Solid-State Electrochemical Process and Performance Optimization of Memristive Materials and Devices
Abstract
:1. Introduction
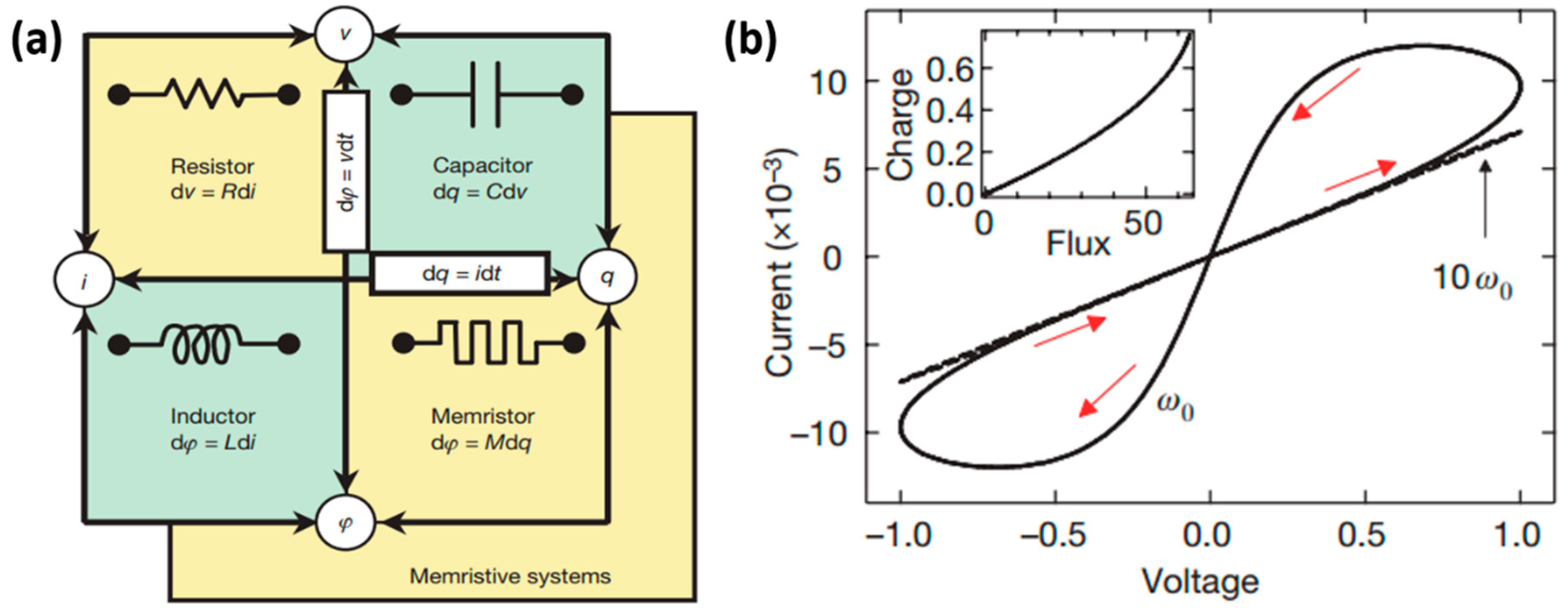
2. Switching Mechanisms of Memristor
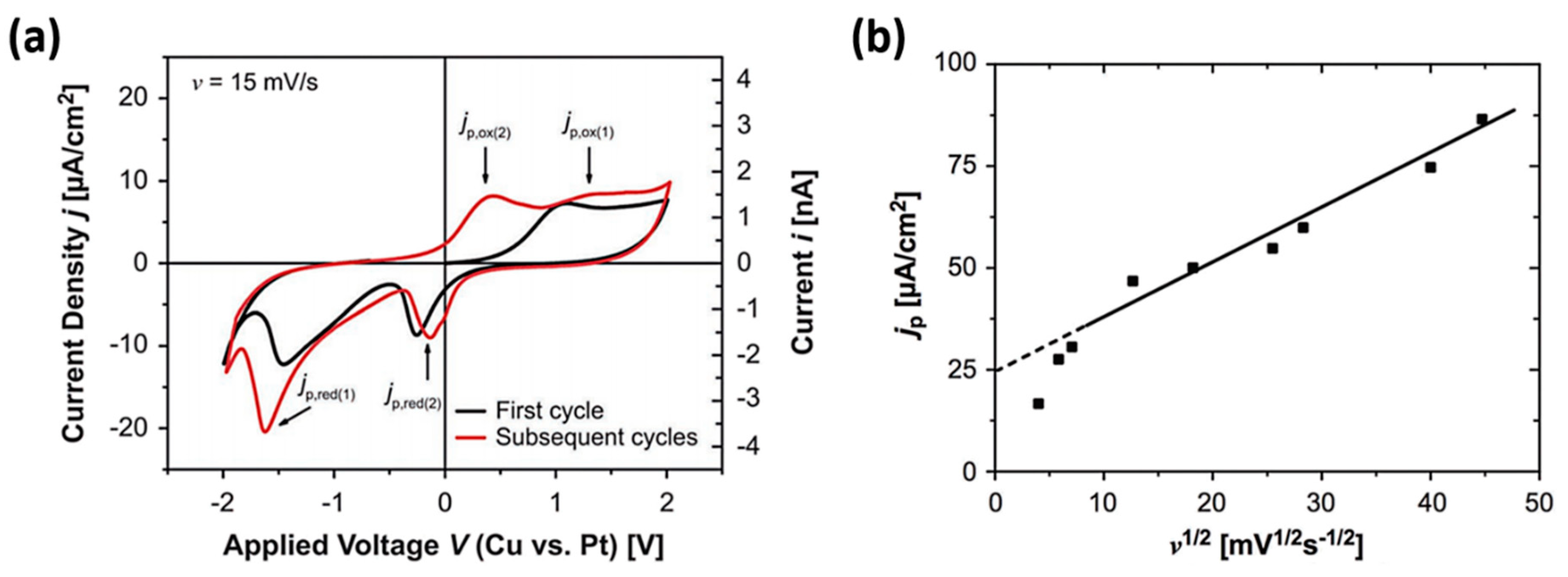
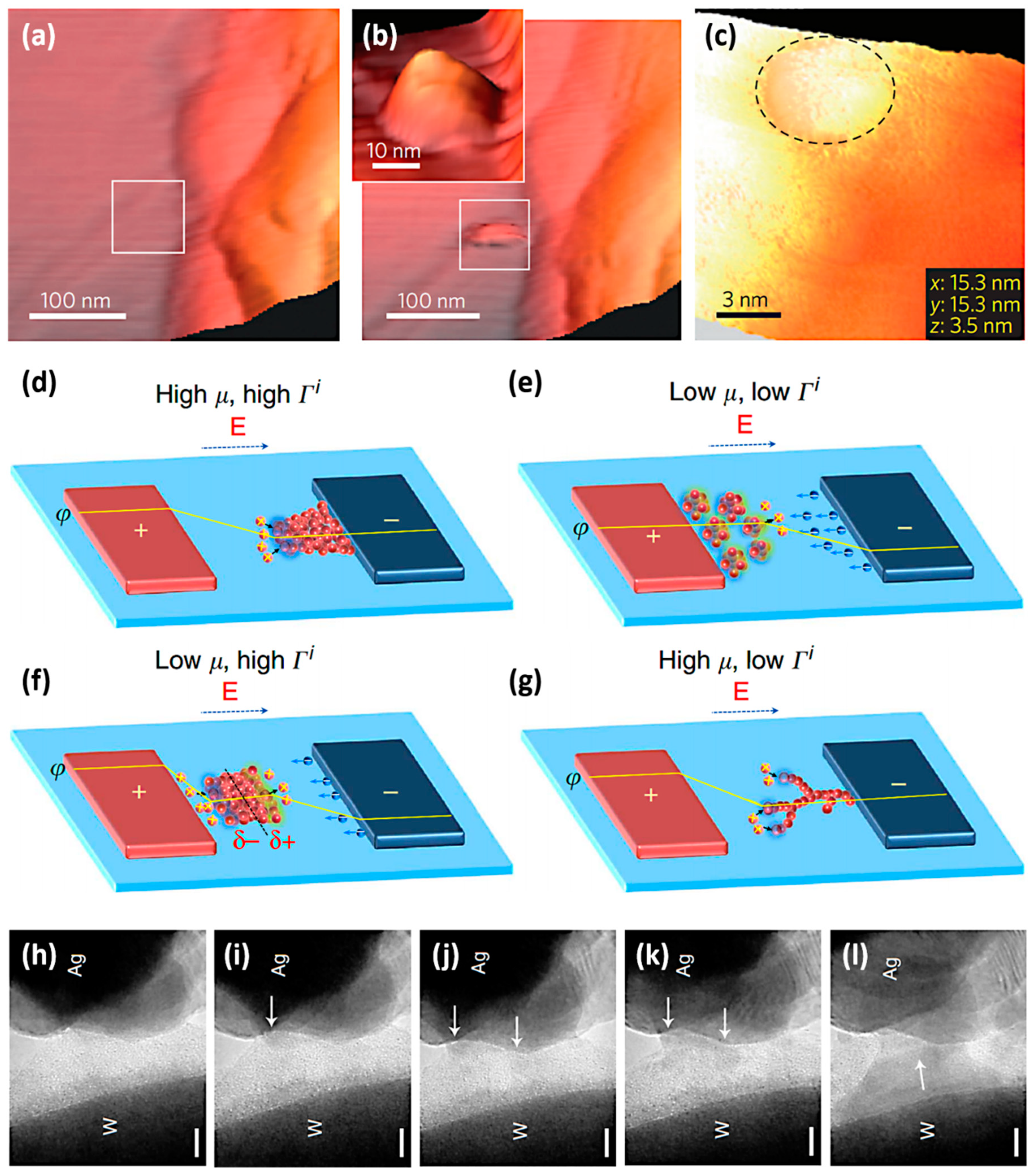
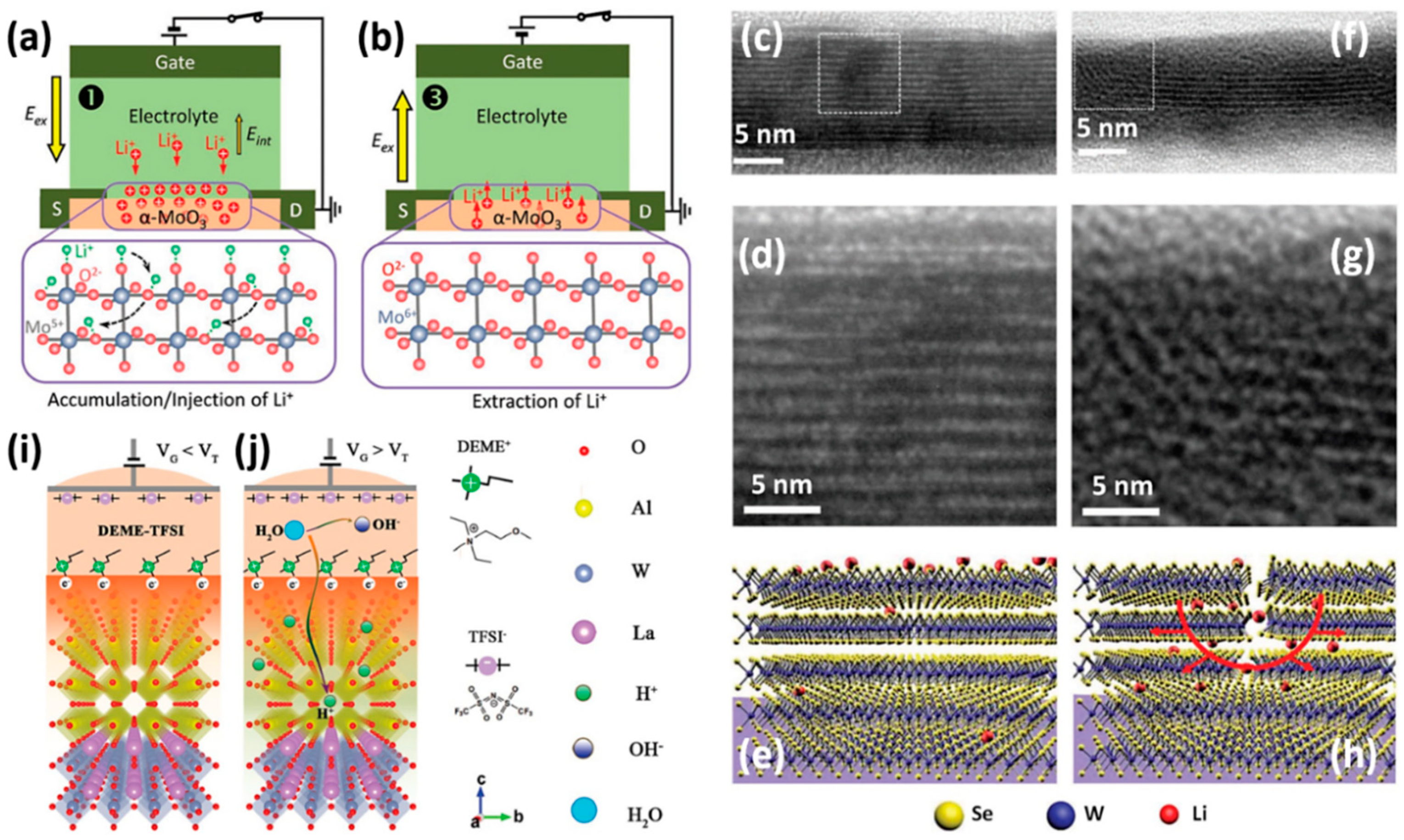
2.1. Dynamic Process of Active Metal Ions in ECM Device
- (1)
- ionization of the active electrode material into cations under the electric filed;
- (2)
- transport of cations toward the inert electrode across the dielectric thin film under high field;
- (3)
- reduction of cations to atoms, leading to the nucleation and growth of metal clusters and eventually the metallic nanofilaments.
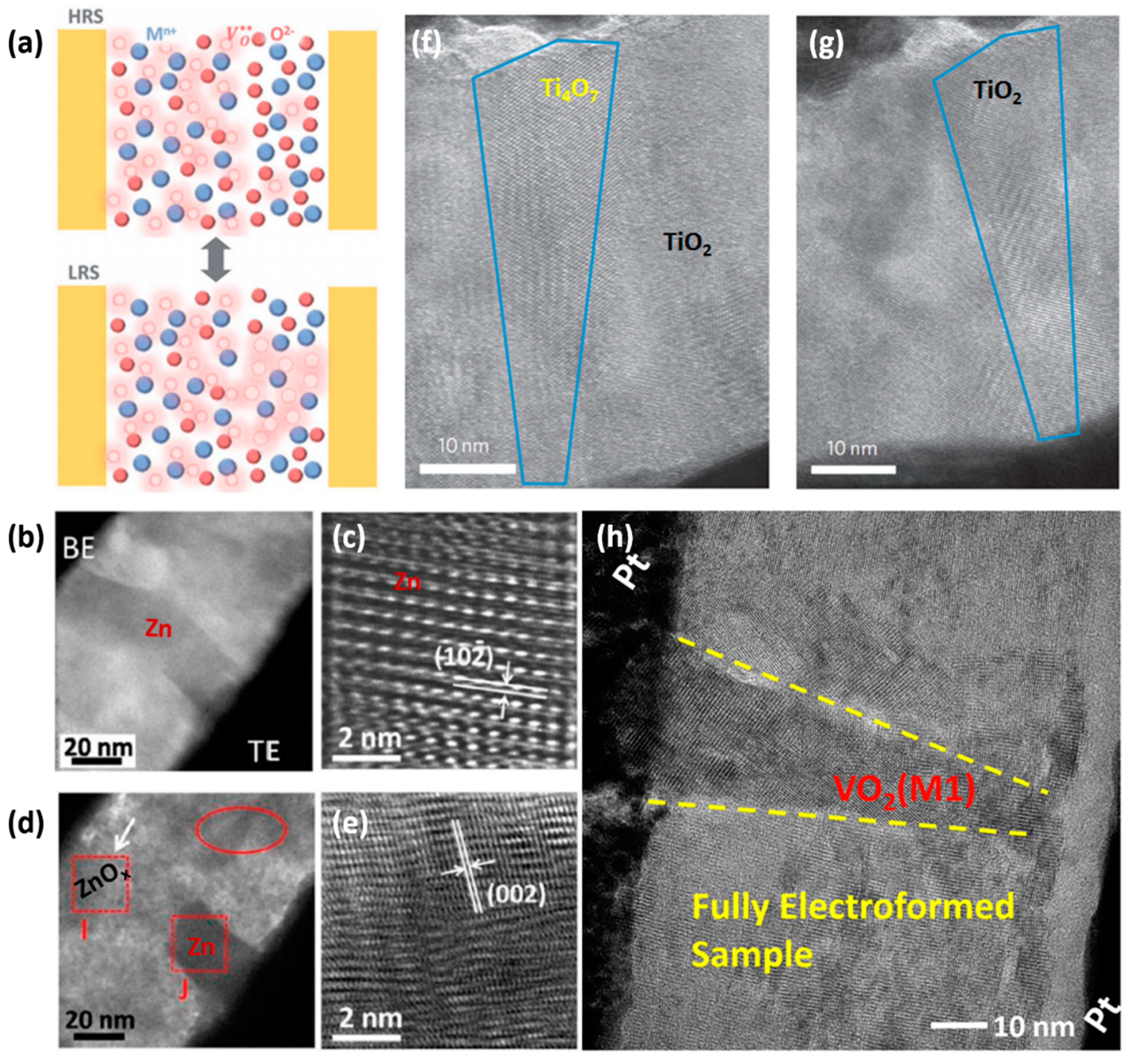
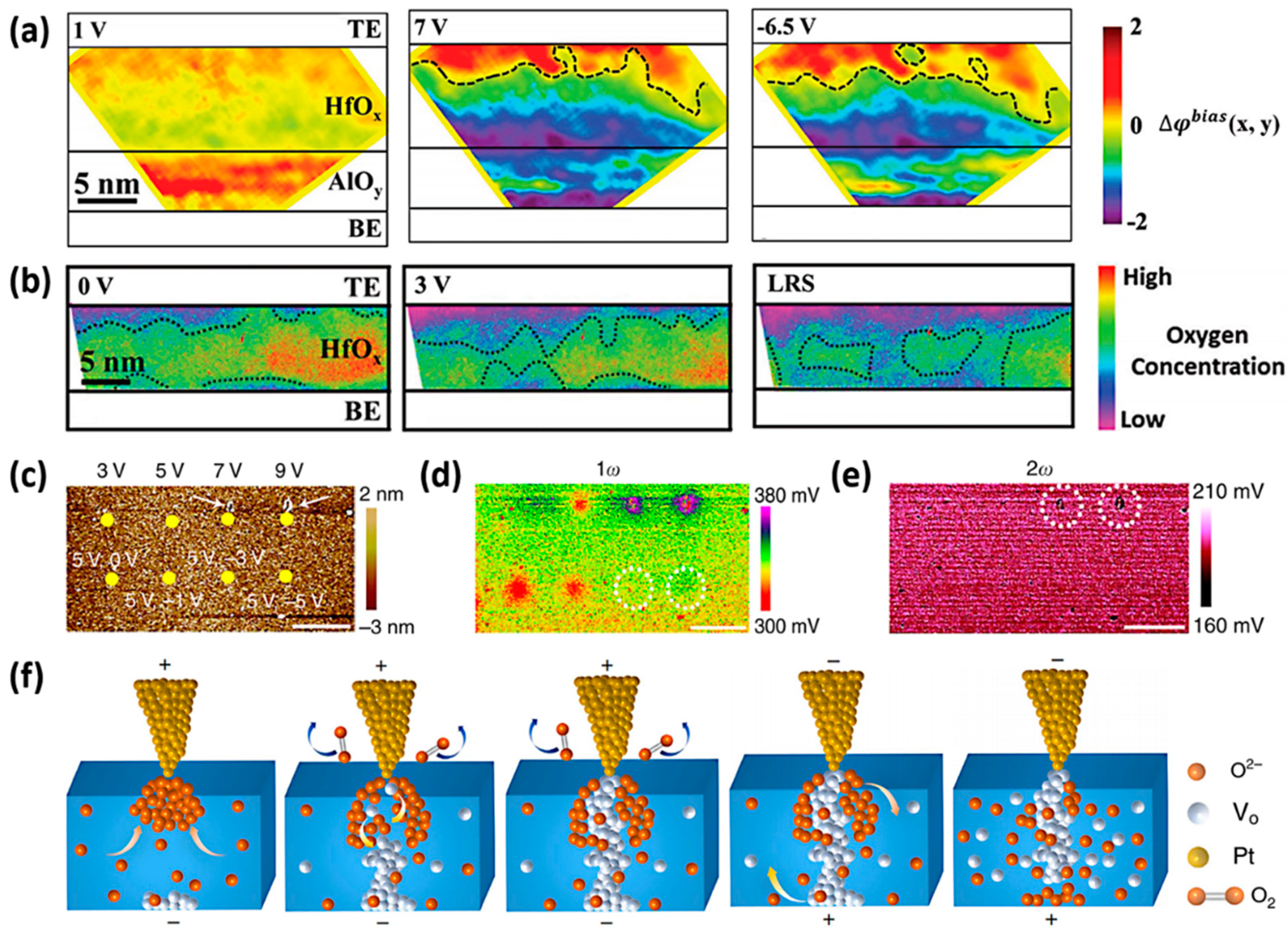
2.2. Dynamic Process of Native Oxygen Ions in Oxide-Based VCM Device
2.3. Dynamic Process of Other Active Ions
2.3.1. Native Active Ions from Dielectric
2.3.2. Active Cations from Ion Gel in Three-Terminal Electrochemical Transistors
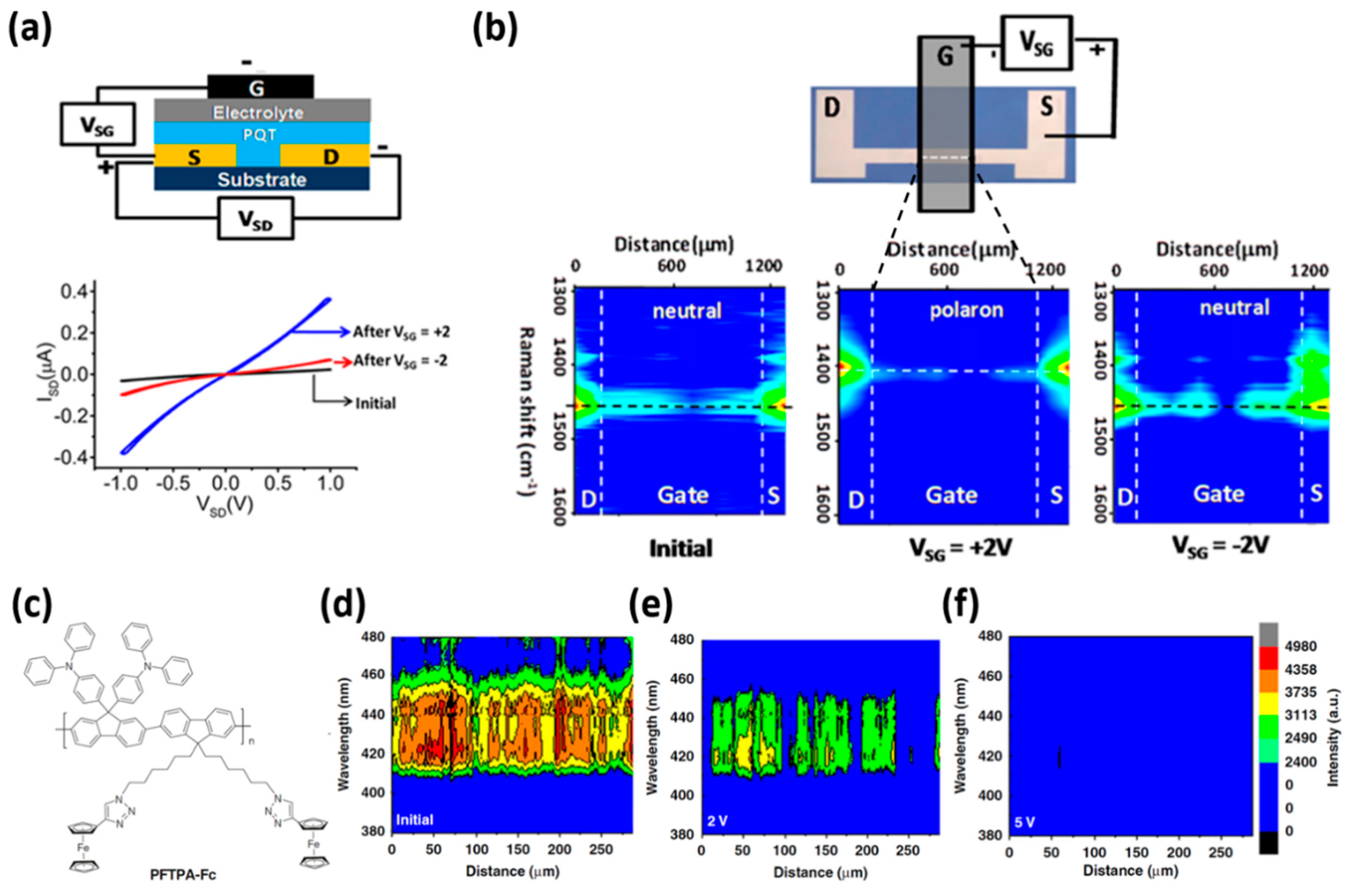
2.4. Switching Mechanisms in Organic Devices
3. Influence Factors of Memristive Performances and Optimization Methods
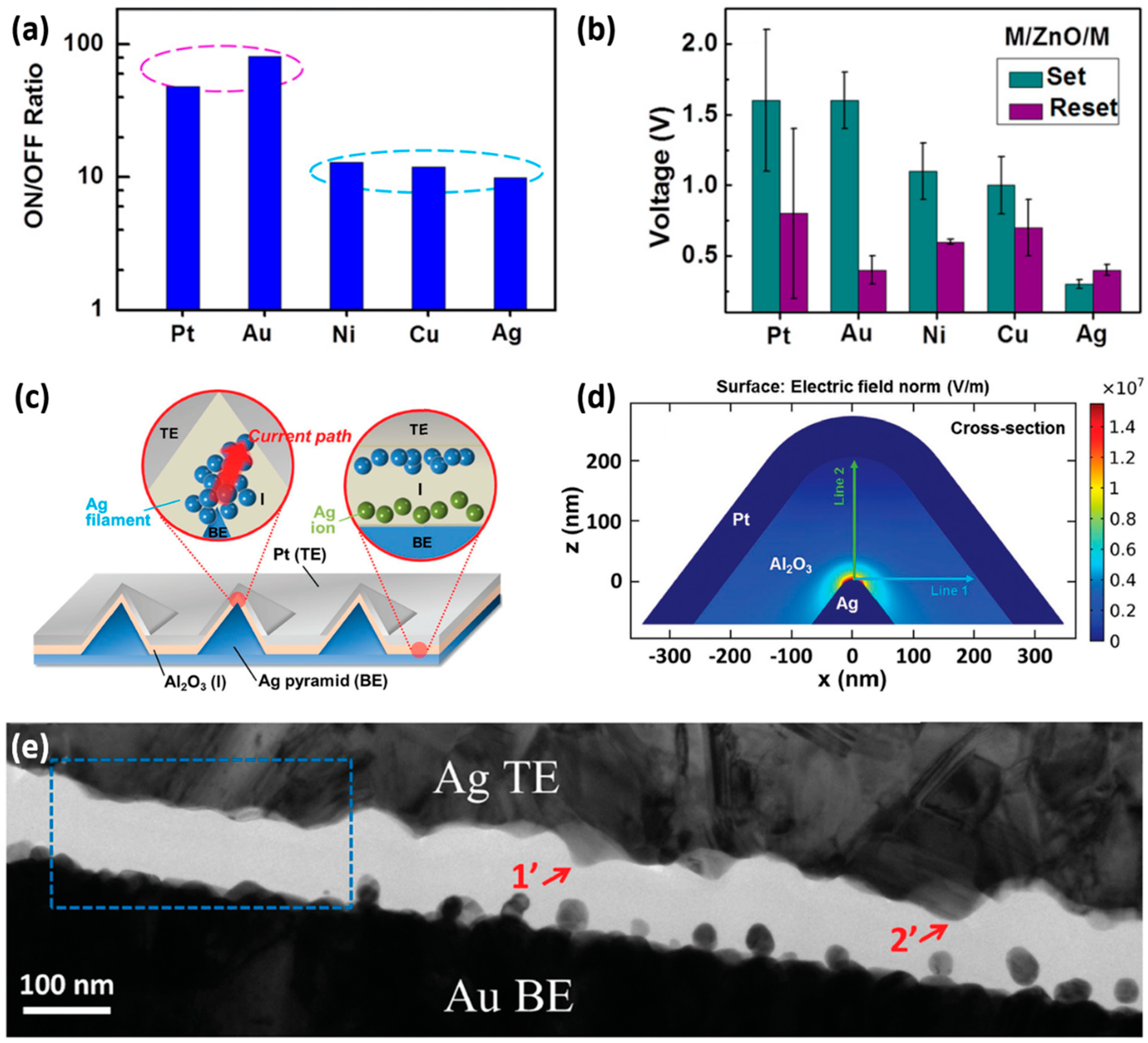
3.1. Electrode Engineering
3.2. Interface Engineering
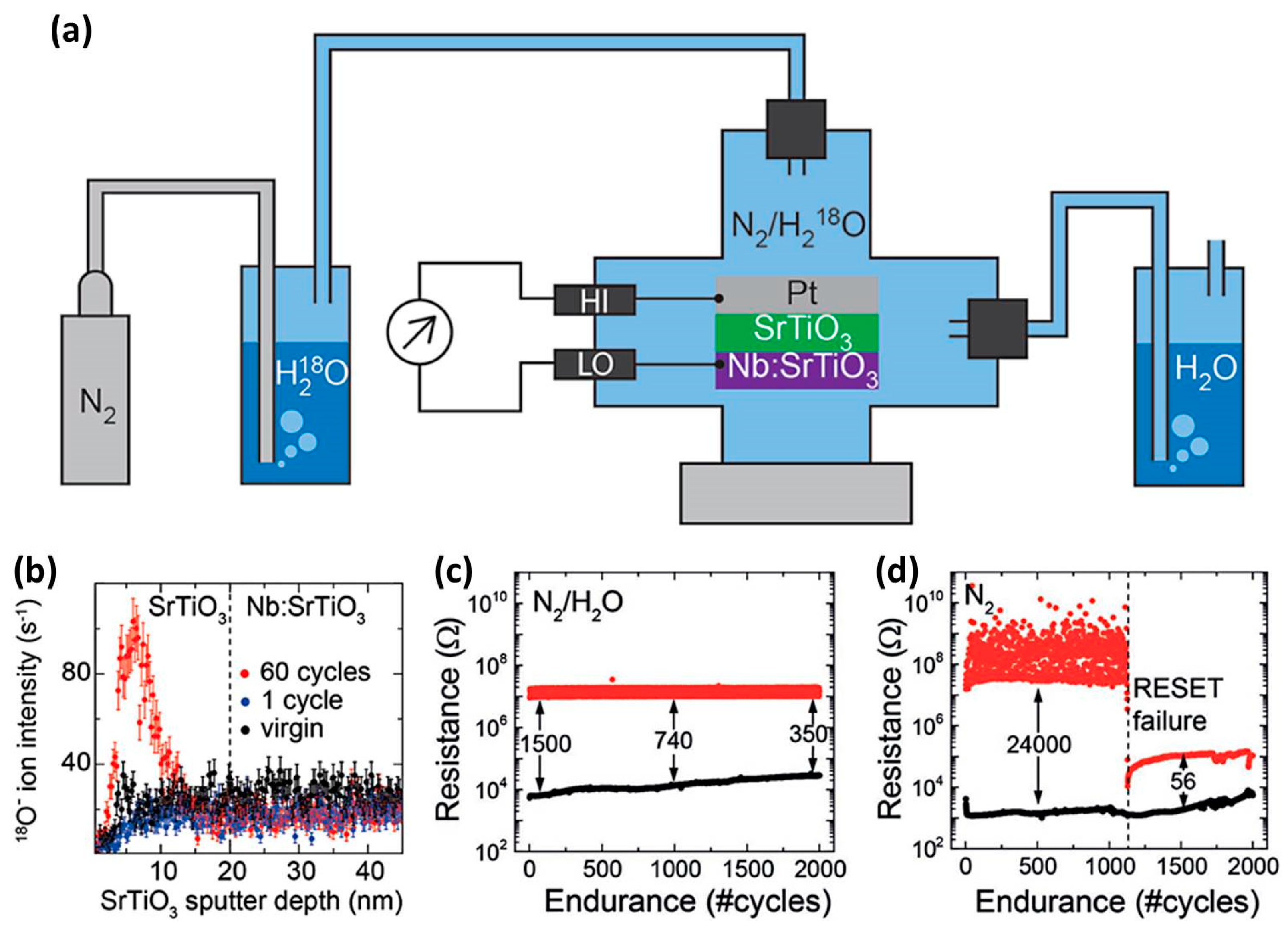
3.3. Ambient Atmosphere
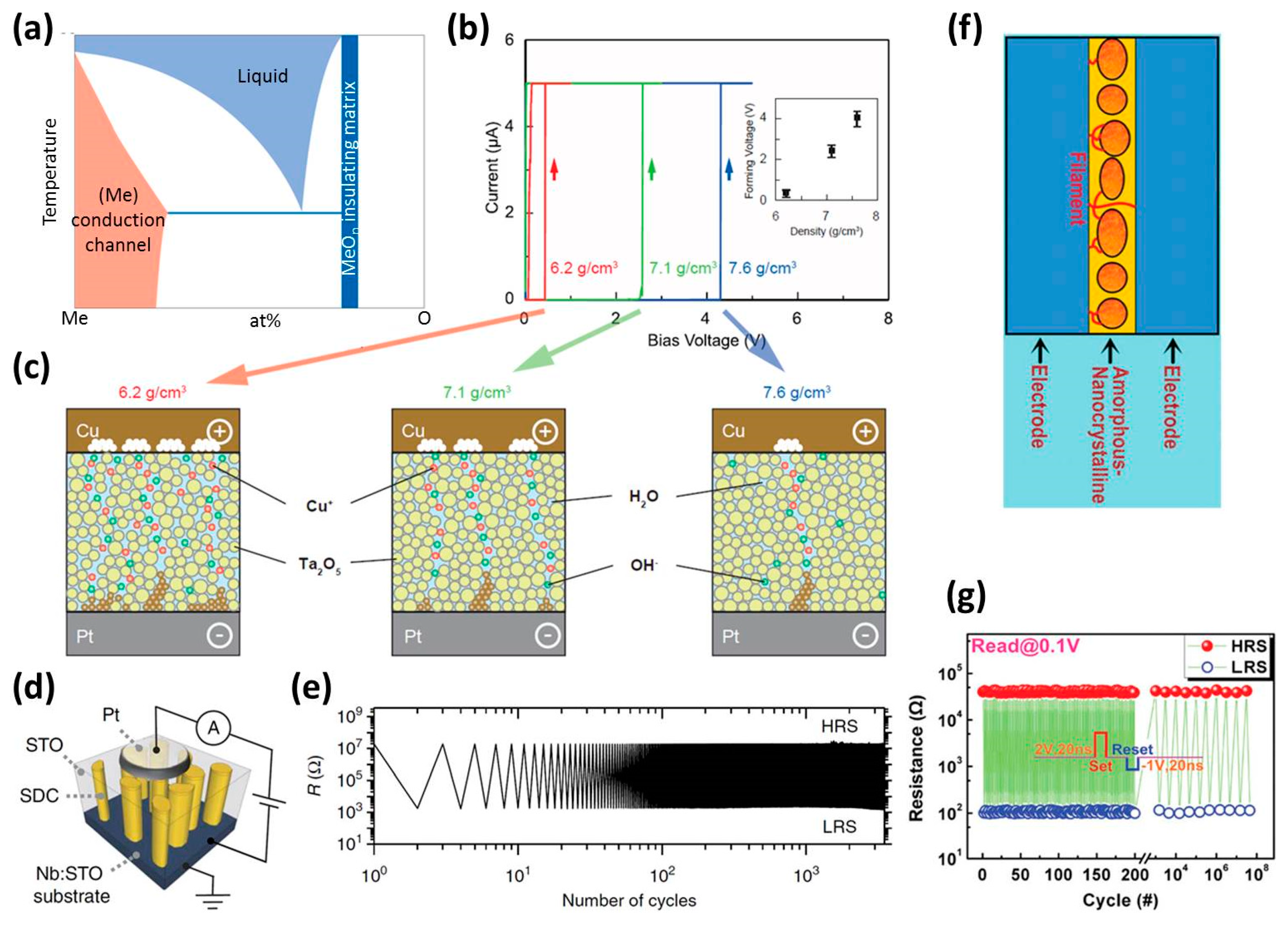
3.4. Selection of Dielectric Materials
3.5. Bias Scheme
4. Applications
5. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chua, L. Memristor-the missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Pan, F.; Liu, Q.; Liu, M.; Zeng, F. Ultrafast and high-density memory application. Nano Lett. 2009, 9, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Strachan, J.P.; Yang, J.J.; Zhang, M.X.; Goldfarb, I.; Torrezan, A.C.; Eschbach, P.; Kelley, R.D.; Medeiros-Ribeiro, G.; Williams, R.S. Anatomy of a nanoscale conduction channel reveals the mechanism of a high-performance memristor. Adv. Mater. 2011, 23, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, L.; Jiang, H.; Jang, M.H.; Lin, P.; Wu, Q.; Barnell, M.; Yang, J.J.; Xin, H.L.; Xia, Q. Three-dimensional crossbar arrays of self-rectifying Si/SiO2/Si memristors. Nat. Commun. 2017, 8, 15666. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Li, C.; Jiang, H.; Xia, W.; Xin, H.; Yang, J.J.; Xia, Q. Memristor crossbar arrays with 6-nm half-pitch and 2-nm critical dimension. Nat. Nanotech. 2018, 14, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yi, X.; Shang, J.; Liu, G.; Li, R.W. Organic and hybrid resistive switching materials and devices. Chem. Soc. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, J.; Snider, G.S.; Kuekes, P.J.; Yang, J.J.; Stewart, D.R.; Williams, R.S. ‘Memristive’ switches enable ‘stateful’ logic operations via material implication. Nature 2010, 464, 873–876. [Google Scholar] [CrossRef]
- Hasegawa, T.; Terabe, K.; Tsuruoka, T.; Aono, M. Atomic switch: Atom/ion movement controlled devices for beyond von-neumann computers. Adv. Mater. 2012, 24, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Strachan, J.P.; Williams, R.S. Chaotic dynamics in nanoscale NbO2 Mott memristors for analogue computing. Nature 2017, 548, 318–321. [Google Scholar] [CrossRef] [PubMed]
- van de Burgt, Y.; Melianas, A.; Keene, S.T.; Malliaras, G.; Salleo, A. Organic electronics for neuromorphic computing. Nat. Electron. 2018, 1, 386–397. [Google Scholar] [CrossRef]
- Wong, H.S.; Salahuddin, S. Memory leads the way to better computing. Nat. Nanotech. 2015, 10, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Hasegawa, T.; Tsuruoka, T.; Terabe, K.; Gimzewski, J.K.; Aono, M. Short-term plasticity and long-term potentiation mimicked in single inorganic synapses. Nat. Mater. 2011, 10, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Joshi, S.; Savel’ev, S.; Song, W.; Midya, R.; Li, Y.; Rao, M.; Yan, P.; Asapu, S.; Zhuo, Y.; et al. Fully memristive neural networks for pattern classification with unsupervised learning. Nat. Electron. 2018, 1, 137–145. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Belkin, D.; Li, Y.; Yan, P.; Hu, M.; Ge, N.; Jiang, H.; Montgomery, E.; Lin, P.; Wang, Z.; et al. Efficient and self-adaptive in-situ learning in multilayer memristor neural networks. Nat. Commun. 2018, 9, 2385. [Google Scholar] [CrossRef]
- van de Burgt, Y.; Lubberman, E.; Fuller, E.J.; Keene, S.T.; Faria, G.C.; Agarwal, S.; Marinella, M.J.; Alec Talin, A.; Salleo, A. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 2017, 16, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, M.; Li, Y.; Jiang, H.; Ge, N.; Montgomery, E.; Zhang, J.; Song, W.; Dávila, N.; Graves, C.E.; et al. Analogue signal and image processing with large memristor crossbars. Nat. Electron. 2017, 1, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Zidan, M.A.; Strachan, J.P.; Lu, W.D. The future of electronics based on memristive systems. Nat. Electron. 2018, 1, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Belkin, D.; Savel’ev, S.E.; Lin, S.; Wang, Z.; Li, Y.; Joshi, S.; Midya, R.; Li, C.; Rao, M.; et al. A novel true random number generator based on a stochastic diffusive memristor. Nat. Commun. 2017, 8, 882. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.J.; Pickett, M.D.; Li, X.; Ohlberg, D.A.; Stewart, D.R.; Williams, R.S. Memristive switching mechanism for metal/oxide/metal nanodevices. Nat. Nanotech. 2008, 3, 429–433. [Google Scholar] [CrossRef]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 833–840. [Google Scholar] [CrossRef]
- Valov, I. Redox-based resistive switching memories (ReRAMs): Electrochemical systems at the atomic scale. Chem. Electro. Chem. 2014, 1, 26–36. [Google Scholar] [CrossRef]
- Pan, F.; Gao, S.; Chen, C.; Song, C.; Zeng, F. Recent progress in resistive random access memories: Materials, switching mechanisms, and performance. Mater. Sci. Eng. R 2014, 83, 1–59. [Google Scholar] [CrossRef]
- Messerschmitt, F.; Kubicek, M.; Rupp, J.L.M. How does moisture affect the physical property of memristance for anionic-electronic resistive switching memories? Adv. Funct. Mater. 2015, 25, 5117–5125. [Google Scholar] [CrossRef]
- Heisig, T.; Baeumer, C.; Gries, U.N.; Mueller, M.P.; La Torre, C.; Luebben, M.; Raab, N.; Du, H.; Menzel, S.; Mueller, D.N.; et al. Oxygen exchange processes between oxide memristive devices and water molecules. Adv. Mater. 2018, 30, 1800957. [Google Scholar] [CrossRef]
- Lee, J.; Lu, W.D. On-demand reconfiguration of nanomaterials: When electronics meets ionics. Adv. Mater. 2017, 30, 1702770. [Google Scholar] [CrossRef]
- Tappertzhofen, S.; Mündelein, H.; Valov, I.; Waser, R. Nanoionic transport and electrochemical reactions in resistively switching silicon dioxide. Nanoscale 2012, 4, 3040. [Google Scholar] [CrossRef] [Green Version]
- Tappertzhofen, S.; Menzel, S.; Valov, I.; Waser, R. Redox processes in silicon dioxide thin films using copper microelectrodes. Appl. Phys. Lett. 2011, 99, 203103. [Google Scholar] [CrossRef] [Green Version]
- Valov, I.; Sapezanskaia, I.; Nayak, A.; Tsuruoka, T.; Bredow, T.; Hasegawa, T.; Staikov, G.; Aono, M.; Waser, R. Atomically controlled electrochemical nucleation at superionic solid electrolyte surfaces. Nat. Mater. 2012, 11, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Valov, I.; Staikov, G. Nucleation and growth phenomena in nanosized electrochemical systems for resistive switching memories. J. Solid State Electrochem. 2012, 17, 365–371. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Li, L.; Pan, X.; Tappertzhofen, S.; Choi, S.; Waser, R.; Valov, I.; Lu, W.D. Electrochemical dynamics of nanoscale metallic inclusions in dielectrics. Nat. Commun. 2014, 5, 4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozicki, M.N.; Park, M.; Mitkova, M. Nanoscale memory elements based on solid-state electrolytes. IEEE Trans. Nanotechnol. 2005, 4, 331–338. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, G.S.; Kim, K.H.; Cho, S.; Yang, W.Y.; Li, X.S.; Moon, J.H.; Lee, K.J.; Kim, K. In situ observation of voltage-induced multilevel resistive switching in solid electrolyte memory. Adv. Mater. 2011, 23, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Gaba, S.; Chang, T.; Pan, X.; Lu, W. Observation of conducting filament growth in nanoscale resistive memories. Nat. Commun. 2012, 3, 732. [Google Scholar] [CrossRef] [Green Version]
- Terabe, K.; Hasegawa, T.; Nakayama, T.; Aono, M. Quantized conductance atomic switch. Nature 2005, 433, 47. [Google Scholar] [CrossRef]
- Nandakumar, S.R.; Minvielle, M.; Nagar, S.; Dubourdieu, C.; Rajendran, B. A 250 mV Cu/SiO2/W memristor with half-integer quantum conductance states. Nano Lett. 2016, 16, 1602. [Google Scholar] [CrossRef]
- Zhu, X.; Su, W.; Liu, Y.; Hu, B.; Pan, L.; Lu, W.; Zhang, J.; Li, R.-W. Observation of conductance quantization in oxide-based resistive switching memory. Adv. Mater. 2012, 24, 3941–3946. [Google Scholar] [CrossRef]
- Xue, W.; Gao, S.; Shang, J.; Yi, X.; Liu, G.; Li, R.-W. Recent Advances of Quantum Conductance in Memristors. Adv. Electron. Mater. 2019. [Google Scholar] [CrossRef]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-based resistive switching memories—Nanoionic mechanisms, prospects, and challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Sharath, S.U.; Vogel, S.; Molina-Luna, L.; Hildebrandt, E.; Wenger, C.; Kurian, J.; Duerrschnabel, M.; Niermann, T.; Niu, G.; Calka, P.; et al. Control of switching modes and conductance quantization in oxygen engineered HfOx based memristive devices. Adv. Funct. Mater. 2017, 27, 1700432. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hsin, C.L.; Huang, C.W.; Chiu, C.H.; Huang, Y.T.; Lin, S.J.; Wu, W.W.; Chen, L.J. Dynamic evolution of conducting nanofilament in resistive switching memories. Nano Lett. 2013, 13, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.S.; Park, G.S.; Lee, B.; Han, S.; et al. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotech. 2010, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, G.; Zhong, Z.; Dai, Y.; Shang, J.; Liu, Y.; Yang, H.; Yi, X.; Tan, H.; Pan, L.; et al. A 1D vanadium dioxide nanochannel constructed via electric-field-induced ion transport and its superior metal-insulator transition. Adv. Mater. 2017, 29, 1702162. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, Z.; Huang, X.; Kumari, N.; Davila, N.; Strachan, J.P.; Vine, D.; Kilcoyne, A.L.D.; Nishi, Y.; Williams, R.S. Conduction channel formation and dissolution due to oxygen thermophoresis/diffusion in hafnium oxide memristors. ACS Nano 2016, 10, 11205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Qin, L.; Zeng, Q.; Qiu, X.; Huang, R. Probing nanoscale oxygen ion motion in memristive systems. Nat. Commun. 2017, 8, 15173. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Gao, B.; Yao, Y.; Guan, X.; Shen, X.; Wang, Y.; Huang, P.; Liu, L.; Liu, X.; Li, J.; et al. Direct observations of nanofilament evolution in switching processes in HfO2-based resistive random access memory by in situ TEM studies. Adv. Mater. 2017, 29, 1602976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ong, C.S.; Xu, X.; Hu, B.; Shang, J.; Yang, H.; Katlakunta, S.; Liu, Y.; Chen, X.; Pan, L.; et al. Direct observation of lithium-ion transport under an electrical field in LixCoO2 nanograins. Sci. Rep. 2013, 3, 1084. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, J.; Lu, W.D. Iodine vacancy redistribution in organic-inorganic halide perovskite films and resistive switching effects. Adv. Mater. 2017, 29, 1700527. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, W.D. Optogenetics-inspired tunable synaptic functions in memristors. ACS Nano 2018, 12, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Jariwala, D.; Kim, I.S.; Chen, K.S.; Marks, T.J.; Lauhon, L.J.; Hersam, M.C. Gate-tunable memristive phenomena mediated by grain boundaries in single-layer MoS2. Nat. Nanotech. 2015, 10, 403–406. [Google Scholar] [CrossRef]
- Sangwan, V.K.; Lee, H.S.; Bergeron, H.; Balla, I.; Beck, M.E.; Chen, K.S.; Hersam, M.C. Multi-terminal memtransistors from polycrystalline monolayer molybdenum disulfide. Nature 2018, 554, 500–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisri, S.Z.; Shimizu, S.; Nakano, M.; Iwasa, Y. Endeavor of iontronics: From fundamentals to applications of ion-controlled electronics. Adv. Mater. 2017, 29, 1607054. [Google Scholar] [CrossRef] [PubMed]
- Leighton, C. Electrolyte-based ionic control of functional oxides. Nat. Mater. 2019, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Shang, D.S.; Liu, N.; Fuller, E.J.; Agrawal, S.; Talin, A.A.; Li, Y.-Q.; Shen, B.-G.; Sun, Y. All-solid-state synaptic transistor with ultralow conductance for neuromorphic computing. Adv. Funct. Mater. 2018, 28, 1804170. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Jia, R.; Liang, Z.; Zhu, W.; Rehman, Z.U.; Bao, L.; Zhang, X.; Cai, Y.; Song, L.; et al. Ion gated synaptic transistors based on 2D van der waals crystals with tunable diffusive dynamics. Adv. Mater. 2018, 30, 1800195. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, F.; Lu, X.F.; Yan, Y.J.; Cho, Y.H.; Ma, L.; Niu, X.; Kim, S.; Son, Y.W.; Feng, D.; et al. Gate-tunable phase transitions in thin flakes of 1T-TaS2. Nat. Nanotech. 2015, 10, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kuzminskii, Y.V.; Voronin, B.M.; Redin, N.N. Iron and nickel phosphorus trisulfides as electroactive materials for primary lithium batteries. J. Power Sources 1995, 55, 133–141. [Google Scholar] [CrossRef]
- Rouxel, J.; Brec, R. Low-dimensional chalcogenides as secondary cathodic materials: Some geometric and electronic aspects. Ann. Rev. Mater. Sci. 1986, 16, 137–162. [Google Scholar] [CrossRef]
- Yang, J.T.; Ge, C.; Du, J.Y.; Huang, H.Y.; He, M.; Wang, C.; Lu, H.B.; Yang, G.Z.; Jin, K.J. Artificial synapses emulated by an electrolyte-gated tungsten-oxide transistor. Adv. Mater. 2018, 1801548. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, Y.; Gao, S.; Zhang, B.; Li, R.-W.; Zhuang, X. Recent advances in resistive switching materials and devices: From memories to memristors. Eng. Sci. 2018, 4, 4–43. [Google Scholar] [CrossRef]
- Goswami, S.; Matula, A.J.; Rath, S.P.; Hedstrom, S.; Saha, S.; Annamalai, M.; Sengupta, D.; Patra, A.; Ghosh, S.; Jani, H.; et al. Robust resistive memory devices using solution-processable metal-coordinated azo aromatics. Nat. Mater. 2017, 16, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Liu, G.; Zhu, X.; Chen, X.; Pan, L.; Tan, H.; Xue, W.; Ji, Z.; Wang, J.; et al. Thermally-stable resistive switching with a large ON/OFF ratio achieved in poly(triphenylamine). Chem. Commun. 2014, 50, 11856–11858. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, B.; Chen, Y.; Zhu, C.X.; Zeng, L.; Chan, D.S.H.; Neoh, K.G. Electrical conductivity switching and memory effects in poly(n-vinylcarbazole) derivatives with pendant azobenzene chromophores and terminal electron acceptor moieties. J. Mater. Chem. 2011, 21, 6027. [Google Scholar] [CrossRef]
- Liu, G.; Wang, C.; Zhang, W.; Pan, L.; Zhang, C.; Yang, X.; Fan, F.; Chen, Y.; Li, R.-W. Organic biomimicking memristor for information storage and processing applications. Adv. Electron. Mater. 2016, 2, 1500298. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, J.; Xue, W.; Tan, H.; Pan, L.; Yang, X.; Guo, S.; Hao, J.; Liu, G.; Li, R.-W. Convertible resistive switching characteristics between memory switching and threshold switching in a single ferritin-based memristor. Chem. Commun. 2016, 52, 4828. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Lim, S.-L.; Song, Y.; Zhu, C.-X.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Nonvolatile polymer memory device based on bistable electrical switching in a thin film of poly(n-vinylcarbazole) with covalently bonded C60. Langmuir 2007, 23, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.L.; Lee, K.H.; Joo, W.J.; Lee, S.; Chae, M.Y. Synthesis and nonvolatile memory behavior of redox-active conjugated polymer-containing ferrocene. J. Am. Chem. Soc. 2007, 129, 9842–9843. [Google Scholar] [CrossRef]
- Xie, L.H.; Ling, Q.D.; Hou, X.Y.; Wei, H. An effective friedel-crafts postfunctionization of poly(n-vinylcarbazole) to tune carrier transportation of supramolecular organic semiconductors based on pi-stacked polymers for nonvolatile flash memory cell. J. Am. Chem. Soc. 2008, 130, 2120–2121. [Google Scholar] [CrossRef]
- Gao, S.; Song, C.; Chen, C.; Zeng, F.; Pan, F. Dynamic processes of resistive switching in metallic filament-based organic memory devices. J. Phys. Chem. C 2012, 116, 17955–17959. [Google Scholar] [CrossRef]
- Gao, S.; Song, C.; Chen, C.; Zeng, F.; Pan, F. Formation process of conducting filament in planar organic resistive memory. Appl. Phys. Lett. 2013, 102, 141606. [Google Scholar] [CrossRef]
- Krishnan, K.; Muruganathan, M.; Tsuruoka, T.; Mizuta, H.; Aono, M. Highly reproducible and regulated conductance quantization in a polymer-based atomic switch. Adv. Funct. Mater. 2017, 27, 1605104. [Google Scholar] [CrossRef]
- Krishnan, K.; Tsuruoka, T.; Mannequin, C.; Aono, M. Mechanism for conducting filament growth in self-assembled polymer thin films for redox-based atomic switches. Adv. Mater. 2016, 28, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhu, X.; Chen, X.; Pan, L.; Peng, S.; Wu, Y.; Shang, J.; Liu, G.; Yan, Q.; Li, R.-W. A multilevel memory based on proton-doped polyazomethine with an excellent uniformity in resistive switching. J. Am. Chem. Soc. 2012, 134, 17408–17411. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pillai, R.G.; Pekas, N.; Wu, Y.; McCreery, R.L. Spatially resolved Raman spectroelectrochemistry of solid-state polythiophene/viologen memory devices. J. Am. Chem. Soc. 2012, 134, 14869–14876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fan, F.; Xue, W.; Liu, G.; Fu, Y.; Zhuang, X.; Xu, X.-H.; Gu, J.; Li, R.-W.; Chen, Y. Redox gated polymer memristive processing memory unit. Nat. Commun. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xiao, W.; Shang, J.; Chen, X.X.; Zhu, X.J.; Pan, L.; Tan, H.W.; Zhang, W.B.; Ji, Z.H.; Liu, G.; et al. Intrinsic and interfacial effect of electrode metals on the resistive switching behaviors of zinc oxide films. Nanotechnology 2014, 25, 425204. [Google Scholar] [CrossRef]
- Sonde, S.; Chakrabarti, B.; Liu, Y.; Sasikumar, K.; Lin, J.; Stan, L.; Divan, R.; Ocola, L.E.; Rosenmann, D.; Choudhury, P.; et al. Silicon compatible Sn-based resistive switching memory. Nanoscale 2018, 10, 9441–9449. [Google Scholar] [CrossRef]
- Chen, C.; Gao, S.; Zeng, F.; Tang, G.S.; Li, S.Z.; Song, C.; Fu, H.D.; Pan, F. Migration of interfacial oxygen ions modulated resistive switching in oxide-based memory devices. J. Appl. Phys. 2013, 114, 014502. [Google Scholar] [CrossRef]
- Yoshida, C.; Tsunoda, K.; Noshiro, H.; Sugiyama, Y. High speed resistive switching in Pt/TiO2/TiN film for nonvolatile memory application. Appl. Phys. Lett. 2007, 91, 223510. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Gou, L.; Clima, S.; Govoreanu, B.; Degraeve, R.; Kar, G.S.; Fantini, A.; Groeseneken, G.; Wouters, D.J.; Jurczak, M. Endurance/Retention Trade-off on HfO2/Metal Cap 1T1R Bipolar RRAM. IEEE Trans. Electron Dev. 2013, 60, 1114. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, M.-X.; Strachan, J.P.; Miao, F.; Pickett, M.D.; Kelley, R.D.; Medeiros-Ribeiro, G.; Williams, R.S. High switching endurance in TaOx memristive devices. Appl. Phys. Lett. 2010, 97, 232102. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Kim, Y.; Antolinez, F.V.; Ha, J.S.; Lee, S.-S.; Park, J.H. Controllable formation of nanofilaments in resistive memories via tip-enhanced electric fields. Adv. Electron. Mater. 2016, 2, 1600233. [Google Scholar] [CrossRef]
- Qian, K.; Nguyen, V.C.; Chen, T.; Lee, P.S. Amorphous-Si-based resistive switching memories with highly reduced electroforming voltage and enlarged memory window. Adv. Electron. Mater. 2016, 2, 1500370. [Google Scholar] [CrossRef]
- Cho, B.; Song, S.; Ji, Y.; Lee, T. Electrical characterization of organic resistive memory with interfacial oxide layers formed by O2 plasma treatment. Appl. Phys. Lett. 2010, 97, 063305. [Google Scholar] [CrossRef]
- Tan, H.; Liu, G.; Zhu, X.; Yang, H.; Chen, B.; Chen, X.; Shang, J.; Lu, W.D.; Wu, Y.; Li, R.-W. An optoelectronic resistive switching memory with integrated demodulating and arithmetic functions. Adv. Mater. 2015, 27, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Chang, T.-C.; Peng, H.-K.; Tseng, H.-C.; Huang, J.-J.; Yang, J.-B.; Chu, A.-K.; Young, T.-F.; Sze, S.M. Insertion of a Si layer to reduce operation current for resistive random access memory applications. Appl. Phys. Lett. 2013, 102, 252902. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, C.B.; Lee, D.; Lee, S.R.; Chang, M.; Hur, J.H.; Kim, Y.B.; Kim, C.J.; Seo, D.H.; Seo, S.; et al. A fast, high-endurance and scalable non-volatile memory device made from asymmetric Ta2O5−x/TaO2−x bilayer structures. Nat. Mater. 2011, 10, 625–630. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.; Chen, Q.; Xue, W.; Yang, H.; Shang, J.; Chen, B.; Zeng, F.; Song, C.; Pan, F.; et al. Improving unipolar resistive switching uniformity with cone-shaped conducting filaments and its logic-in-memory application. ACS Appl. Mater. Interfaces 2018, 10, 6453–6462. [Google Scholar] [CrossRef]
- Lee, J.; Du, C.; Sun, K.; Kioupakis, E.; Lu, W.D. Tuning ionic transport in memristive devices by graphene with engineered nanopores. ACS Nano 2016, 10, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, J.; Xiao, X.; Liu, Q.; Shao, L.; Chen, D.; Liu, S.; Niu, J.; Zhang, X.; Wang, Y.; et al. Breaking the current-retention dilemma in cation-based resistive switching devices utilizing graphene with controlled defects. Adv. Mater. 2018, 30, 1705193. [Google Scholar] [CrossRef] [PubMed]
- Lubben, M.; Karakolis, P.; Ioannou-Sougleridis, V.; Normand, P.; Dimitrakis, P.; Valov, I. Graphene-modified interface controls transition from VCM to ECM switching modes in Ta/TaOx based memristive devices. Adv. Mater. 2015, 27, 6202–6207. [Google Scholar] [CrossRef] [PubMed]
- Lübben, M.; Wiefels, S.; Waser, R.; Valov, I. Processes and effects of oxygen and moisture in resistively switching TaOx and HfOx. Adv. Electron. Mater. 2018, 4, 1700458. [Google Scholar] [CrossRef]
- Jeong, D.S.; Schroeder, H.; Breuer, U.; Waser, R. Characteristic electroforming behavior in Pt/TiO2/Pt resistive switching cells depending on atmosphere. J. Appl. Phys. 2008, 104, 123716. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Terabe, K.; Hasegawa, T.; Valov, I.; Waser, R.; Aono, M. Effects of moisture on the switching characteristics of oxide-based, gapless-type atomic switches. Adv. Funct. Mater. 2012, 22, 70–77. [Google Scholar] [CrossRef]
- Knorr, N.; Wirtz, R.; Rosselli, S.; Nelles, G. Field-absorbed water induced electrochemical processes in organic thin film junctions. J. Phys. Chem. C 2010, 114, 15791–15796. [Google Scholar] [CrossRef]
- Tappertzhofen, S.; Valov, I.; Tsuruoka, T.; Hasegawa, T.; Waser, R.; Aono, M. Generic relevance of counter charges for cation-based nanoscale resistive switching memories. ACS Nano 2013, 7, 6396–6402. [Google Scholar] [CrossRef]
- Cooper, D.; Baeumer, C.; Bernier, N.; Marchewka, A.; La Torre, C.; Dunin-Borkowski, R.E.; Menzel, S.; Waser, R.; Dittmann, R. Anomalous resistance hysteresis in oxide ReRAM: Oxygen evolution and reincorporation revealed by in situ TEM. Adv. Mater. 2017, 29, 1700212. [Google Scholar] [CrossRef]
- Yang, J.J.; Strukov, D.B.; Stewart, D.R. Memristive devices for computing. Nat. Nanotech. 2013, 8, 13–24. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Y.S.; Chen, P.S.; Gu, P.Y.; Hsu, Y.Y.; Wang, S.M.; Liu, W.H.; Tsai, C.H.; Sheu, S.S.; Chiang, P.C.; et al. Evidence and solution of over-RESET problem for HfOx based resistive memory with sub-ns switching speed and high endurance. In Proceedings of the 2010 International Electron Devices Meeting, San Francisco, CA, USA, 6–8 December 2010; pp. 19.7.1–19.7.4. [Google Scholar]
- Choi, S.; Tan, S.H.; Li, Z.; Kim, Y.; Choi, C.; Chen, P.Y.; Yeon, H.; Yu, S.; Kim, J. SiGe epitaxial memory for neuromorphic computing with reproducible high performance based on engineered dislocations. Nat. Mater. 2018, 17, 335–340. [Google Scholar] [CrossRef]
- Pan, L.; Liu, G.; Li, H.; Meng, S.; Han, L.; Shang, J.; Chen, B.; Platero-Prats, A.E.; Lu, W.; Zou, X.; et al. A resistance-switchable and ferroelectric metal-organic framework. J. Am. Chem. Soc. 2014, 136, 17477–17483. [Google Scholar] [CrossRef]
- Pan, L.; Ji, Z.; Yi, X.; Zhu, X.; Chen, X.; Shang, J.; Liu, G.; Li, R.-W. Metal-organic framework nanofilm for mechanically flexible information storage applications. Adv. Funct. Mater. 2015, 25, 2677–2685. [Google Scholar] [CrossRef]
- Nayak, A.; Unayama, S.; Tai, S.; Tsuruoka, T.; Waser, R.; Aono, M.; Valov, I.; Hasegawa, T. Nanoarchitectonics for controlling the number of dopant atoms in solid electrolyte nanodots. Adv. Mater. 2018, 30, 1703261. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Valov, I.; Tappertzhofen, S.; van den Hurk, J.; Hasegawa, T.; Waser, R.; Aono, M. Redox reactions at Cu,Ag/Ta2O5 interfaces and the effects of Ta2O5 film density on the forming process in atomic switch structures. Adv. Funct. Mater. 2015, 25, 6374–6381. [Google Scholar] [CrossRef]
- Cho, S.; Yun, C.; Tappertzhofen, S.; Kursumovic, A.; Lee, S.; Lu, P.; Jia, Q.; Fan, M.; Jian, J.; Wang, H.; et al. Self-assembled oxide films with tailored nanoscale ionic and electronic channels for controlled resistive switching. Nat. Commun. 2016, 7, 12373. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Liu, G.; Yang, H.; Zhu, X.; Chen, X.; Tan, H.; Hu, B.; Pan, L.; Xue, W.; Li, R.-W. Thermally stable transparent resistive random access memory based on all-oxide heterostructures. Adv. Funct. Mater. 2014, 24, 2171–2179. [Google Scholar] [CrossRef]
- Shang, J.; Xue, W.; Ji, Z.; Liu, G.; Niu, X.; Yi, X.; Pan, L.; Zhan, Q.; Xu, X.H.; Li, R.-W. Highly flexible resistive switching memory based on amorphous-nanocrystalline hafnium oxide films. Nanoscale 2017, 9, 7037–7046. [Google Scholar] [CrossRef]
- Zhu, X.; Li, D.; Liang, X.; Lu, W.D. Ionic modulation and ionic coupling effects in MoS2 device for neuromorphic computing. Nat. Mater. 2018, 18, 141–148. [Google Scholar] [CrossRef]
- Pan, C.; Ji, Y.; Xiao, N.; Hui, F.; Tang, K.; Guo, Y.; Xie, X.; Puglisi, F.M.; Larcher, L.; Miranda, E.; et al. Coexistence of grain-boundaries-assisted bipolar and threshold resistive switching in multilayer hexagonal boron nitride. Adv. Funct. Mater. 2017, 27, 1604811. [Google Scholar] [CrossRef]
- Wang, M.; Cai, S.; Pan, C.; Wang, C.; Lian, X.; Zhuo, Y.; Xu, K.; Cao, T.; Pan, X.; Wang, B.; et al. Robust memristors based on layered two-dimensional materials. Nat. Electron. 2018, 1, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Bessonov, A.A.; Kirikova, M.N.; Petukhov, D.I.; Allen, M.; Ryhanen, T.; Bailey, M.J. Layered memristive and memcapacitive switches for printable electronics. Nat. Mater. 2015, 14, 199–204. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Krylyuk, S.; Milligan, C.A.; Zhu, Y.; Zemlyanov, D.Y.; Bendersky, L.A.; Burton, B.P.; Davydov, A.V.; Appenzeller, J. Electric-field induced structural transition in vertical MoTe2- and Mo1−xWxTe2-based resistive memories. Nat. Mater. 2019, 18, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Wong, H.S.P.; Pop, E.; Ielmini, D.; Strukov, D.; Regan, B.C.; Larcher, L.; Villena, M.A.; Yang, J.J.; Goux, L.; et al. Recommended methods to study resistive switching devices. Adv. Electron. Mater. 2018, 1800143. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, P.; Lv, H.B.; Xu, J.; Song, Y.L.; Fu, X.F.; Tang, T.A.; Chen, B.A.; Lin, Y.Y. Improvement of resistive switching in CuxO using new RESET mode. IEEE Trans. Electron Dev. 2008, 29, 681–683. [Google Scholar] [CrossRef]
- Luo, W.-C.; Liu, J.-C.; Lin, Y.-C.; Lo, C.-L.; Huang, J.-J.; Lin, K.-L.; Hou, T.-H. Statistical model and rapid prediction of RRAM set speed–disturb dilemma. IEEE Trans. Electron Dev. 2013, 60, 3760–3766. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Long, S.; Yu, Z.; Li, Y.; Xu, D.; Lv, H.; Liu, Q.; Miranda, E.; Suñé, J.; et al. A physical model for the statistics of the set switching time of resistive RAM measured with the width-adjusting pulse operation method. IEEE Trans. Electron Dev. 2015, 36, 1303–1305. [Google Scholar] [CrossRef]
- Nili, H.; Adam, G.C.; Hoskins, B.; Prezioso, M.; Kim, J.; Mahmoodi, M.R.; Bayat, F.M.; Kavehei, O.; Strukov, D.B. Hardware-intrinsic security primitives enabled by analogue state and nonlinear conductance variations in integrated memristors. Nat. Electron. 2018, 1, 197–202. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Zhang, R.; Yan, P.; Lin, P.; Li, Y.; Yang, J.J.; Holcomb, D.; Xia, Q. A provable key destruction scheme based on memristive crossbar arrays. Nat. Electron. 2018, 1, 548–554. [Google Scholar] [CrossRef]
- Zidan, M.A.; Jeong, Y.; Lee, J.; Chen, B.; Huang, S.; Kushner, M.J.; Lu, W.D. A general memristor-based partial differential equation solver. Nat. Electron. 2018, 1, 411–420. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.; Xu, X.-H.; Liu, G. Solid-State Electrochemical Process and Performance Optimization of Memristive Materials and Devices. Chemistry 2019, 1, 44-68. https://doi.org/10.3390/chemistry1010005
Xue W, Xu X-H, Liu G. Solid-State Electrochemical Process and Performance Optimization of Memristive Materials and Devices. Chemistry. 2019; 1(1):44-68. https://doi.org/10.3390/chemistry1010005
Chicago/Turabian StyleXue, Wuhong, Xiao-Hong Xu, and Gang Liu. 2019. "Solid-State Electrochemical Process and Performance Optimization of Memristive Materials and Devices" Chemistry 1, no. 1: 44-68. https://doi.org/10.3390/chemistry1010005



