Intrabolus Pressure Has Better Correlation Than Eosinophilia with Dysphagia Severity in Fibrostenotic Eosinophilic Esophagitis: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Baseline Demographics and PPI Use
2.2. Endoscopic Severity and Esophageal Caliber
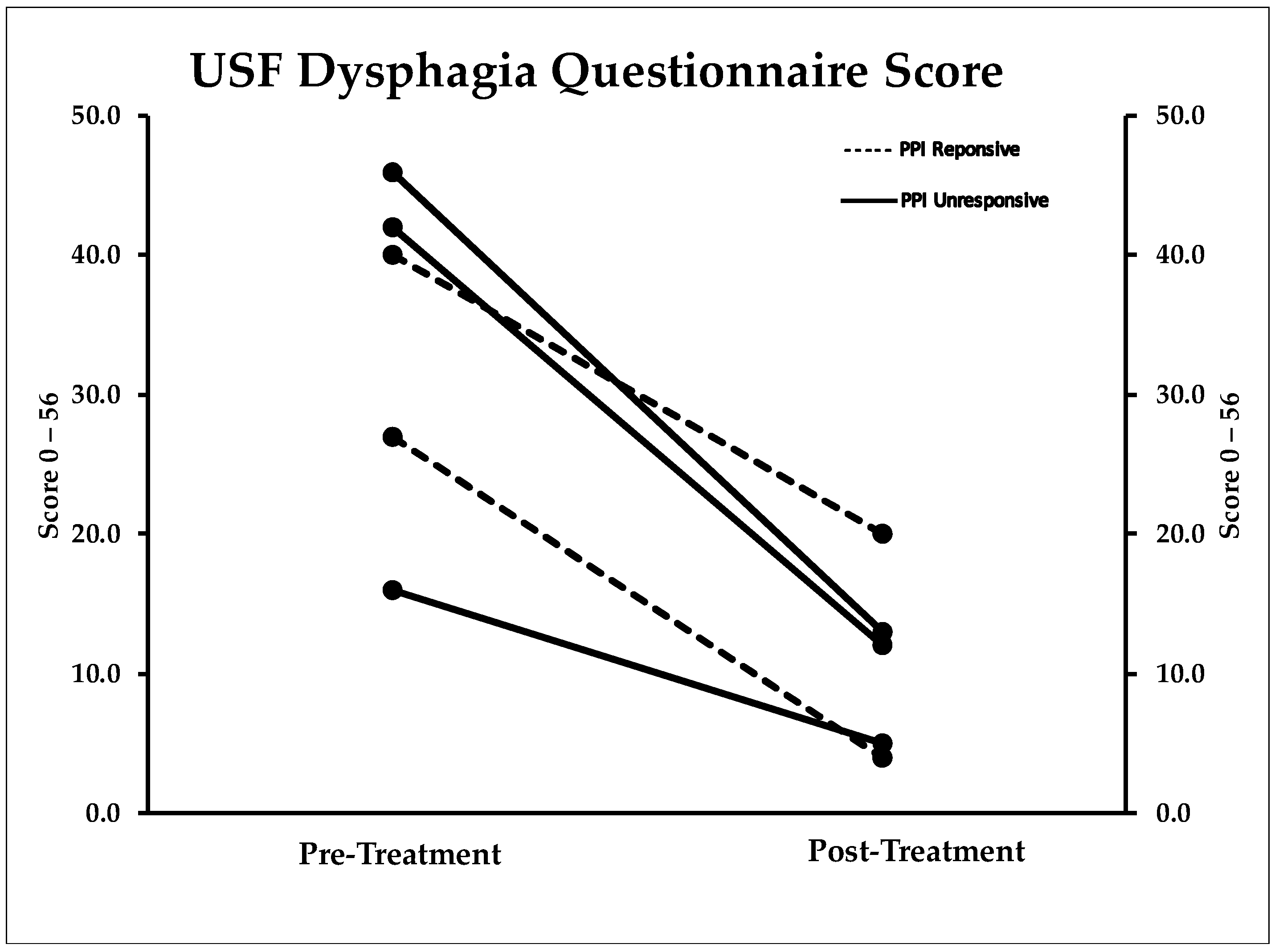
2.3. Dysphagia Questionnaire
2.4. Mucosal Eosinophilia
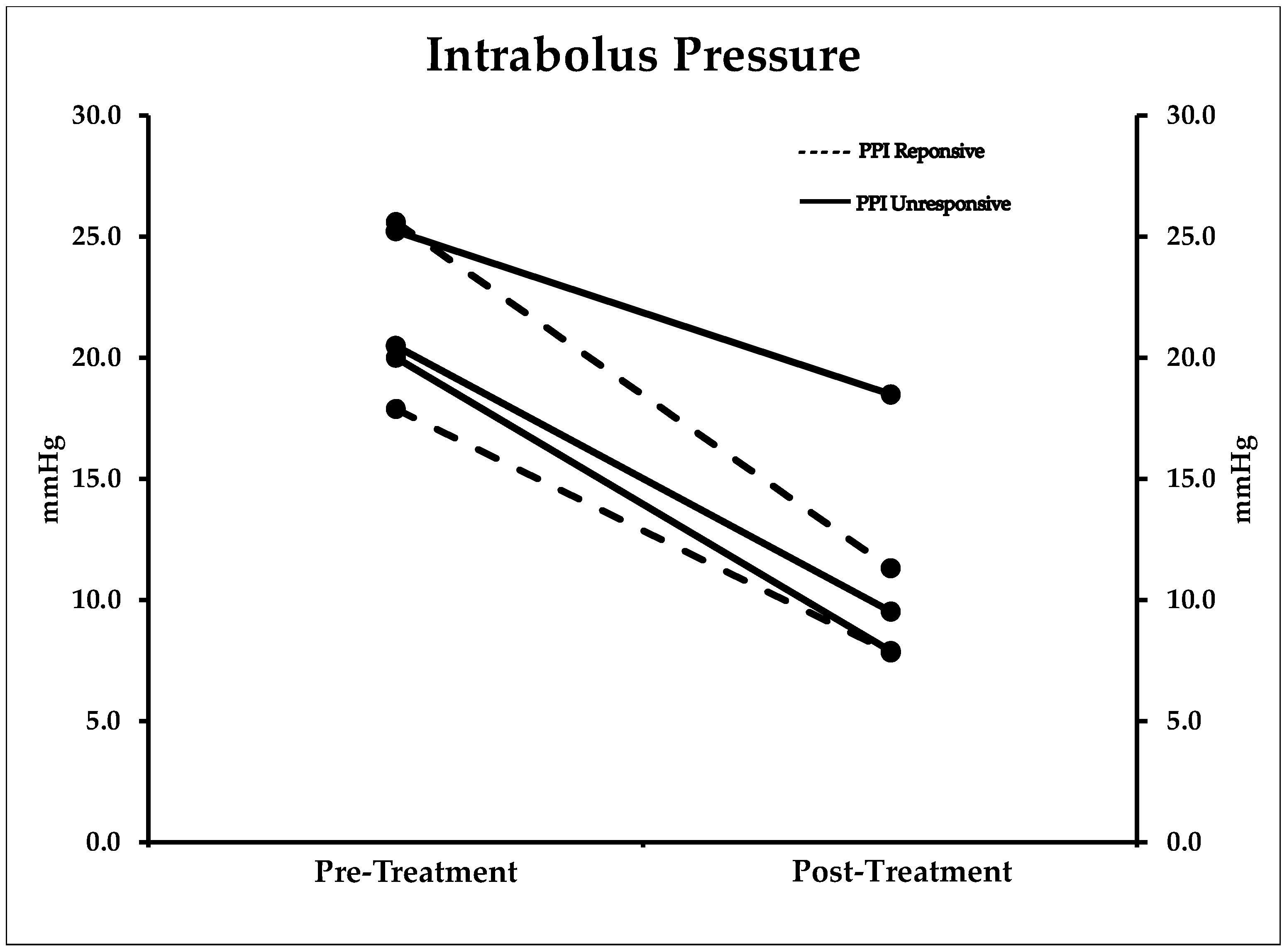
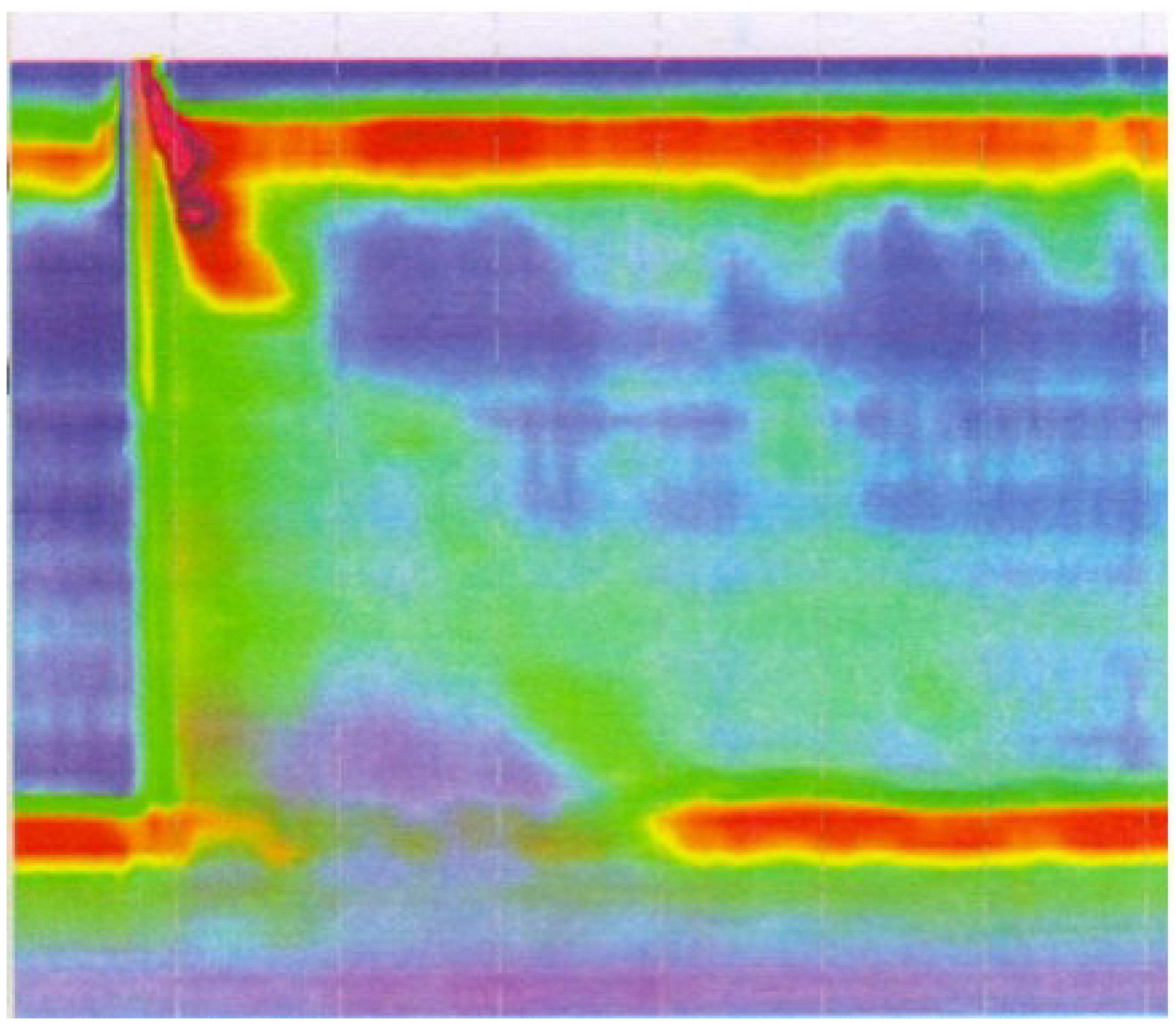
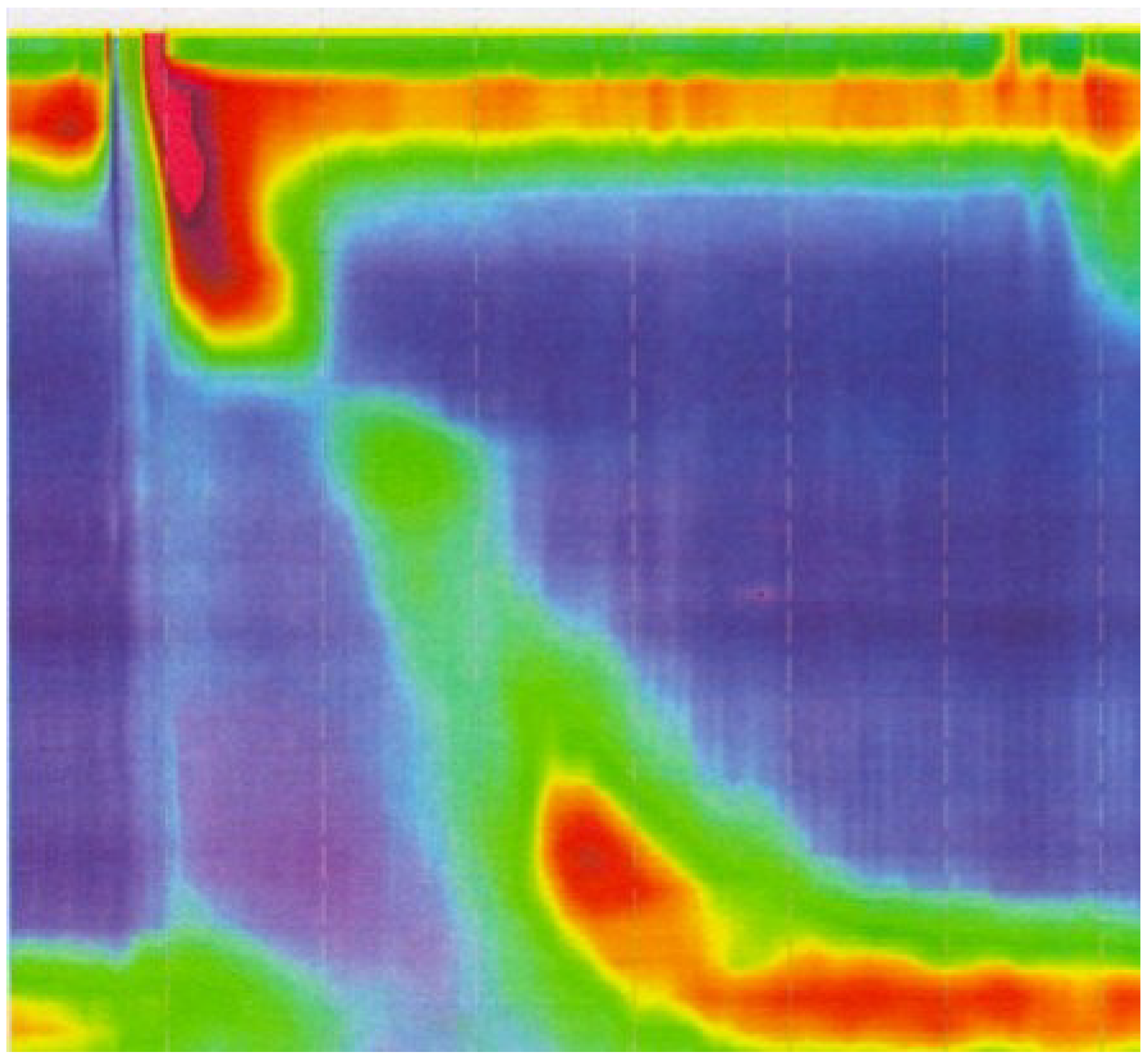
2.5. Manometry
2.6. Clinical Vignette
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. University of South Florida (USF) Dysphagia Questionnaire
| Sore throat | Cough | Tooth/oral pain while eating |
| Fever | Nasal congestion |
- □
- Yes (if yes, please circle answer above)
- □
- No
- □
- Very frequently (nearly every meal)
- □
- Frequently (3–5 days/week)
- □
- Sometimes (1–2 days/week)
- □
- Rarely (1–2 meals/14-days)
- □
- Never
- □
- I worry about choking with every meal
- □
- Trouble swallowing has caused me to avoid certain foods
- □
- I am able to eat most foods, but have to modify their consistency
- □
- I am able to eat most foods, and rarely experience trouble swallowing
- □
- I am able to eat food without any difficulty swallowing or change in diet
- (A)
- Indicate how difficult it would be to swallow each of the following food items without changing its consistency (e.g., using food processor, crushing, liquefying, etc.)
- No difficulty: I Can eat this food without any trouble swallowing
- Minimal difficulty: I experience some difficulty when swallowing this food
- Moderate difficulty: It takes some time to completely swallow this food
- Severe difficulty: I experience extreme difficulty when swallowing this food. I worry about choking with this food.
- (B)
- Indicate how frequently you would experience difficulty in swallowing each of the food items without changing its consistency (e.g., using food processor, crushing, liquefying, etc.)
- Never have any problems: 0 episodes of swallowing difficulties with this food
- Rarely: <2 episodes/week of swallowing difficulty with this food
- Frequently: 3–5 episodes/week of swallowing difficulty with this food
- Always: Experience difficulty swallowing this food every time
 |  |  |  |  |  |  |  | |
|---|---|---|---|---|---|---|---|---|
| Steak (beef, poultry) | Pudding | Ground Beef | Steamed Rice | Apple | White Bread | Oatmeal | French Fries | |
| Level of difficulty swallowing this food |
|
|
|
|
|
|
|
|
| Frequency of difficulty in swallowing this food |
|
|
|
|
|
|
|
|
References
- Patel, R.V.; Hirano, I. New developments in the diagnosis, therapy, and monitoring of eosinophilic esophagitis. Curr. Treat. Options Gastroenterol. 2018, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Spichtin, H.P.; Bernoulli, R.; Loosli, J.; Vogtlin, J. [idiopathic eosinophilic esophagitis: A frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz. Med. Wochenschr. 1994, 124, 1419–1429. [Google Scholar] [PubMed]
- Mansoor, E.; Cooper, G.S. The 2010–2015 prevalence of eosinophilic esophagitis in the USA: A population-based study. Dig. Dis. Sci. 2016, 61, 2928–2934. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Hirano, I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2017, 154, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2013, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Prados-Manzano, R.; Gonzalez-Cordero, P.L. The role of proton pump inhibitor therapy in the management of eosinophilic esophagitis. Expert Rev. Clin. Immunol. 2016, 12, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J.; Cheng, E.; Schoepfer, A.; Al-Haddad, S.; Bellizzi, A.M.; Dawson, H.; El-Zimaity, H.; Guindi, M.; Penagini, R.; Safrooneva, E.; et al. Eosinophilic esophagitis: Current perspectives from diagnosis to management. Ann. N. Y. Acad. Sci. 2016, 1380, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Lipowska, A.M.; Kavitt, R.T. Current diagnostic and treatment strategies for eosinophilic esophagitis. Gastroenterol. Hepatol. 2017, 13, 527–535. [Google Scholar]
- Richter, J.E. Endoscopic treatment of eosinophilic esophagitis. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J.; Molina-Infante, J.; Lucendo, A.J.; Cantrell, S.E.; Tmanova, L.; Douglas, K.M. Systematic review with meta-analysis: Endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017, 46, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Gentile, N.; Katzka, D.; Ravi, K.; Trenkner, S.; Enders, F.; Killian, J.; Kryzer, L.; Talley, N.J.; Alexander, J. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment. Pharmacol. Ther. 2014, 40, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Quader, F.; Reddy, C.; Patel, A.; Gyawali, C.P. Elevated intrabolus pressure identifies obstructive processes when integrated relaxation pressure is normal on esophageal high-resolution manometry. Am. J. Phys. Gastrointest. Liver Phys. 2017, 313, G73–G79. [Google Scholar] [CrossRef] [PubMed]
- Colizzo, J.M.; Clayton, S.B.; Richter, J.E. Intrabolus pressure on high-resolution manometry distinguishes fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. Dis. Esophagus 2016, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Holloway, R.H.; Smout, A.J.; Omari, T.I. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol. Motil. 2013, 25, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.; Pandolfino, J.E.; Kahrilas, P.J.; Patankar, N.A. Simulation studies of the role of esophageal mucosa in bolus transport. Biomech. Model. Mechanobiol. 2017, 16, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portmann, S.; Simon, H.U.; Straumann, A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013, 145, 1230–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipka, S.; Kumar, A.; Richter, J.E. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J. Clin. Gastroenterol. 2016, 50, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.A.; Jung, K.W.; Arora, A.S.; Enders, F.; Katzka, D.A.; Kephardt, G.M.; Kita, H.; Kryzer, L.A.; Romero, Y.; Smyrk, T.C.; et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2012, 10, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Richter, J.E.; Kelsen, S.; Thomas, R. Esophageal dilation: Simple and effective treatment for adults with eosinophilic esophagitis and esophageal rings and narrowing. Dis. Esophagus 2010, 23, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E. Esophageal dilation in eosinophilic esophagitis. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Moole, H.; Jacob, K.; Duvvuri, A.; Moole, V.; Dharmapuri, S.; Boddireddy, R.; Uppu, A.; Puli, S.R. Role of endoscopic esophageal dilation in managing eosinophilic esophagitis: A systematic review and meta-analysis. Medicine 2017, 96, e5877. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Aigner, T.; Neureiter, D.; Stolte, M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: A retrospective and comparative study on pathological biopsy. J. Clin. Pathol. 2006, 59, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Furuta, G.T.; Masterson, J.C. Deeper than the epithelium: Role of matrix and fibroblasts in pediatric and adult eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Persad, R.; Huynh, H.Q.; Hao, L.; Ha, J.R.; Sergi, C.; Srivastava, R.; Persad, S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFKappaB pathway. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Pandolfino, J.E.; Boeckxstaens, G.E. Functional lumen imaging probe for the management of esophageal disorders: Expert review from the clinical practice updates committee of the aga institute. Clin. Gastroenterol. Hepatol. 2017, 15, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Nicodeme, F.; Hirano, I.; Chen, J.; Robinson, K.; Lin, Z.; Xiao, Y.; Gonsalves, N.; Kwasny, M.J.; Kahrilas, P.J.; Pandolfino, J.E. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2013, 11, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Straumann, A.; Panczak, R.; Coslovsky, M.; Kuehni, C.E.; Maurer, E.; Haas, N.A.; Romero, Y.; Hirano, I.; Alexander, J.A.; et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014, 147, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age (Year) | Sex | Race | Symptom Duration (Years) | PPI Therapy | PPI Response | Baseline EREFS | FS Location(s) | Dilation Sessions (n) |
|---|---|---|---|---|---|---|---|---|
| 41 | M | Caucasian | 26 | Dexlansoprazole 60 mg Daily | Responsive | 4 | Distal | 2 |
| 53 | M | Caucasian | 36 | Lansoprazole 30 mg BID | Responsive | 5 | Distal | 3 |
| 32 | M | Caucasian | 7 | Esomeprazole 40 mg BID | Unresponsive | 6 | Mid/Distal | 3 |
| 27 | F | Caucasian | 13 | Esomeprazole 40 mg BID | Unresponsive | 7 | Distal | 4 |
| 36 | M | Caucasian | 20 | Dexlansoprazole 60 mg Daily | Unresponsive | 3 | Distal | 2 |
| Baseline * (SD) | Post-Therapy * (SD) | p-Value (CI) † | |
|---|---|---|---|
| Mean eos/hpf proximal esophagus | 57.8 (58.8) | 26.6 (50.2) | 0.07 (−5.3–67.7) |
| Mean eos/hpf distal esophagus | 78.2 (58.6) | 71.6 (107.9) | 0.83 (−76.3–89.5) |
| Esophageal Diameter (mm) | 10.8 (1.9) | 17.2 (2.6) | 0.001 (−8.4–−4.3) * |
| USF Dysphagia Score ‡ | 34.2 (12.4) | 10.8 (6.5) | 0.004 (12.6–34.2) * |
| EREFS Score § | 5 (1.5) | 2.4 | 0.007 (1.1–4.0) * |
| IRP mmHg | 8.26 (2.7) | 6.9 (4.2) | 0.42 (−2.7–5.2) |
| DCI mmHg·cm·s | 2319.3 (1403.6) | 2187.9 (1542.5) | 0.70 (−762.4–1025.1) |
| IBP mmHg | 21.8 (3.3) | 11.0 (4.4) | 0.001 (7.3–14.3) * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colizzo, J.; Clayton, S.; Kumar, A.; Richter, J. Intrabolus Pressure Has Better Correlation Than Eosinophilia with Dysphagia Severity in Fibrostenotic Eosinophilic Esophagitis: A Pilot Study. Gastrointest. Disord. 2019, 1, 3-14. https://doi.org/10.3390/gidisord1010002
Colizzo J, Clayton S, Kumar A, Richter J. Intrabolus Pressure Has Better Correlation Than Eosinophilia with Dysphagia Severity in Fibrostenotic Eosinophilic Esophagitis: A Pilot Study. Gastrointestinal Disorders. 2019; 1(1):3-14. https://doi.org/10.3390/gidisord1010002
Chicago/Turabian StyleColizzo, Jason, Steven Clayton, Ambuj Kumar, and Joel Richter. 2019. "Intrabolus Pressure Has Better Correlation Than Eosinophilia with Dysphagia Severity in Fibrostenotic Eosinophilic Esophagitis: A Pilot Study" Gastrointestinal Disorders 1, no. 1: 3-14. https://doi.org/10.3390/gidisord1010002





