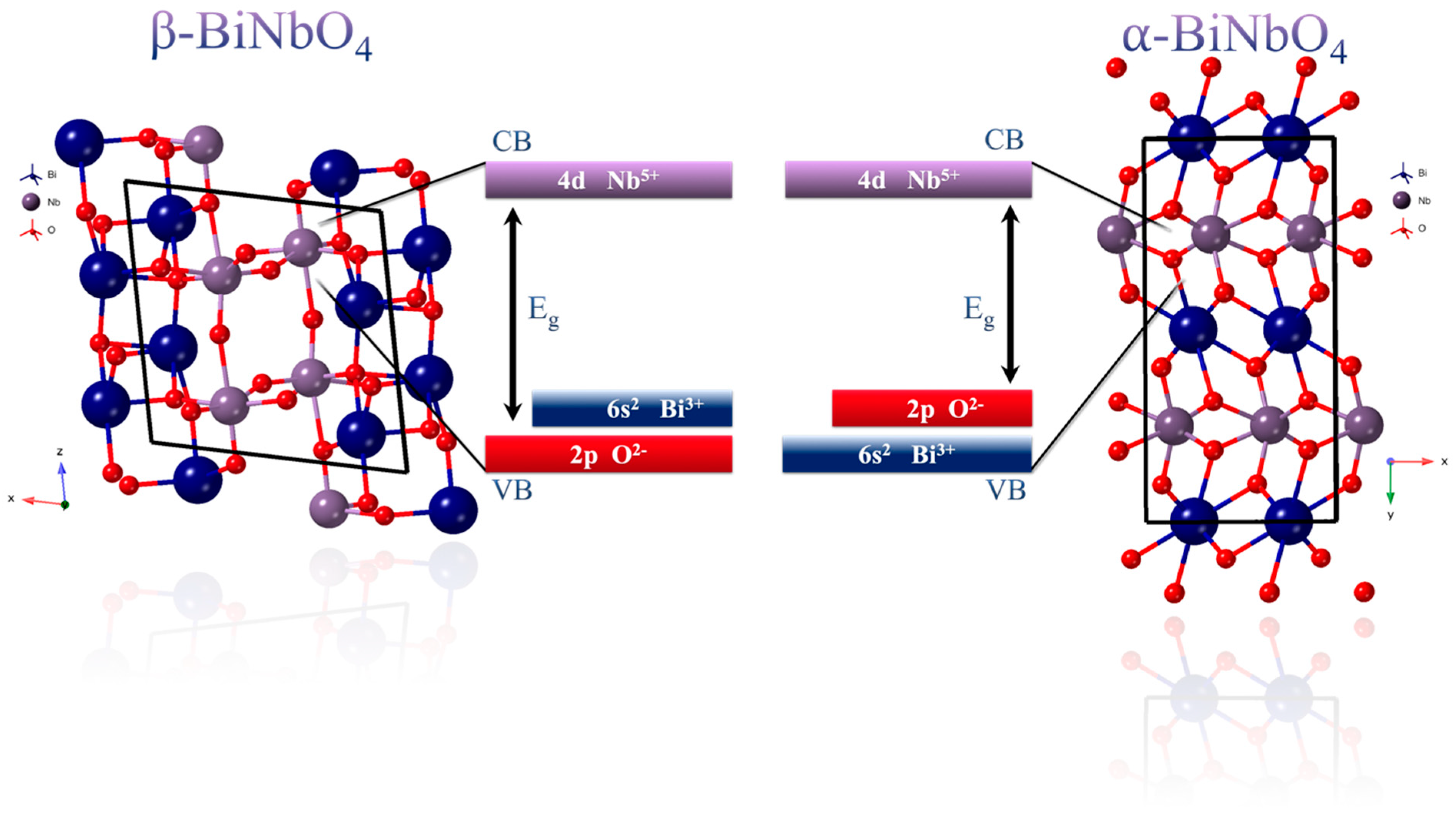
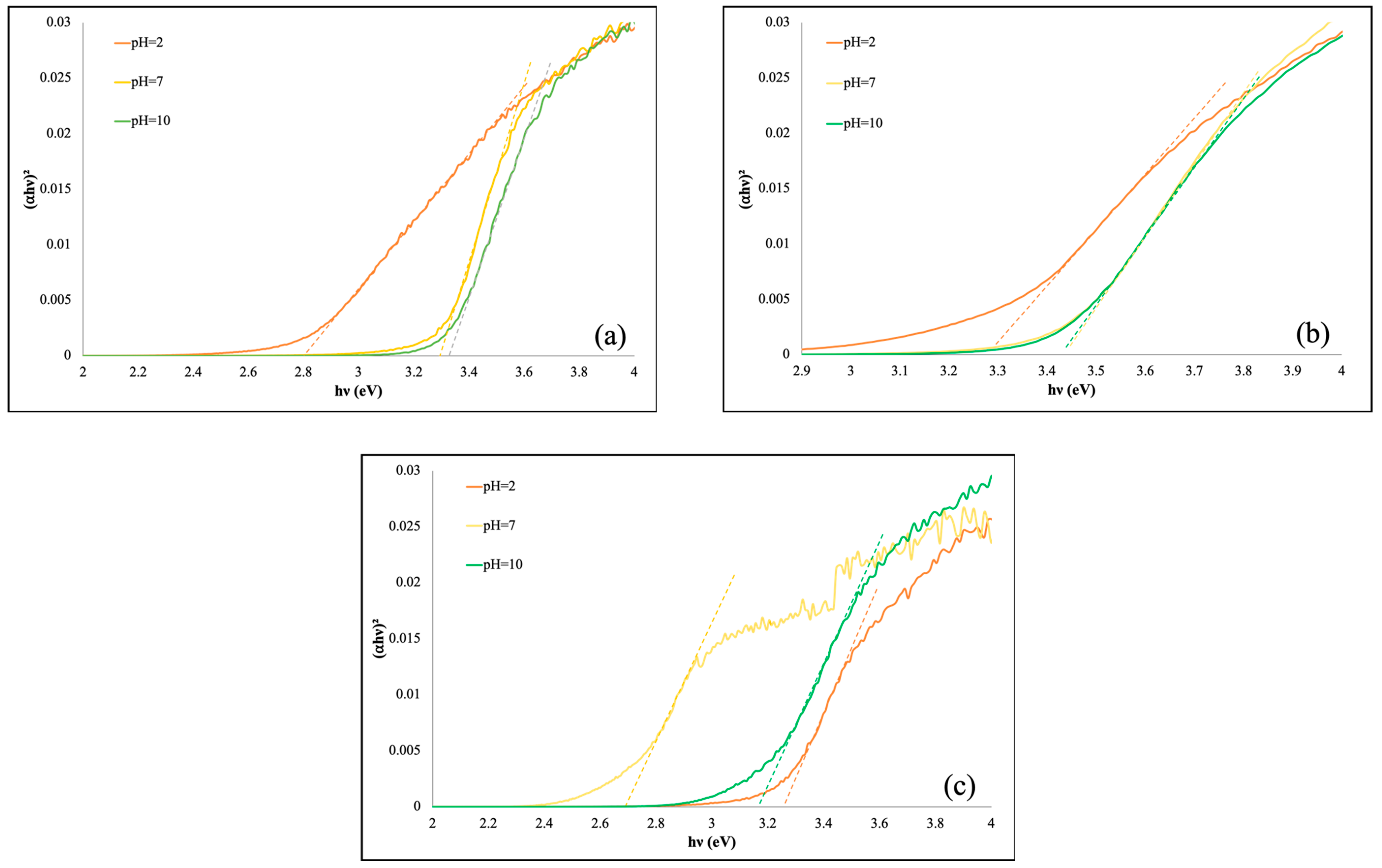
3.2. UV-Vis DRS
3.3. SEM and Energy-Dispersive X-Ray (EDS) Analysis
3.4. N2 Adsorption–Desorption Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pahalagedara, M.N.; Pahalagedara, L.R.; Kuo, C.H.; Dharmarathna, S.; Suib, S.L. Ordered mesoporous mixed metal oxides: Remarkable effect of pore size on catalytic activity. Langmuir 2014, 30, 8228–8237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Yao, H.B.; Zhang, Q.; Gong, J.Y.; Liu, S.J.; Yu, S.H. Hierarchical FeWO4 microcrystals: Solvothermal synthesis and their photocatalytic and magnetic properties. Inorg. Chem. 2009, 48, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.K.; Hassan, J.; Wahab, Z.A.; Azis, R.S. Electrical conductivity and dielectric behaviour of manganese and vanadium mixed oxide prepared by conventional solid state method. Eng. Sci. Technol. Int. J. 2016, 19, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.K.; Jaswal, V.S.; Singh, K.; Singh, R. Applications of metal/mixed metal oxides as photocatalyst: A review. Orient. J. Chem. 2016, 32, 2035–2042. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of mixed metal oxides in catalysis science—Versatile applications in organic synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Mao, N.; Jiao, Y. CuAl Hydrotalcite Formed CuAl-Mixed Metal Oxides for Photocatalytic Removal of Rhodamine B and Cr(VI). ChemistrySelect 2018, 3, 12676–12681. [Google Scholar] [CrossRef]
- Rodriguez, J.A. Electronic and chemical properties of mixed-metal oxides: Basic principles for the design of DeNOx and DeSOx catalysts. Catal. Today 2003, 85, 177–192. [Google Scholar] [CrossRef]
- Cui, H.; Zayat, M.; Levy, D. Sol-gel synthesis of nanoscaled spinels using propylene oxide as a gelation agent. J. Sol-Gel Sci. Technol. 2005, 35, 175–181. [Google Scholar] [CrossRef]
- Elia, A.; Aispuro, P.M.; Quaranta, N.; Martín-Martínez, J.M.; Vázquez, P. Synthesis and characterization of new silica-titania mixed oxides obtained by sol-gel technique. Macromol. Symp. 2011, 301, 136–145. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Parghi, K.; Shrikhande, J.J.; Pandey, R.K.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Jayaram, R.V. Synthesis and characterization of versatile MgO-ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol. 2011, 1, 1653–1664. [Google Scholar] [CrossRef]
- Sheets, W.C.; Stampler, E.S.; Kabbour, H.; Bertoni, M.I.; Cario, L.; Mason, T.O.; Marks, T.J.; Poeppelmeier, K.R. Facile Synthesis of BiCuOS by Hydrothermal Methods. Inorg. Chem. 2007, 46, 2333–2335. [Google Scholar] [CrossRef]
- Zyryanov, V.V. Mechanochemical synthesis and thermal behavior of metastable mixed oxides in the CaO-Sb2O3-Bi2O3 system. Inorg. Mater. 2003, 39, 1163–1171. [Google Scholar] [CrossRef]
- Babu, R.; Kelkar, S.; Kashid, V.; Achary, S.N.; Salunke, H.G.; Gupta, N.M. Photophysical, bandstructural, and textural properties of o-FeNbO4 in relation to its cocatalyst-assisted photoactivity for water oxidation. RSC Adv. 2014, 4, 33435–33445. [Google Scholar] [CrossRef]
- Rao, K.S.; Buddhudu, S. Structural, Thermal and Dielectric Properties of BiNbO4 Ceramic Powder. Ferroelectr. Lett. Sect. 2010, 37, 101–109. [Google Scholar] [CrossRef]
- Dunkle, S.S.; Suslick, K.S. Photodegradation of BiNbO4 Powder during Photocatalytic Reactions. Scanning 2009, 113, 1031–10345. [Google Scholar] [CrossRef]
- Aurivillius, B. X-ray investigations on BiNbO4, BiTaO4 and BiSbO4. Ark. Kemi. 1951, 3, 153–161. [Google Scholar]
- Roth, R.S.; Waring, J.L. Phase equilibrium relations in the binary system bismuth sesquioxide-niobium pentoxide. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1962, 66A, 451. [Google Scholar] [CrossRef]
- Diehl, R.; Carpentier, C. The Crystal Structure of Triclinic β-BiNbO4. Acta Crystallogr. 1973, B34, 1105–1111. [Google Scholar]
- Popolitov, V.I.; Ivanova, L.A.; Stephanovitch, S.Y.; Chetchkin, V.V.; Lobachev, A.N.; Venevtsev, Y.N. Ferroelectrics ab04: Synthesis of single crystals and ceramics; dielectric and nonlinear optical properties. Ferroelectrics 1974, 8, 519–520. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Calabrese, J.C. Crystal Structure of The Low Temperature Form of Bismuth Niobium Oxide [⍺-BiNbO4]. Mater. Res. Bull. 1993, 28, 523–529. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, H.; Yao, X.; Wei, X.; Xiang, F.; Pang, L. Phase transformation in BiNbO4 ceramics. Appl. Phys. Lett. 2007, 90, 172910. [Google Scholar] [CrossRef]
- Zhai, H.F.; Qian, X.; Kong, J.Z.; Li, A.D.; Gong, Y.P.; Li, H.; Wu, D. Abnormal phase transition in BiNbO4 powders prepared by a citrate method. J. Alloys Compd. 2011, 509, 10230–10233. [Google Scholar] [CrossRef]
- Zhai, H.F.; Li, A.D.; Kong, J.Z.; Li, X.F.; Zhao, J.; Guo, B.L.; Yin, J.; Li, Z.S.; Wu, D. Preparation and visible-light photocatalytic properties of BiNbO4 and BiTaO4 by a citrate method. J. Solid State Chem. 2013, 202, 6–14. [Google Scholar] [CrossRef]
- Xu, C.; He, D.; Liu, C.; Wang, H.; Zhang, L.; Wang, P.; Yin, S. High pressure and high temperature study the phase transitions of BiNbO4. Solid State Commun. 2013, 156, 21–24. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, C.; He, D.W.; Sen Fu, M.; Guo, J.; Zhou, H.F.; Pang, L.X.; Yao, X. Dielectric properties and phase transitions of BiNbO4 ceramic. Scr. Mater. 2014, 81, 40–43. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; He, D.; Zhang, J.; Hu, Q.; Qi, L. Exploring the phase transition of BiNbO4: A high pressure x-ray diffraction study. Solid State Commun. 2017, 265, 15–18. [Google Scholar] [CrossRef]
- Zou, Z.; Arakawa, H.; Ye, J. Substitution Effect of Ta5+ by Nb5+ on Photocatalytic, Photophysical, and Structural Properties of BiTa1–x NbxO4 (0≦x≦1). J. Mater. Res. 2002, 17, 1446–1454. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Photocatalytic water splitting into H2 and/or O2 under UV and visible light irradiation with a semiconductor photocatalyst. Int. J. Hydrog. Energy 2003, 28(6), 663–669. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Optical and structural properties of the BiTa1−xNbxO4 (0≦x≦1) compounds. Solid State Commun. 2001, 119, 471–475. [Google Scholar] [CrossRef]
- Wiegel, M.; Middel, W.; Blasse, G. Influence of ns2 ions on the luminescence of niobates and tantalates. J. Mater. Chem. 1995, 5, 981–983. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The reflection of X-rays by crystals. Proc. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character. 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchem. Ein Lehrb; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Ting, C.-C.; Chen, S.-Y.; Liu, D.-M. Structural evolution and optical properties of TiO2 thin films prepared by thermal oxidation of sputtered Ti films. J. Appl. Phys. 2000, 88, 4628–4633. [Google Scholar] [CrossRef]
- Radha, R.; Gupta, U.N.; Samuel, V.; Muthurajan, H.; Kumar, H.H.; Ravi, V. A co-precipitation technique to prepare BiNbO4 powders. Ceram. Int. 2008, 34, 1565–1567. [Google Scholar] [CrossRef]
- Schreiner, W.N. A standard test method for the determination of RIR values by X-ray diffraction. Powder Diffr. 1995, 10, 25–33. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley publishing Co., Inc.: Boston, MA, USA, 1978. [Google Scholar]
- Fluri, A.; Pergolesi, D.; Roddatis, V.; Wokaun, A.; Lippert, T. In situ stress observation in oxide films and how tensile stress influences oxygen ion conduction. Nat. Commun. 2016, 7, 10692. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Yang, J.W.; Simin, G.; Gaska, R.; Shur, M.S.; zur Loye, H.-C.; Tamulaitis, G.; Zukauskas, A.; Smith, D.J.; Chandrasekhar, D. Lattice and energy band engineering in AlInGaN/GaN heterostructures. Appl. Phys. Lett. 2000, 76, 1161–1163. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Macquart, R.; Zhou, Q.; Kennedy, B.J. Structural and spectroscopic studies of BiTa1− xNbxO4. J. Solid State Chem. 2003, 174, 310–318. [Google Scholar] [CrossRef]
- Maruyama, Y.; Izawa, C.; Watanabe, T. Synthesis of BiNbO4 by the Flux Method. ISRN Mater. Sci. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
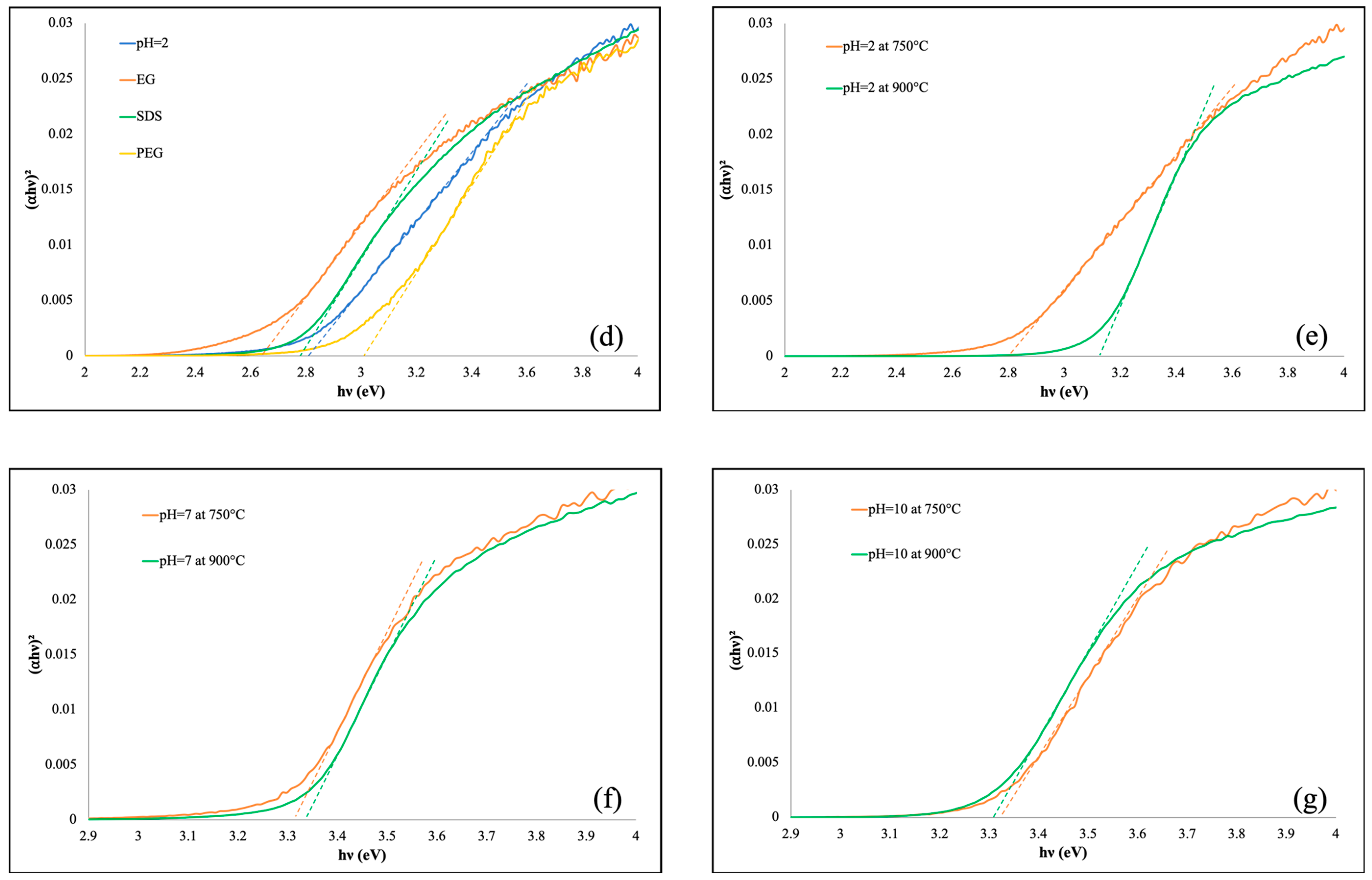
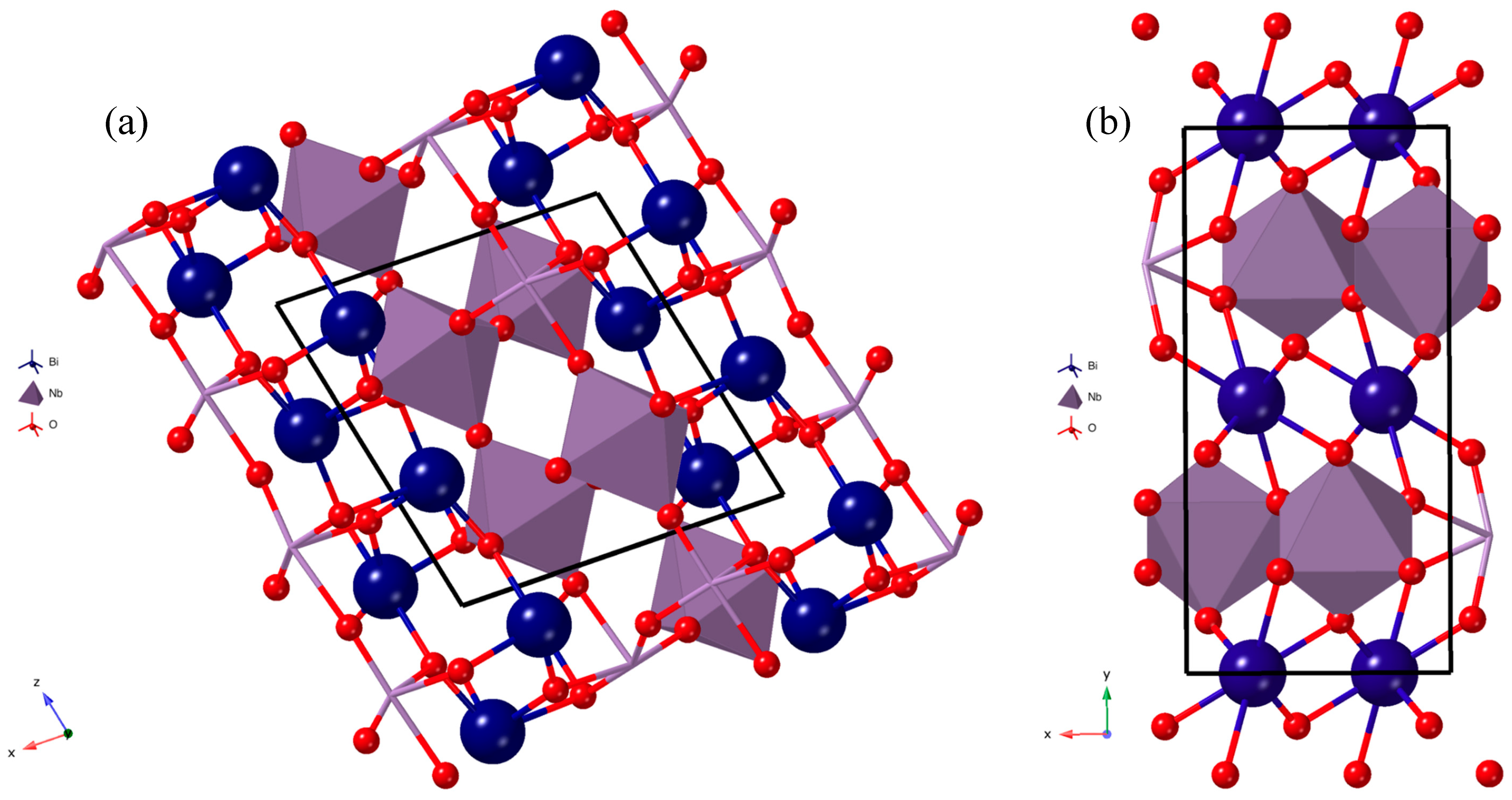
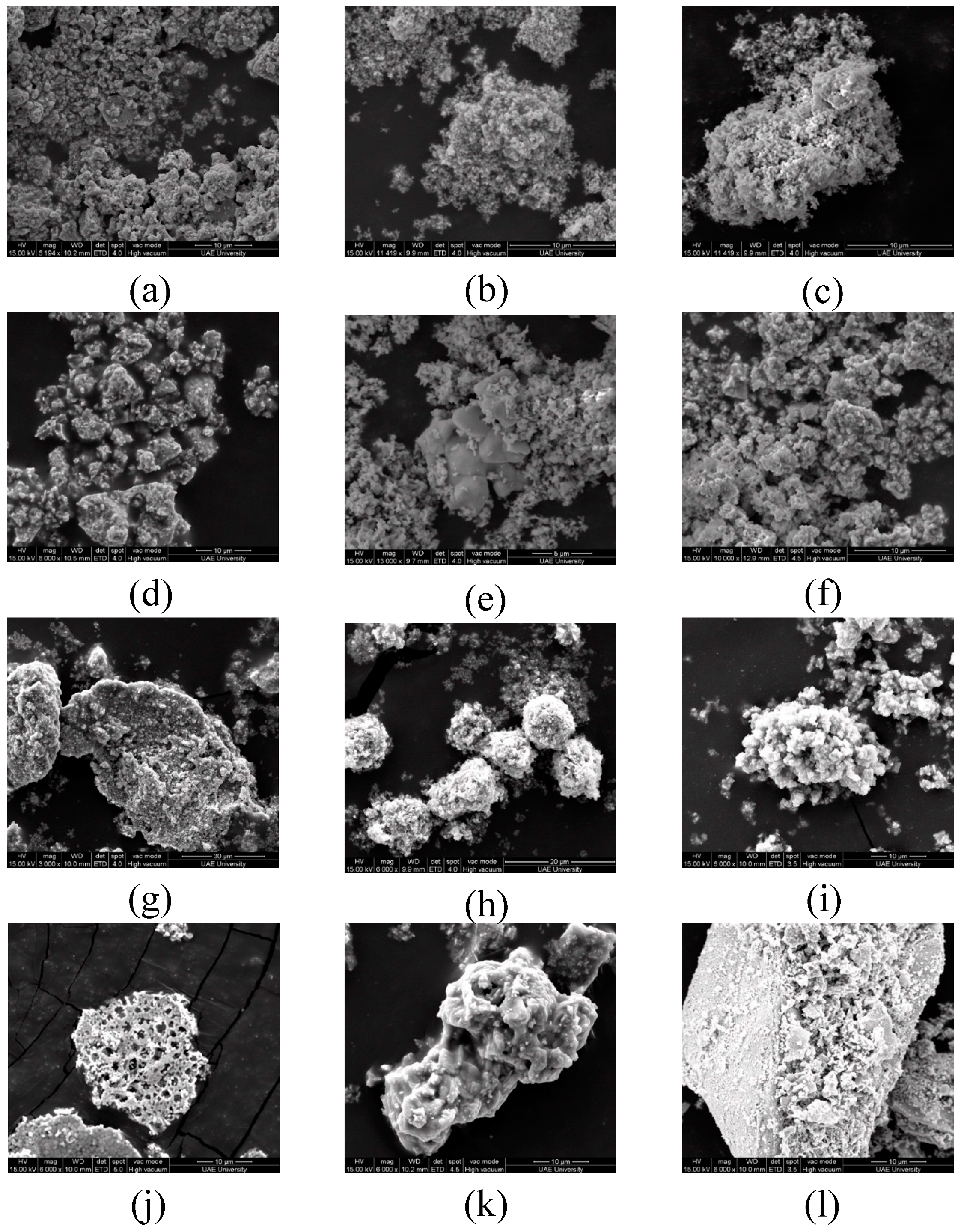
| Co-Precipitation Method at 750 °C | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Band Gap Energy (eV) | SBET (m2g−1) | Pore Size (nm) | Vpores (10−3 cm3g−1) | Crystalline Size (nm) | Lattice Constants | |||||||
| SYS | a (Å) | b (Å) | c (Å) | Vol (Å3) | |||||||||
| pH = 2 | 2.86 | 1.0 | 7.6 | 1.9 | 23.1 | T | 7.66 | 5.54 | 7.91 | 90.09 | 78.11 | 86.65 | 327.88 |
| O | 4.99 | 11.701 | 5.687 | 90 | 90 | 90 | 332.10 | ||||||
| pH = 7 | 3.3 | 3.1 | 6.1 | 4.7 | 35.7 | T | 7.51 | 5.51 | 7.90 | 90.14 | 77.09 | 91.93 | 318.53 |
| O | 4.96 | 11.65 | 5.66 | 90 | 90 | 90 | 309.25 | ||||||
| pH = 10 | 3.3 | 2.9 | 5.7 | 4.1 | 34.3 | T | 7.26 | 5.50 | 8.03 | 91.94 | 74.04 | 82.61 | 305.58 |
| O | 4.97 | 11.68 | 5.68 | 90 | 90 | 90 | 330.04 | ||||||
| Hydrothermal Approach at 750 °C | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Band Gap Energy (eV) | SBET (m2g−1) | Pore Size (nm) | Vpores (10−3 cm3g−1) | Crystalline Size (nm) | Lattice Constants | |||||||
| SYS | a (Å) | b (Å) | c (Å) | Vol (Å3) | |||||||||
| pH = 2 | 3.26 | 6.2 | 5.9 | 9.2 | 13.5 | T | 7.12 | 5.19 | 7.47 | 89.88 | 76.34 | 87.53 | 267.62 |
| O | 4.94 | 11.63 | 5.65 | 90 | 90 | 90 | 324.60 | ||||||
| pH = 7 | 3.43 | 12.9 | 7.5 | 24.3 | 23.1 | T | - | - | - | - | - | - | - |
| O | - | - | - | - | - | - | - | ||||||
| pH = 10 | 3.43 | 20.5 | 7.6 | 39.1 | 21.1 | T | 7.28 | 5.19 | 7.48 | 86.03 | 77.63 | 87.13 | 274.79 |
| O | 4.91 | 11.81 | 5.73 | 90 | 90 | 90 | 332.27 | ||||||
| Citrate Method at 750 °C | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Band Gap Energy (eV) | SBET (m2g−1) | Pore Size (nm) | Vpores (10−3 cm3g−1) | Crystalline Size (nm) | Lattice Constants | |||||||
| SYS | a (Å) | b (Å) | c (Å) | Vol (Å3) | |||||||||
| pH = 2 | 3.25 | 4.6 | 7.4 | 8.8 | 11.8 | T | 7.39 | 5.20 | 7.35 | 90.86 | 78.39 | 86.01 | 275.13 |
| O | 4.84 | 11.84 | 5.74 | 90 | 90 | 90 | 328.93 | ||||||
| pH = 7 | 2.69 | 4.5 | 7.0 | 8.0 | - | T | - | - | - | - | - | - | - |
| O | 5.03 | 11.70 | 5.64 | 90 | 90 | 90 | 331.92 | ||||||
| pH = 10 | 3.16 | 4.6 | 7.4 | 8.8 | 21.8 | T | 7.26 | 5.17 | 7.44 | 89.59 | 77.14 | 86.65 | 271.62 |
| O | 4.79 | 11.75 | 5.73 | 90 | 90 | 90 | 322.50 | ||||||
| Co-Precipitation Method at 750 °C with Surfactant | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Band Gap Energy (eV) | SBET (m2g−1) | Pore Size (nm) | Vpores (10−3 cm3g−1) | Crystalline Size (nm) | Lattice Constants | |||||||
| SYS | a (Å) | b (Å) | c (Å) | Vol (Å3) | |||||||||
| pH = 2 | 2.80 | 1.0 | 7.6 | 1.9 | 23.1 | T | 7.66 | 5.54 | 7.91 | 90.09 | 78.11 | 86.65 | 327.88 |
| O | 4.99 | 11.70 | 5.69 | 90 | 90 | 90 | 332.10 | ||||||
| SDS | 2.77 | 1.6 | 6.3 | 2.5 | 41.1 | T | 7.33 | 5.16 | 7.43 | 89.79 | 78.54 | 85.4 | 274.45 |
| O | 4.95 | 11.72 | 5.71 | 90 | 90 | 90 | 331.26 | ||||||
| PEG | 3.30 | 1.2 | 5.7 | 1.7 | 32.9 | T | - | - | - | - | - | - | - |
| O | 4.92 | 11.67 | 5.67 | 90 | 90 | 90 | 325.19 | ||||||
| EG | 2.6 | 0.6 | 7.8 | 1.2 | 18.6 | T | 7.63 | 5.51 | 7.92 | 90.56 | 102.0 | 86.34 | 325.29 |
| O | 4.97 | 11.75 | 5.67 | 90 | 90 | 90 | 331.49 | ||||||
| Co-Precipitation Method at 900 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Band Gap Energy (eV) | Crystalline Size (nm) | Lattice Constants | |||||||
| SYS | a (Å) | b (Å) | c (Å) | Vol (Å3) | ||||||
| pH = 2 | 3.13 | 43.5 | T | - | - | - | - | - | - | - |
| O | 4.97 | 11.69 | 5.67 | 90 | 90 | 90 | 329.4 | |||
| pH = 7 | 3.3 | 34.3 | T | 7.57 | 5.52 | 7.91 | 90.12 | 77.33 | 87.60 | 322.73 |
| O | 4.94 | 11.69 | 5.67 | 90 | 90 | 90 | 327.43 | |||
| pH = 10 | 3.3 | 47.7 | T | - | - | - | - | - | - | - |
| O | - | - | - | - | - | - | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakiro, M.; Hussein Ahmed, S.; Alzamly, A. Effect of pH, Surfactant, and Temperature on Mixed-Phase Structure and Band Gap Properties of BiNbO4 Nanoparticles Prepared Using Different Routes. Chemistry 2019, 1, 89-110. https://doi.org/10.3390/chemistry1010008
Bakiro M, Hussein Ahmed S, Alzamly A. Effect of pH, Surfactant, and Temperature on Mixed-Phase Structure and Band Gap Properties of BiNbO4 Nanoparticles Prepared Using Different Routes. Chemistry. 2019; 1(1):89-110. https://doi.org/10.3390/chemistry1010008
Chicago/Turabian StyleBakiro, Maram, Salwa Hussein Ahmed, and Ahmed Alzamly. 2019. "Effect of pH, Surfactant, and Temperature on Mixed-Phase Structure and Band Gap Properties of BiNbO4 Nanoparticles Prepared Using Different Routes" Chemistry 1, no. 1: 89-110. https://doi.org/10.3390/chemistry1010008




