Influence of Nanoconfinement on the Hydrogen Release Processes from Sodium Alanate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NaAlH4/Carbon Composites
2.2. Temperature Controlled Decomposition
3. Results
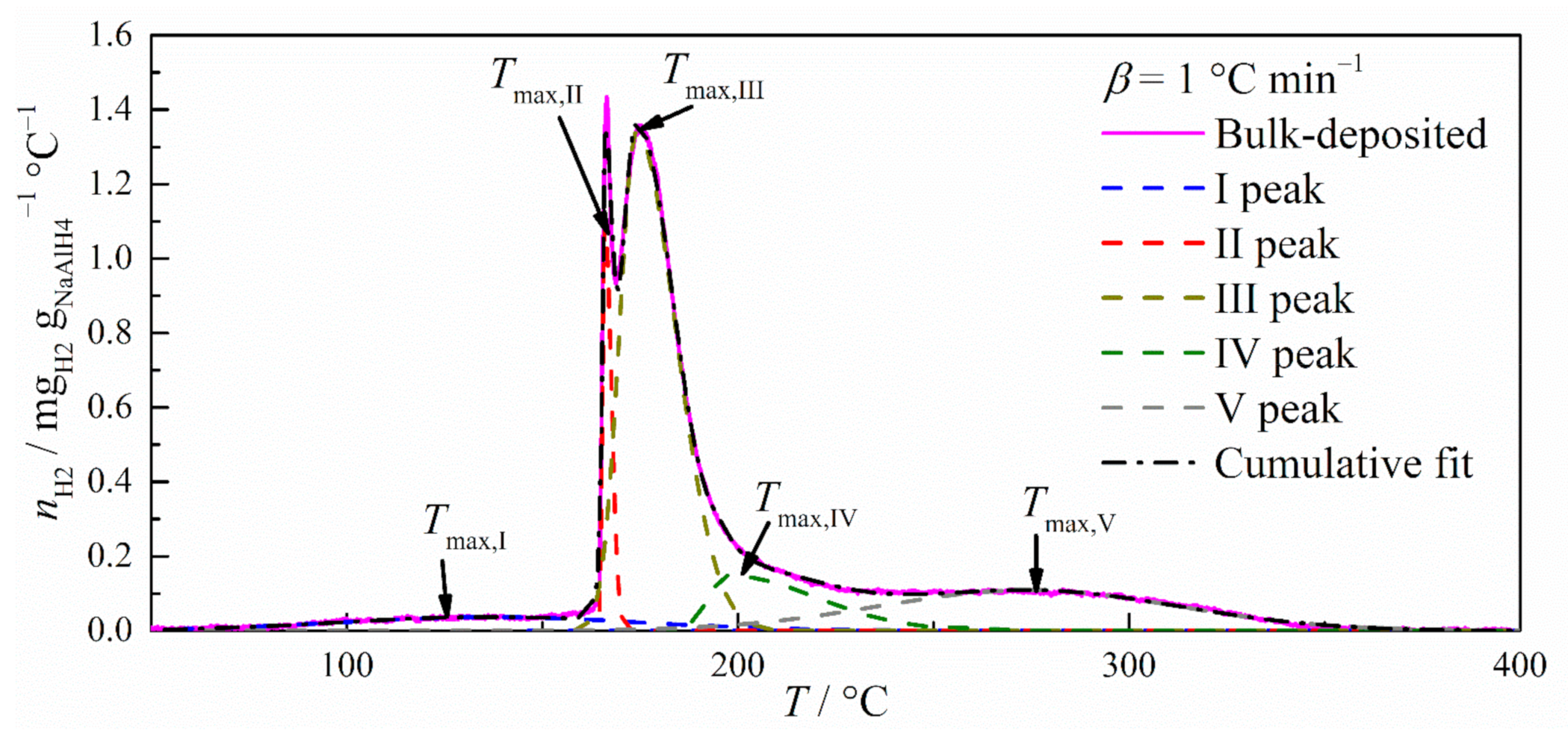
3.1. Hydrogen Release from NaAlH4/Carbon Composites
3.2. Modelling of Decomposition Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Von Helmolt, R.; Eberle, U. Fuel cell vehicles: Status 2007. J. Power Sources 2007, 165, 833–843. [Google Scholar] [CrossRef]
- Peighambardoust, S.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.; Peng, J.-K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment of compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef] [Green Version]
- Makridis, S.S. Hydrogen storage and compression. In Methane and Hydrogen for Energy Storage; Carriveau, R., Ting, D.S.-K., Eds.; Institution of Engineering and Technology: London, UK, 2016; pp. 1–28. [Google Scholar]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Tanaka, I. Decomposition reactions forNaAlH4, Na3AlH6, and NaH: First-principles study. Phys. Rev. B 2005, 71, 024117. [Google Scholar] [CrossRef]
- Gao, J.; Adelhelm, P.; Verkuijlen, M.H.W.; Rongeat, C.; Herrich, M.; Van Bentum, P.J.M.; Gutfleisch, O.; Kentgens, A.P.M.; De Jong, K.P.; De Jongh, P.E. Confinement of NaAlH4 in Nanoporous Carbon: Impact on H2 Release, Reversibility, and Thermodynamics. J. Phys. Chem. C 2010, 114, 4675–4682. [Google Scholar] [CrossRef]
- Baldeé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; De Jong, K.P. Sodium Alanate Nanoparticles—Linking Size to Hydrogen Storage Properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, R.K.; Maharrey, S.; Stavila, V.; Highley, A.; Alam, T.; Majzoub, E.; Allendorf, M. Thermodynamics and kinetics of NaAlH4 nanocluster decomposition. Phys. Chem. Chem. Phys. 2012, 14, 8160–8169. [Google Scholar] [CrossRef]
- Zheng, S.; Fang, F.; Zhou, G.; Chen, G.; Ouyang, L.; Zhu, M.; Sun, D. Hydrogen Storage Properties of Space-Confined NaAlH4Nanoparticles in Ordered Mesoporous Silica. Chem. Mater. 2008, 20, 3954–3958. [Google Scholar] [CrossRef]
- Bendyna, J.K.; Dyjak, S.; Notten, P.H. The influence of ball-milling time on the dehydrogenation properties of the NaAlH4–MgH2 composite. Int. J. Hydrogen Energy 2015, 40, 4200–4206. [Google Scholar] [CrossRef] [Green Version]
- Andrei, C.; Walmsley, J.C.; Brinks, H.; Holmestad, R.; Srinivasan, S.; Jensen, C.; Hauback, B. Electron-microscopy studies of NaAlH4 with TiF3 additive: Hydrogen-cycling effects. Appl. Phys. A 2005, 80, 709–715. [Google Scholar] [CrossRef]
- Palm, R.; Kurig, H.; Aruväli, J.; Lust, E. NaAlH4 /microporous carbon composite materials for reversible hydrogen storage. Microporous Mesoporous Mater. 2018, 264, 8–12. [Google Scholar] [CrossRef]
- Baldé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; De Jong, K.P. Facilitated Hydrogen Storage in NaAlH4 Supported on Carbon Nanofibers. Angew. Chem. 2006, 118, 3581–3583. [Google Scholar] [CrossRef] [Green Version]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloys Compd. 2013, 566, 137–141. [Google Scholar] [CrossRef]
- Shaw, L.L.; Ren, R.; Markmaitree, T.; Osborn, W. Effects of mechanical activation on dehydrogenation of the lithium amide and lithium hydride system. J. Alloys Compd. 2008, 448, 263–271. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li–Mg–N–H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Ismail, M. Study on the hydrogen storage properties and reaction mechanism of NaAlH4–MgH2–LiBH4 ternary-hydride system. Int. J. Hydrogen Energy 2014, 39, 8340–8346. [Google Scholar] [CrossRef]
- Gu, J.; Gao, M.; Pan, H.; Liu, Y.-F.; Li, B.; Yang, Y.; Liang, C.; Fu, H.; Guo, Z.X. Improved hydrogen storage performance of Ca(BH4)2: A synergetic effect of porous morphology and in situ formed TiO2. Energy Environ. Sci. 2012, 6, 847–858. [Google Scholar] [CrossRef]
- Valentoni, A.; Mulas, G.; Enzo, S.; Garroni, S. Remarkable hydrogen storage properties of MgH2 doped with VNbO5. Phys. Chem. Chem. Phys. 2018, 20, 4100–4108. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Ali, N.; Ismail, M. Modification of NaAlH4 properties using catalysts for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2021, 46, 766–782. [Google Scholar] [CrossRef]
- Sandrock, G.; Gross, K.; Thomas, G. Effect of Ti-catalyst content on the reversible hydrogen storage properties of the sodium alanates. J. Alloys Compd. 2002, 339, 299–308. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced Hydrogen Storage Kinetics of LiBH4 in Nanoporous Carbon Scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Fang, F.; Yu, X.Y.; A Zhang, Q.; Ouyang, L.Z.; Zhu, M.; Sun, D. De-/re-hydrogenation features of NaAlH4 confined exclusively in nanopores. Acta Mater. 2011, 59, 1829–1838. [Google Scholar] [CrossRef]


| Material | Most likely H2 Release step | Temperature Range of Tmax/°C | Ea/kJ mol−1 | Additional Comments |
|---|---|---|---|---|
| Bulk | Equation (1) | 167–172 | – | Low amount, most likely only surface layer, no clear dependence of Tmax on β |
| Bulk | Equation (1) | 230–243 | 270 ± 10 | Over a wide T range, from the whole phase |
| Bulk | Equation (2) | 240–264 | 150 ± 60 | Surface layer, low amount |
| Bulk | Equation (2) | 245–279 | 120 ± 20 | From the whole phase |
| Bulk | Equation (3) | 308–337 | 150 ± 30 | From the whole phase |
| Bulk-deposited | Equation (1) | 129–165 | – | Over a wide T range, no clear dependence of Tmax on β. Most likely decomposition of nano- and surface layer. |
| Bulk-deposited | Equation (1) | 165–174 | 400 ± 50 | H2 release over a very narrow T range |
| Bulk-deposited | Equation (1) | 174–190 | 170 ± 20 | From the whole phase |
| Bulk-deposited | Equation (2) | 180–227 | 80 ± 10 | From the whole phase |
| Bulk-deposited | Equation (3) | 263–325 | 90 ± 20 | From the whole phase |
| Nanoconfined | Equation (1) | 51–57 | 70 ± 40 | Only present at high β |
| Nanoconfined | Equations (1) and (2) | 41–94 | 30 ± 5 | Overlap strongly, both over a wide T range |
| Nanoconfined | Equations (1) and (2) | 102–180 | 45 ± 5 | |
| Nanoconfined | Equation (3) | 407–452 | 210 ± 30 | Low amount |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuul, K.; Palm, R. Influence of Nanoconfinement on the Hydrogen Release Processes from Sodium Alanate. Reactions 2021, 2, 1-9. https://doi.org/10.3390/reactions2010001
Tuul K, Palm R. Influence of Nanoconfinement on the Hydrogen Release Processes from Sodium Alanate. Reactions. 2021; 2(1):1-9. https://doi.org/10.3390/reactions2010001
Chicago/Turabian StyleTuul, Kenneth, and Rasmus Palm. 2021. "Influence of Nanoconfinement on the Hydrogen Release Processes from Sodium Alanate" Reactions 2, no. 1: 1-9. https://doi.org/10.3390/reactions2010001





