Assessment of the Acute Effects of Carbonated Beverage Consumption on Symptoms and Objective Markers of Gastric Reflux
Abstract
:1. Introduction
2. Results
3. Discussion
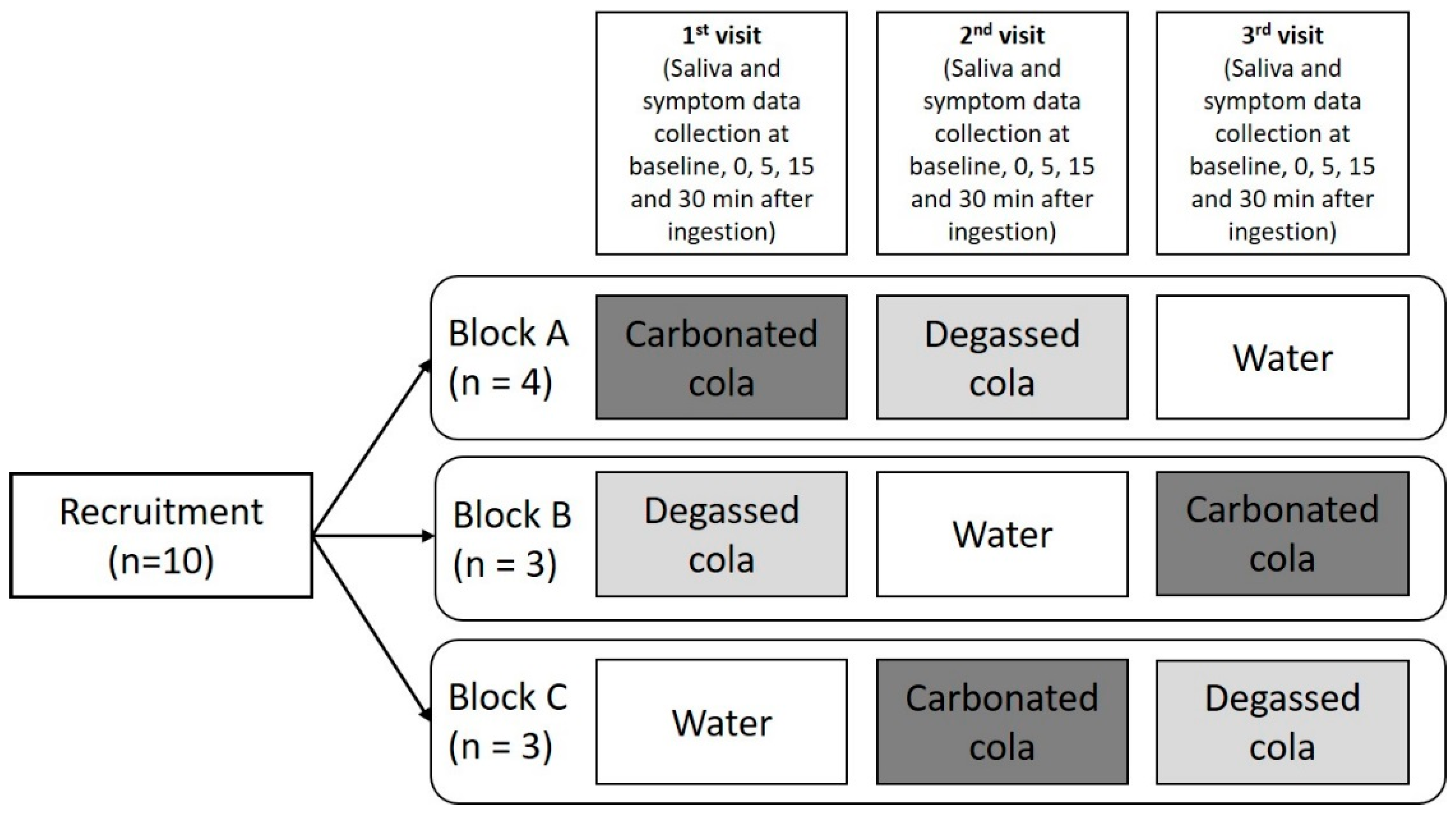
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dent, J.; El-Serag, H.; Wallander, M.A.; Johansson, S. Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2005, 54, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, M.J.; Carrion, A.F. Gastro-Oesophageal Reflux Disease; BMJ Best Practice, BMJ Publishing Group Ltd.: London, UK, 2018. [Google Scholar]
- Broers, C.; Tack, J.; Pauwels, A. Review article: Gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Aliment. Pharmacol. Ther. 2018, 47, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.M.; Allen, J.E.; Blumin, J.H.; Warner, E.A.; Pellegrini, C.A.; Chan, W.W. Respiratory manifestations of gastroesophageal reflux disease. Ann. N. Y. Acad. Sci. 2013, 1300, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G. The lower oesophageal sphincter. Neurogastroenterol. Motil. 2005, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Chang, C.S.; Fock, K.M.; Ke, M.; Park, H.J.; Lam, S.K. Gastro-oesophageal reflux disease in Asia. J. Gastroenterol. Hepatol. 2000, 15, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, G.R.; Talley, N.J.; Fett, S.L.; Zinsmeister, A.R.; Melton, L.J. Risk factors associated with symptoms of gastroesophageal reflux. Am. J. Med. 1999, 106, 642–649. [Google Scholar] [CrossRef]
- Nilsson, M.; Johnsen, R.; Ye, W.; Hveem, K.; Lagergren, J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut 2004, 53, 1730–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloway, R.H.; Hongo, M.; Berger, K.; McCallum, R.W. Gastric distention: A mechanism for postprandial gastroesophageal reflux. Gastroenterology 1985, 89, 779–784. [Google Scholar] [CrossRef]
- Straathof, J.; Ringers, J.; Lamers, C.; Masclee, A. Provocation of transient lower esophageal sphincter relaxations by gastric distension with air. Am. J. Gastroenterol. 2001, 96, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Block, G.; Quesenberry, C.P.; Buffler, P.; Corley, D.A. Dietary guideline adherence for gastroesophageal reflux disease. BMC Gastroenterol. 2014, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Chung, S.J.; Lee, J.H.; Kim, Y.-H.; Chang, D.K.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Rhee, P.-L. Relationship between gastroesophageal reflux symptoms and dietary factors in Korea. J. Neurogastroenterol. Motil. 2011, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Hamoui, N.; Lord, R.V.; Hagen, J.A.; Theisen, J.; DeMeester, T.R.; Crookes, P.F. Response of the lower esophageal sphincter to gastric distention by carbonated beverages. J. Gastrointest. Surg. 2006, 10, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Meshram, M.; Gopan, A.; Ganjewar, V.; Kumar, P.; Bhatia, S.J. Ingestion of a carbonated beverage decreases lower esophageal sphincter pressure and increases frequency of transient lower esophageal sphincter relaxation in normal subjects. Indian J. Gastroenterol. 2012, 31, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Savarese, M.F.; Sarnelli, G.; Vollono, G.; Rocco, A.; Coccoli, P.; Cirillo, C.; Asciore, L.; Nardone, G.; Buyckx, M. Sweetened carbonated drinks do not alter upper digestive tract physiology in healthy subjects. Neurogastroenterol. Motil. 2008, 20, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Gerson, L.; Hershcovici, T.; Stave, C.; Fass, R. Systematic review: The effects of carbonated beverages on gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2010, 31, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N. Test and treat or treat and test in reflux disease? Aliment. Pharmacol. Ther. 2003, 17, 57–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, J.O.; Gabieta-Somnez, S.; Yazaki, E.; Kang, J.-Y.; Woodcock, A.; Dettmar, P.; Mabary, J.; Knowles, C.H.; Sifrim, D. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut 2015, 64, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Lively, M.O.; Johnston, N.; Dettmar, P.W.; Koufman, J.A. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope 2005, 115, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.L.; Johnston, N. Pepsin as a marker of extraesophageal reflux. Ann. Otol. Rhinol. Laryngol. 2010, 119, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Potluri, S.; Chang, A.; MacNeal, R.; Manus, C.; Parkman, H.P.; Bromer, M.; Fisher, R.; Neuman, T.; Kueppers, F.; Miller, L.S. Use of a pepsin assay in saliva and sputum to determine gastroesophageal reflux into the proximal esophagus, oral pharynx and lung. Am. J. Gastroenterol. 2001, 96, S23. [Google Scholar] [CrossRef]
- Na, S.Y.; Kwon, O.E.; Lee, Y.C.; Eun, Y.G. Optimal timing of saliva collection to detect pepsin in patients with laryngopharyngeal reflux. Laryngoscope 2016, 126, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Sereg-Bahar, M.; Jerin, A.; Jansa, R.; Stabuc, B.; Hocevar-Boltezar, I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux—A prospective comparative study. Clin. Otolaryngol. 2015, 40, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.M.; Robertson, A.G.N.; Bredenoord, A.J.; Brownlee, I.A.; Stovold, R.; Brodlie, M.; Forrest, I.; Dark, J.H.; Pearson, J.P.; Ward, C. Aspiration and allograft injury secondary to gastroesophageal reflux occur in the immediate post-lung transplantation period (Prospective Clinical Trial). Ann. Surg. 2013, 258, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Saritas Yuksel, E.; Hong, S.K.S.; Strugala, V.; Slaughter, J.C.; Goutte, M.; Garrett, C.G.; Dettmar, P.W.; Vaezi, M.F. Rapid salivary pepsin test: Blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope 2012, 122, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.; Konečný, Š.; Ďurč, P.; Lačná, J.; Greguš, M.; Foret, F.; Skřičková, J.; Doubková, M.; Kindlová, D.; Pokojová, E.; et al. Evaluation of Important Analytical Parameters of the Peptest Immunoassay that Limit its Use in Diagnosing Gastroesophageal Reflux Disease. J. Clin. Gastroenterol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Peters, J.; Hsieh, C.C.; Tamhankar, A.; Arain, M.; Hagen, J.; DeMeester, S.; DeMeester, T. When are reflux episodes symptomatic? Dis. Esophagus 2007, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, F.; Hu, Z.; Wu, J.; Wang, Z.; Yan, C.; Zhang, C.; Tang, J. The diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: A preliminary study from China. BMC Gastroenterol. 2017, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.; Dettmar, P.W.; Ondrey, F.G.; Nanchal, R.; Lee, S.H.; Bock, J.M. Pepsin: Biomarker, mediator, and therapeutic target for reflux and aspiration. Ann. N. Y. Acad. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hirschowitz, B.I. Gastric acid and pepsin secretion in patients with Barrett’s esophagus and appropriate controls. Dig. Dis. Sci. 1996, 41, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, N.A.; Rhee, P.L.; Mittal, R.K. Distension during gastroesophageal reflux: Effects of acid inhibition and correlation with symptoms. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G469–G474. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, N.; Wells, C.W.; Samuels, T.L.; Blumin, J.H. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann. Otol. Rhinol. Laryngol. 2009, 118, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.M.; Castelo, P.M.; Carpenter, G.H.; Gavião, M.B.D. Masticatory function, taste, and salivary flow in young healthy adults. J. Oral Sci. 2016, 58, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, S.; Hong, G.; Iwasaki, K.; Izumi, M.; Matsuyama, Y.; Chiba, M.; Toda, T.; Kudo, T.A. Gustatory salivation is associated with body mass index, daytime sleepiness, and snoring in healthy young adults. Tohoku J. Exp. Med. 2016, 240, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Li-Hui, W.; Chuan-Quan, L.; Long, Y.; Ru-Liu, L.; Long-Hui, C.; Wei-Wen, C. Gender differences in the saliva of young healthy subjects before and after citric acid stimulation. Clin. Chim. Acta 2016, 460, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.J.; Orlando, R.C. The pathogenesis of heartburn in nonerosive reflux disease: A unifying hypothesis. Gastroenterology 2005, 128, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Tougas, G. Functional heartburn: The stimulus, the pain, and the brain. Gut 2002, 51, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Richter, J. Heartburn, dysphagia, odynophagia, and other esophageal symptoms. In Gastrointestinal Disease, 5th ed.; WE Saunders Company: Philadelphia, PA, USA, 1993. [Google Scholar]
- Feldman, M.; Barnett, C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gastroenterology 1995, 108, 125–131. [Google Scholar] [CrossRef]
- Bredenoord, A.J.; Smout, A.J. Physiologic and pathologic belching. Clin. Gastroenterol. Hepatol. 2007, 5, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Barham, C.; Gotley, D.; Mills, A.; Alderson, D. Precipitating causes of acid reflux episodes in ambulant patients with gastro-oesophageal reflux disease. Gut 1995, 36, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Sifrim, D.; Castell, D.; Dent, J.; Kahrilas, P. Gastro-oesophageal reflux monitoring: Review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004, 53, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Clinical Excellence. Dyspepsia and Gastrooesophageal Reflux Disease: Investigation and Management of Dyspepsia, Symptoms Suggestive of Gastro-Oesophageal Reflux Disease, or Both (CG184). Available online: https://www.nice.org.uk/guidance/cg184 (accessed on 12 July 2018).
- Ploutz-Snyder, L.; Foley, J.; Ploutz-Snyder, R.; Kanaley, J.; Sagendorf, K.; Meyer, R. Gastric gas and fluid emptying assessed by magnetic resonance imaging. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Postma, G.N.; Halum, S.L. Laryngeal and pharyngeal complications of gastroesophageal reflux disease. GI Motil. Online 2006. [Google Scholar] [CrossRef]
- Decalmer, S.; Stovold, R.; Houghton, L.A.; Pearson, J.; Ward, C.; Kelsall, A.; Jones, H.; McGuinness, K.; Woodcock, A.; Smith, J.A. Chronic cough: Relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest 2012, 142, 958–964. [Google Scholar] [CrossRef] [PubMed]
 Carbonated cola;
Carbonated cola;  Degassed cola;
Degassed cola;  Water) over after ingestion compared to baseline (pre-ingestion). No statistically significant difference was observed at a p-value of 0.05.
Water) over after ingestion compared to baseline (pre-ingestion). No statistically significant difference was observed at a p-value of 0.05.
 Carbonated cola;
Carbonated cola;  Degassed cola;
Degassed cola;  Water) over after ingestion compared to baseline (pre-ingestion). No statistically significant difference was observed at a p-value of 0.05.
Water) over after ingestion compared to baseline (pre-ingestion). No statistically significant difference was observed at a p-value of 0.05.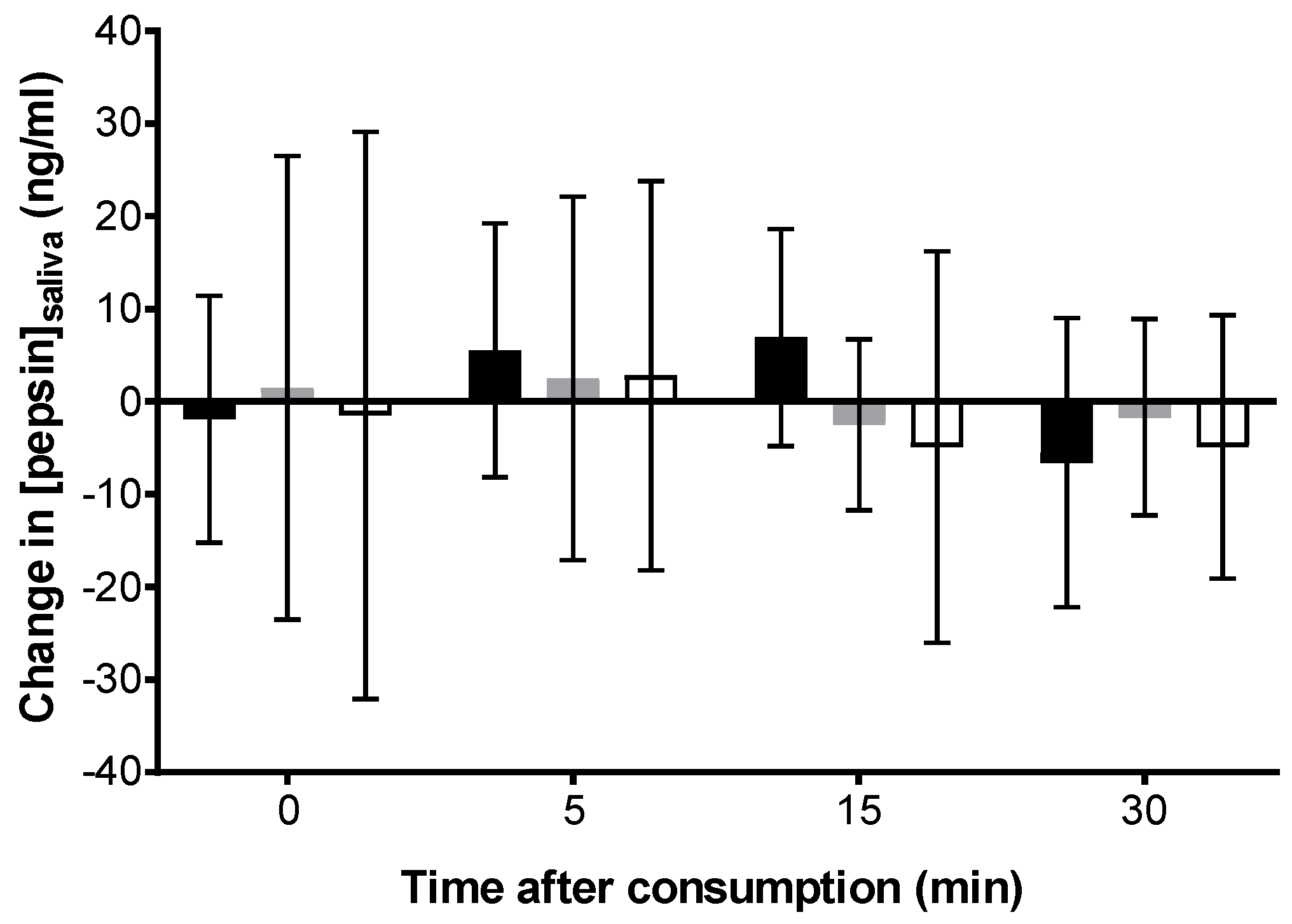
 Carbonated cola;
Carbonated cola;  Degassed cola;
Degassed cola;  Water). * Carbonated cola value statistically different from water (p < 0.05) at this time-point. Comparisons to degassed cola are presented in-text.
Water). * Carbonated cola value statistically different from water (p < 0.05) at this time-point. Comparisons to degassed cola are presented in-text.
 Carbonated cola;
Carbonated cola;  Degassed cola;
Degassed cola;  Water). * Carbonated cola value statistically different from water (p < 0.05) at this time-point. Comparisons to degassed cola are presented in-text.
Water). * Carbonated cola value statistically different from water (p < 0.05) at this time-point. Comparisons to degassed cola are presented in-text.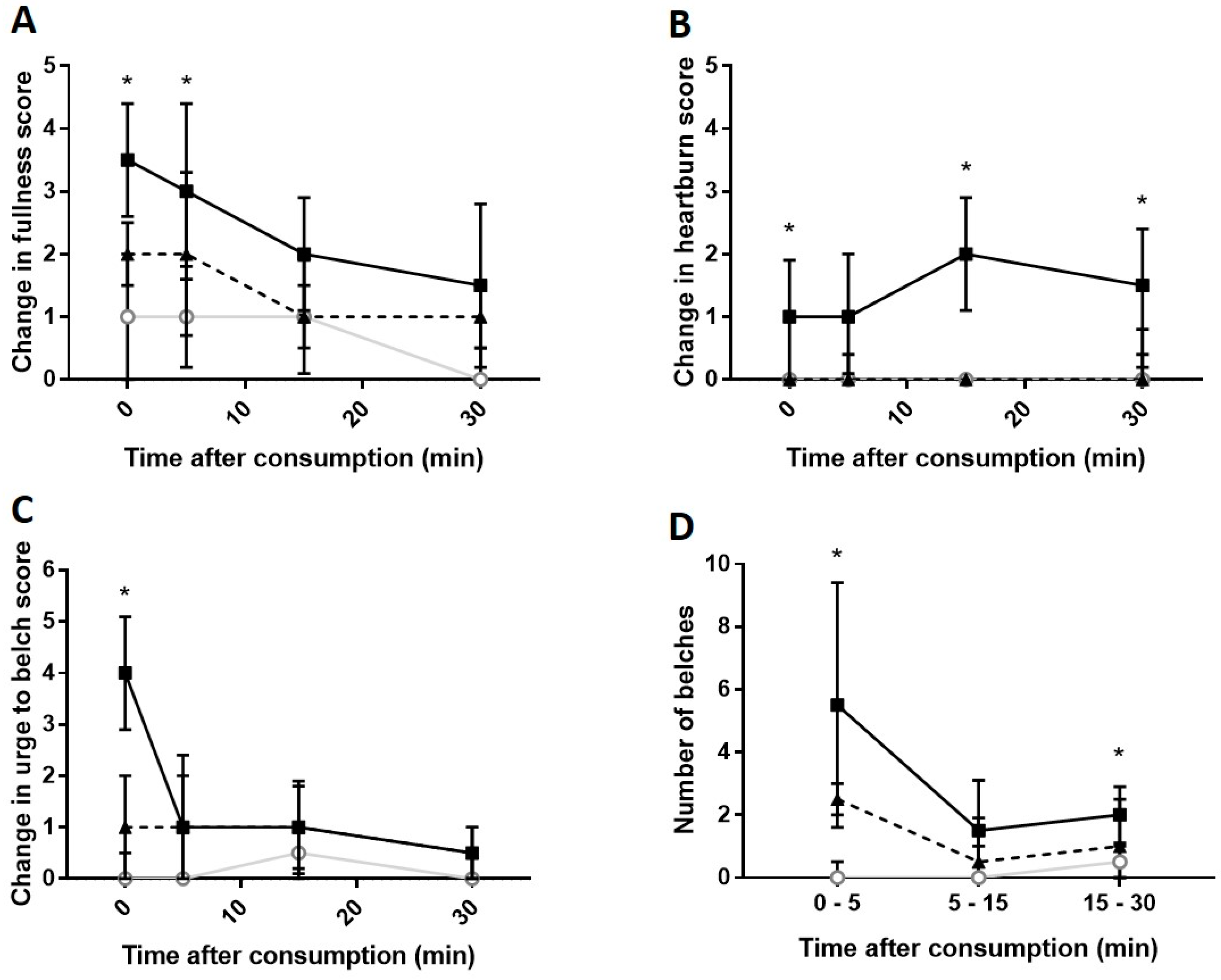
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.X.B.; Brownlee, I.A. Assessment of the Acute Effects of Carbonated Beverage Consumption on Symptoms and Objective Markers of Gastric Reflux. Gastrointest. Disord. 2019, 1, 30-38. https://doi.org/10.3390/gidisord1010004
Lim SXB, Brownlee IA. Assessment of the Acute Effects of Carbonated Beverage Consumption on Symptoms and Objective Markers of Gastric Reflux. Gastrointestinal Disorders. 2019; 1(1):30-38. https://doi.org/10.3390/gidisord1010004
Chicago/Turabian StyleLim, Shi Xiang Brandon, and Iain A. Brownlee. 2019. "Assessment of the Acute Effects of Carbonated Beverage Consumption on Symptoms and Objective Markers of Gastric Reflux" Gastrointestinal Disorders 1, no. 1: 30-38. https://doi.org/10.3390/gidisord1010004




