Changes in the Spatial Structure of Synchronization Connections in EEG During Nocturnal Sleep Apnea
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. The Wavelet Bicoherence (WB)
4.2.2. Assessment of Spatial WB Connections
4.2.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EEG | Electroencephalography |
| OSA | Obstructive Sleep Apnea |
| PSG | Polisomnography |
| WB | Wavelet Bicoherence |
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.C.; Crecelius, A.R.; Larson, D.G.; Luckasen, G.J.; Dinenno, F.A. Integrative Cardiovascular Physiology and Pathophysiology: Impaired peripheral vasodilation during graded systemic hypoxia in healthy older adults: Role of the sympathoadrenal system. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H832. [Google Scholar] [CrossRef]
- Yasuma, F.; Noda, A.; Hayano, J. Blood Pressure Regulation and Hypertension in Obstructive Sleep Apnea Syndrome: A Historical Perspective. Intern. Med. 2024, 63, 3131–3136. [Google Scholar] [CrossRef]
- Ryskova, L.; Pospisilova, K.; Vavra, J.; Wolf, T.; Dvorak, A.; Vitek, L.; Polak, J. Contribution of glucose and glutamine to hypoxia-induced lipid synthesis decreases, while contribution of acetate increases, during 3T3-L1 differentiation. Sci. Rep. 2024, 14, 28193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, P.Y.; Su, M.C.; Chen, Y.L.; Chang, Y.T.; Chin, C.H.; Lin, I.C.; Chen, Y.M.; Wang, T.Y.; Lin, Y.Y.; et al. Long non-coding RNA FKSG29 regulates oxidative stress and endothelial dysfunction in obstructive sleep apnea. Mol. Cell. Biochem. 2024, 479, 2723–2740. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, C.; Yu, Y. The correlation between rapid eye movement sleep and nocturnal hypertension in patients with obstructive sleep apnea: A retrospective study. Medicine 2024, 103, e40740. [Google Scholar] [CrossRef]
- Donkor, N.; Gardner, J.J.; Bradshaw, J.L.; Cunningham, R.L.; Inman, D.M. Ocular inflammation and oxidative stress as a result of chronic intermittent hypoxia: A rat model of sleep apnea. Antioxidants 2024, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; Marco, T.D.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 28, e010440. [Google Scholar] [CrossRef]
- Knowlden, A.P.; Winchester, L.J.; MacDonald, H.V.; Geyer, J.D.; Higginbotham, J.C. Associations Among Cardiometabolic Risk Factors, Sleep Duration, and Obstructive Sleep Apnea in a Southeastern US Rural Community: Cross-Sectional Analysis from the SLUMBRx-PONS Study. JMIR Form. Res. 2024, 8, e54792. [Google Scholar] [CrossRef]
- Henning, R.J.; Anderson, W.M. Sleep apnea is a common and dangerous cardiovascular risk factor. Curr. Probl. Cardiol. 2024, 50, 102838. [Google Scholar] [CrossRef]
- Lam, A.; D’Rozario, A.L.; Palmer, J.R.; McKinnon, A.; Dalton, M.R.; Espinosa, N.; Mowszowski, L.; Phillips, C.L.; Grunstein, R.R.; Naismith, S.L. Hypoxemia during rapid eye movement sleep mediates memory impairment in older adults at risk for dementia via CA1 hippocampal volume loss. Eur. J. Neurol. 2024, 31, e16491. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, N.; Baril, A.A.; Osorio, R.S.; Kaminska, M.; Carrier, J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am. J. Respir. Crit. Care Med. 2019, 199, 142–148. [Google Scholar] [CrossRef]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef]
- May, A.M.; Mehra, R. Obstructive sleep apnea: Role of intermittent hypoxia and inflammation. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2014; Volume 35, pp. 531–544. [Google Scholar]
- Spector, A.R.; Farrer, T.J. Neurocognitive and Neuropsychological Effects of OSA. In Management of Obstructive Sleep Apnea: An Evidence-Based, Multidisciplinary Textbook; Kim, K.B., Movahed, R., Malhotra, R.K., Stanley, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 45–56. [Google Scholar] [CrossRef]
- Sircu, V.; Colesnic, S.I.; Covantsev, S.; Corlateanu, O.; Sukhotko, A.; Popovici, C.; Corlateanu, A. The burden of comorbidities in obstructive sleep apnea and the pathophysiologic mechanisms and effects of CPAP. Clocks Sleep 2023, 5, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Krishnan Paramasivan, V.; Manimaran, V. Bidirectional Interactions Between Obstructive Sleep Apnea and Chronic Kidney Disease—A Review. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 5066–5070. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Ayappa, I.; Ayas, N.; Collop, N.; Kirsch, D.; Mcardle, N.; Mehra, R.; Pack, A.I.; Punjabi, N.; White, D.P.; et al. Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep 2021, 44, zsab030. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, J.; Chen, J.; Wu, D.; Luo, K.; Shen, G.; Fang, Y.; Zhang, W.; Huang, G.; Su, X.; et al. Brain morphology and functional connectivity alterations in patients with severe obstructive sleep apnea. Sleep Med. 2023, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Selskii, A.; Drapkina, O.; Agaltsov, M.; Posnenkova, O.; Simonyan, M.; Zhuravlev, M.; Runnova, A. Adaptation of recurrence plot method to study a polysomnography: Changes in EEG activity in obstructive sleep apnea syndrome. Eur. Phys. J. Spec. Top. 2023, 232, 703–714. [Google Scholar] [CrossRef]
- Runnova, A.; Zhuravlev, M.; Orlova, A.; Agaltsov, M.; Drapkina, O.; Kiselev, A. Structural abnormalities of brain electrical activity during night sleep in patients with obstructive apnoea syndrome. Eur. Phys. J. Spec. Top. 2024, 233, 531–542. [Google Scholar] [CrossRef]
- Liu, C.R.; Yang, C.Y.; Sharma, D.; Chen, T.H.; Huang, X.Q.; Hung, T.M.; Kuo, T.B.; Jou, J.H. Associations between Sleep Duration and Autonomic Nervous System Regulation in Patients with Probable Alzheimer’s Disease: A Cross-Sectional Pilot Study. Clocks Sleep 2024, 6, 533–545. [Google Scholar] [CrossRef]
- Yu, Y. Links between Sleep Apnoea and Insomnia in a British Cohort. Clocks Sleep 2023, 5, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Kumar, R.; Woo, M.A.; Valladares, E.M.; Yan-Go, F.L.; Harper, R.M. Brain structural changes in obstructive sleep apnea. Sleep 2008, 31, 967–977. [Google Scholar]
- Tummala, S.; Roy, B.; Park, B.; Kang, D.W.; Woo, M.A.; Harper, R.M.; Kumar, R. Associations between brain white matter integrity and disease severity in obstructive sleep apnea. J. Neurosci. Res. 2016, 94, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Tae, W.S.; Lee, M.J.; Kang, J.W.; Park, H.S.; Lee, J.Y.; Suh, M.; Hong, S.B. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 2010, 33, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.; Jackson, M.; Twigg, G.; Ghiassi, R.; McRobbie, D.; Quest, R.; Pardoe, H.; Pell, G.; Abbott, D.; Rochford, P.; et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 2010, 65, 908–914. [Google Scholar] [CrossRef]
- Torelli, F.; Moscufo, N.; Garreffa, G.; Placidi, F.; Romigi, A.; Zannino, S.; Bozzali, M.; Fasano, F.; Giulietti, G.; Djonlagic, I.; et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2011, 54, 787–793. [Google Scholar] [CrossRef]
- Fox, N.C.; Schott, J.M. Imaging cerebral atrophy: Normal ageing to Alzheimer’s disease. Lancet 2004, 363, 392–394. [Google Scholar] [CrossRef]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef]
- Salat, D.H.; Greve, D.N.; Pacheco, J.L.; Quinn, B.T.; Helmer, K.G.; Buckner, R.L.; Fischl, B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage 2009, 44, 1247–1258. [Google Scholar] [CrossRef]
- Weihs, A.; Frenzel, S.; Wittfeld, K.; Obst, A.; Stubbe, B.; Habes, M.; Szentkirályi, A.; Berger, K.; Fietze, I.; Penzel, T.; et al. Associations between sleep apnea and advanced brain aging in a large-scale population study. Sleep 2021, 44, zsaa204. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Erus, G.; Toledo, J.B.; Zhang, T.; Bryan, N.; Launer, L.J.; Rosseel, Y.; Janowitz, D.; Doshi, J.; Van der Auwera, S.; et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016, 139, 1164–1179. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Janowitz, D.; Erus, G.; Toledo, J.; Resnick, S.; Doshi, J.; Van der Auwera, S.; Wittfeld, K.; Hegenscheid, K.; Hosten, N.; et al. Advanced brain aging: Relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl. Psychiatry 2016, 6, e775. [Google Scholar] [CrossRef]
- Meissner, A. Hypertension and the brain: A risk factor for more than heart disease. Cerebrovasc. Dis. 2016, 42, 255–262. [Google Scholar] [CrossRef]
- Yoon, S.; Cho, H.; Kim, J.; Lee, D.W.; Kim, G.H.; Hong, Y.S.; Moon, S.; Park, S.; Lee, S.; Lee, S.; et al. Brain changes in overweight/obese and normal-weight adults with type 2 diabetes mellitus. Diabetologia 2017, 60, 1207–1217. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Duan, W.; Li, H.; Kong, L.; Shu, Y.; Li, P.; Li, K.; Xie, W.; Zeng, Y.; et al. Abnormal functional connectivity of hippocampal subdivisions in obstructive sleep apnea: A resting-state functional magnetic resonance imaging study. Front. Neurosci. 2022, 16, 850940. [Google Scholar] [CrossRef]
- Pal, A.; Ogren, J.A.; Aguila, A.P.; Aysola, R.; Kumar, R.; Henderson, L.A.; Harper, R.M.; Macey, P.M. Functional organization of the insula in men and women with obstructive sleep apnea during Valsalva. Sleep 2021, 44, zsaa124. [Google Scholar] [CrossRef]
- Joo, E.Y.; Jeon, S.; Kim, S.T.; Lee, J.M.; Hong, S.B. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep 2013, 36, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.H.; Tsai, Y.H.; Chen, C.F.; Lin, Y.C.; Yang, C.T.; Tsai, Y.H.; Yang, C.Y. Mapping gray matter reductions in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Sleep 2014, 37, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 2011, 183, 1419–1426. [Google Scholar] [CrossRef]
- Lundblad, L.C.; Fatouleh, R.H.; Hammam, E.; McKenzie, D.K.; Macefield, V.G.; Henderson, L.A. Brainstem changes associated with increased muscle sympathetic drive in obstructive sleep apnoea. Neuroimage 2014, 103, 258–266. [Google Scholar] [CrossRef]
- Fatouleh, R.H.; Hammam, E.; Lundblad, L.C.; Macey, P.M.; McKenzie, D.K.; Henderson, L.A.; Macefield, V.G. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin. 2014, 6, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Baril, A.A.; Gagnon, K.; Brayet, P.; Montplaisir, J.; De Beaumont, L.; Carrier, J.; Lafond, C.; L’Heureux, F.; Gagnon, J.F.; Gosselin, N. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am. J. Respir. Crit. Care Med. 2017, 195, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Haris, N.; Kumar, R.; Thomas, M.A.; Woo, M.A.; Harper, R.M. Obstructive sleep apnea and cortical thickness in females and males. PLoS ONE 2018, 13, e0193854. [Google Scholar] [CrossRef]
- Huynh, N.T.; Prilipko, O.; Kushida, C.A.; Guilleminault, C. Volumetric brain morphometry changes in patients with obstructive sleep apnea syndrome: Effects of CPAP treatment and literature review. Front. Neurol. 2014, 5, 58. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Mapa, M.S. Functional connectivity and cognitive decline: A review of rs-fMRI, EEG, MEG, and graph theory approaches in aging and dementia. Explor. Med. 2024, 5, 797–821. [Google Scholar] [CrossRef]
- Blum, L.; Hofmann, A.; Rosenbaum, D.; Elshehabi, M.; Suenkel, U.; Fallgatter, A.J.; Ehlis, A.C.; Metzger, F.G. Effects of aging on functional connectivity in a neurodegenerative risk cohort: Resting state versus task measurement using near-infrared spectroscopy. Sci. Rep. 2022, 12, 11262. [Google Scholar] [CrossRef]
- Katayama, O.; Stern, Y.; Habeck, C.; Coors, A.; Lee, S.; Harada, K.; Makino, K.; Tomida, K.; Morikawa, M.; Yamaguchi, R.; et al. Detection of neurophysiological markers of cognitive reserve: An EEG study. Front. Aging Neurosci. 2024, 16, 1401818. [Google Scholar] [CrossRef]
- Albert, M.S. Changes in cognition. Neurobiol. Aging 2011, 32, S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Cieri, F.; Chiacchiaretta, P.; Cera, N.; Lauriola, M.; Di Giannantonio, M.; Tartaro, A.; Ferretti, A. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: Comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018, 12, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Köhler, S.; Sistermans, N.; Koene, T.; Pijnenburg, Y.; van der Flier, W.; Scheltens, P.; Aalten, P.; Verhey, F.; Visser, P.J.; et al. The trajectory of cognitive decline in the pre-dementia phase in memory clinic visitors: Findings from the 4C-MCI study. Psychol. Med. 2015, 45, 1509–1519. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, D.; Li, M.; Zhang, X.; Rong, W.; Wang, P.; Li, L.; He, S.; Xu, Y.; et al. EEG Power Spectral Density in NREM Sleep is Associated with the Degree of Hypoxia in Patients with Obstructive Sleep Apnea. Nat. Sci. Sleep 2023, 15, 979–992. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, M.; Niu, P.; Li, S.; Hu, Z.; Shi, C.; Li, Y. Phase-Amplitude Coupling in Theta and Beta Bands: A Potential Electrophysiological Marker for Obstructive Sleep Apnea. Nat. Sci. Sleep 2024, 16, 1469–1482. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Zhang, T.; Wen, J.; Pang, F.; Luo, Y. Characterization of the abnormal cortical effective connectivity in patients with sleep apnea hypopnea syndrome during sleep. Comput. Methods Programs Biomed. 2021, 204, 106060. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Y.; Lian, J.; Pang, F.; Wen, J.; Luo, Y. Regional characterization of functional connectivity in patients with sleep apnea hypopnea syndrome during sleep. Physiol. Meas. 2021, 42, 075004. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, M.; Agaltsov, M.; Kiselev, A.; Simonyan, M.; Novikov, M.; Selskii, A.; Ukolov, R.; Drapkina, O.; Orlova, A.; Penzel, T.; et al. Compensatory mechanisms of reduced interhemispheric EEG connectivity during sleep in patients with apnea. Sci. Rep. 2023, 13, 8444. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, W.; Chen, X.; Wan, X.; Lei, X. Aberrant awake spontaneous brain activity in obstructive sleep apnea: A review focused on resting-state EEG and resting-state fMRI. Front. Neurol. 2020, 11, 768. [Google Scholar] [CrossRef]
- Fortin, M.; Lina, J.M.; Desjardins, M.È.; Gagnon, K.; Baril, A.A.; Carrier, J.; Gosselin, N. Waking EEG functional connectivity in middle-aged and older adults with obstructive sleep apnea. Sleep Med. 2020, 75, 88–95. [Google Scholar] [CrossRef]
- Rial, R.; González, J.; Gené, L.; Akaârir, M.; Esteban, S.; Gamundí, A.; Barceló, P.; Nicolau, C. Asymmetric sleep in apneic human patients. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 304, R232–R237. [Google Scholar] [CrossRef]
- Rial, R.V.; Barbal, F.; Cañellas, F.; Gamundi, A.; Akaârir, M.; Nicolau, M.C. Human sleep apneas and animal diving reflexes: The comparative link. Sleep Breath. 2000, 4, 0031–0042. [Google Scholar] [CrossRef]
- Boccaletti, S.; Kurths, J.; Osipov, G.; Valladares, D.; Zhou, C. The synchronization of chaotic systems. Phys. Rep. 2002, 366, 1–101. [Google Scholar] [CrossRef]
- Hramov, A.E.; Koronovskii, A.A. An approach to chaotic synchronization. Chaos Interdiscip. J. Nonlinear Sci. 2004, 14, 603–610. [Google Scholar] [CrossRef]
- Schiecke, K.; Wacker, M.; Benninger, F.; Feucht, M.; Leistritz, L.; Witte, H. Matching pursuit-based time-variant bispectral analysis and its application to biomedical signals. IEEE Trans. Biomed. Eng. 2015, 62, 1937–1948. [Google Scholar] [CrossRef]
- Sheppard, L.; Vuksanović, V.; McClintock, P.; Stefanovska, A. Oscillatory dynamics of vasoconstriction and vasodilation identified by time-localized phase coherence. Phys. Med. Biol. 2011, 56, 3583. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Zhuravlev, M.O.; Runnova, A.E.; Protasov, P.; Maksimenko, V.A.; Frolov, N.S.; Pisarchik, A.N.; Hramov, A.E. Betweenness centrality in multiplex brain network during mental task evaluation. Phys. Rev. E 2018, 98, 062413. [Google Scholar] [CrossRef]
- Hramov, A.E.; Koronovskii, A.A.; Makarov, V.A.; Pavlov, A.N.; Sitnikova, E. Wavelets in Neuroscience; Springer Series in Synergetics; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Peters, R. Ageing and the brain: This article is part of a series on ageing edited by Professor Chris Bulpitt. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ulyanov, V.; Zhuravlev, M.; Kiselev, A.; Musatov, V.; Musatova, T.; Akimova, N.; Parsamyan, R.; Runnova, A. EEG markers of attention sustainability detected in neuropsychological testing in different age groups. Eur. Phys. J. Spec. Top. 2024, 233, 519–530. [Google Scholar] [CrossRef]
- Karavaev, A.; Kiselev, A.; Runnova, A.; Zhuravlev, M.; Borovkova, E.; Prokhorov, M.; Ponomarenko, V.; Pchelintseva, S.; Efremova, T.Y.; Koronovskii, A.; et al. Synchronization of infra-slow oscillations of brain potentials with respiration. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 081102. [Google Scholar] [CrossRef]
- Zhuravlev, M.; Egorov, E.; Moskalenko, O.; Zhuravleva, Y.; Akimova, N.; Kiselev, A.; Drapkina, O.; Runnova, A. Wavelet analysis of intermittent dynamics in nocturnal electrocardiography and electroencephalography data. Chaos Interdiscip. J. Nonlinear Sci. 2024, 34, 081105. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.; Wilkinson, L.S. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006, 1126, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, J.; Jagannath, M. Brain connectivity analysis based classification of obstructive sleep apnea using electroencephalogram signals. Sci. Rep. 2024, 14, 5561. [Google Scholar] [CrossRef]
- Luo, Y.g.; Wang, D.; Liu, K.; Weng, J.; Guan, Y.; Chan, K.C.; Chu, W.C.; Shi, L. Brain structure network analysis in patients with obstructive sleep apnea. PLoS ONE 2015, 10, e0139055. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Fukunaga, M.; de Zwart, J.A.; van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum. Brain Mapp. 2008, 29, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villar, G.; Daneault, V.; Martineau-Dussault, M.È.; Baril, A.A.; Gagnon, K.; Lafond, C.; Gilbert, D.; Thompson, C.; Marchi, N.A.; Lina, J.M.; et al. Altered resting-state functional connectivity patterns in late middle-aged and older adults with obstructive sleep apnea. Front. Neurol. 2023, 14, 1215882. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Li, H.; Shu, Y.; Li, K.; Xie, W.; Zeng, Y.; Huang, L.; Zeng, L.; Liu, X.; Peng, D. Functional connectivity changes in the insular subregions of patients with obstructive sleep apnea after 6 months of continuous positive airway pressure treatment. Neural Plast. 2023, 2023, 5598047. [Google Scholar] [CrossRef]
- D’Rozario, A.L.; Kao, C.H.; Phillips, C.L.; Mullins, A.E.; Memarian, N.; Yee, B.J.; Duffy, S.L.; Cho, G.; Wong, K.K.; Kremerskothen, K.; et al. Region-specific changes in brain activity and memory after continuous positive airway pressure therapy in obstructive sleep apnea: A pilot high-density electroencephalography study. Sleep 2023, 46, zsad255. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kaneko, N.; Usuda, N.; Kato, T.; Khoo, H.M.; Fukuma, R.; Oshino, S.; Tani, N.; Kishima, H.; Yanagisawa, T.; et al. M/EEG source localization for both subcortical and cortical sources using a convolutional neural network with a realistic head conductivity model. APL Bioeng. 2024, 8, 046104. [Google Scholar] [CrossRef]
- Le Van Quyen, M.; Foucher, J.; Lachaux, J.P.; Rodriguez, E.; Lutz, A.; Martinerie, J.; Varela, F.J. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. Neurosci. Methods 2001, 111, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Hramov, A.E.; Koronovskii, A.A.; Sitnikova, Y.; Makarov, V.A.; Ovchinnikov, A.A. Wavelet analysis in neurodynamics. Physics-Uspekhi 2012, 55, 845–875. [Google Scholar] [CrossRef]
- Sitnikova, E.; Hramov, A.; Grubov, V.; Koronovsky, A. Time-frequency characteristics and dynamics of sleep spindles in WAG/Rij rats with absence epilepsy. Brain Res. 2014, 1543, 290–299. [Google Scholar] [CrossRef]
- Bandrivskyy, A.; Bernjak, A.; McClintock, P.; Stefanovska, A. Wavelet phase coherence analysis: Application to skin temperature and blood flow. Cardiovasc. Eng. Int. J. 2004, 4, 89–93. [Google Scholar] [CrossRef]

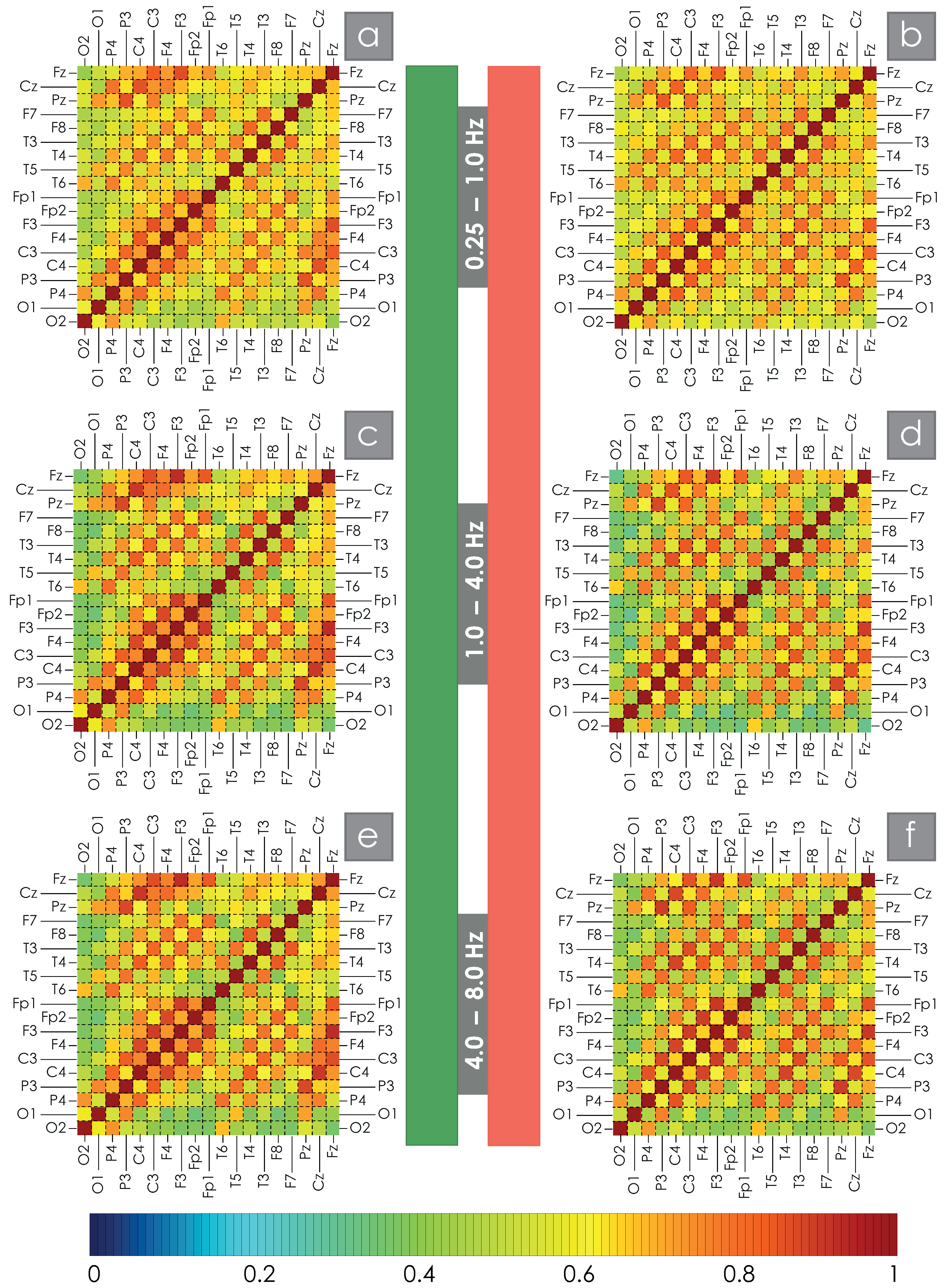
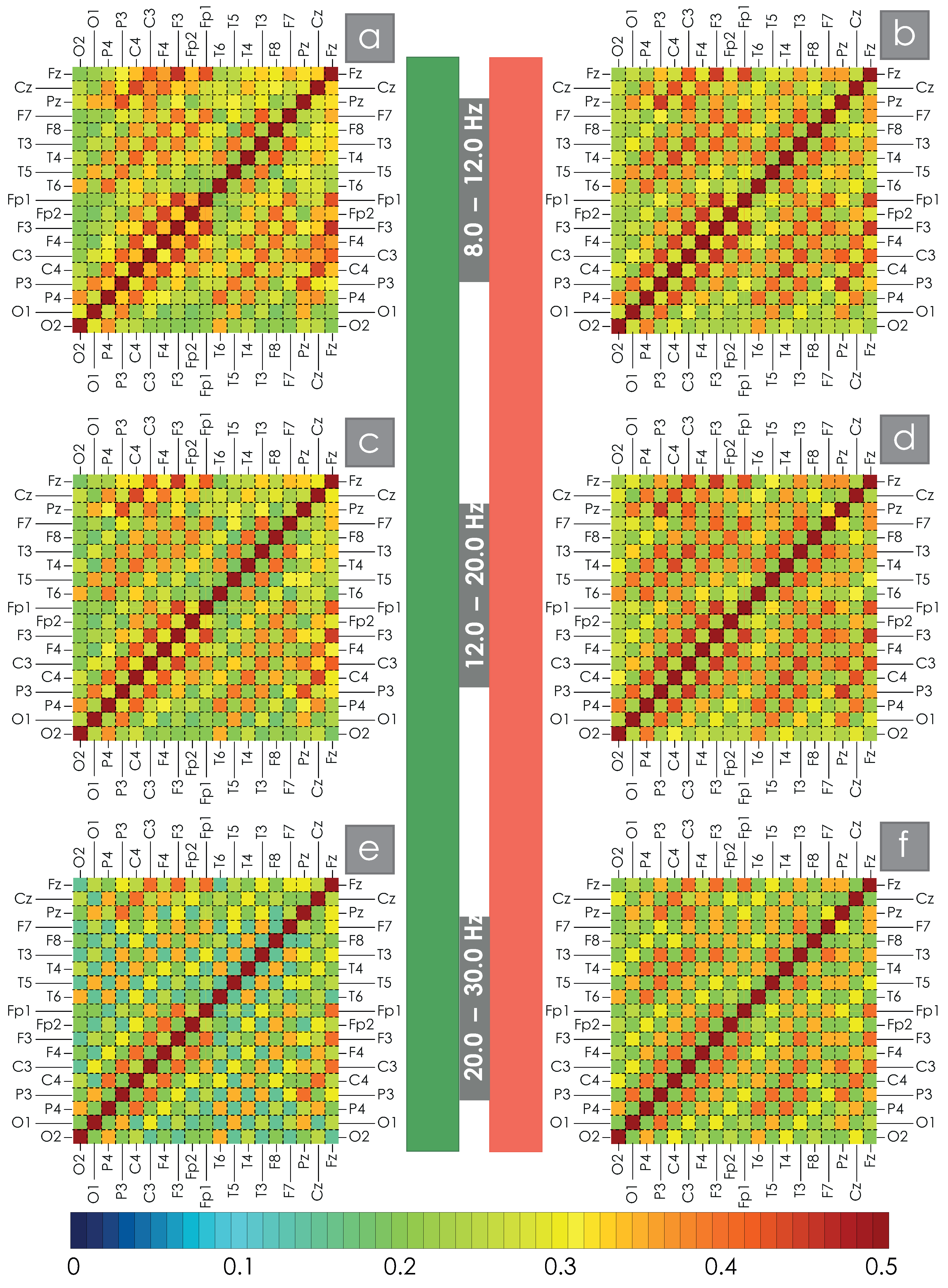
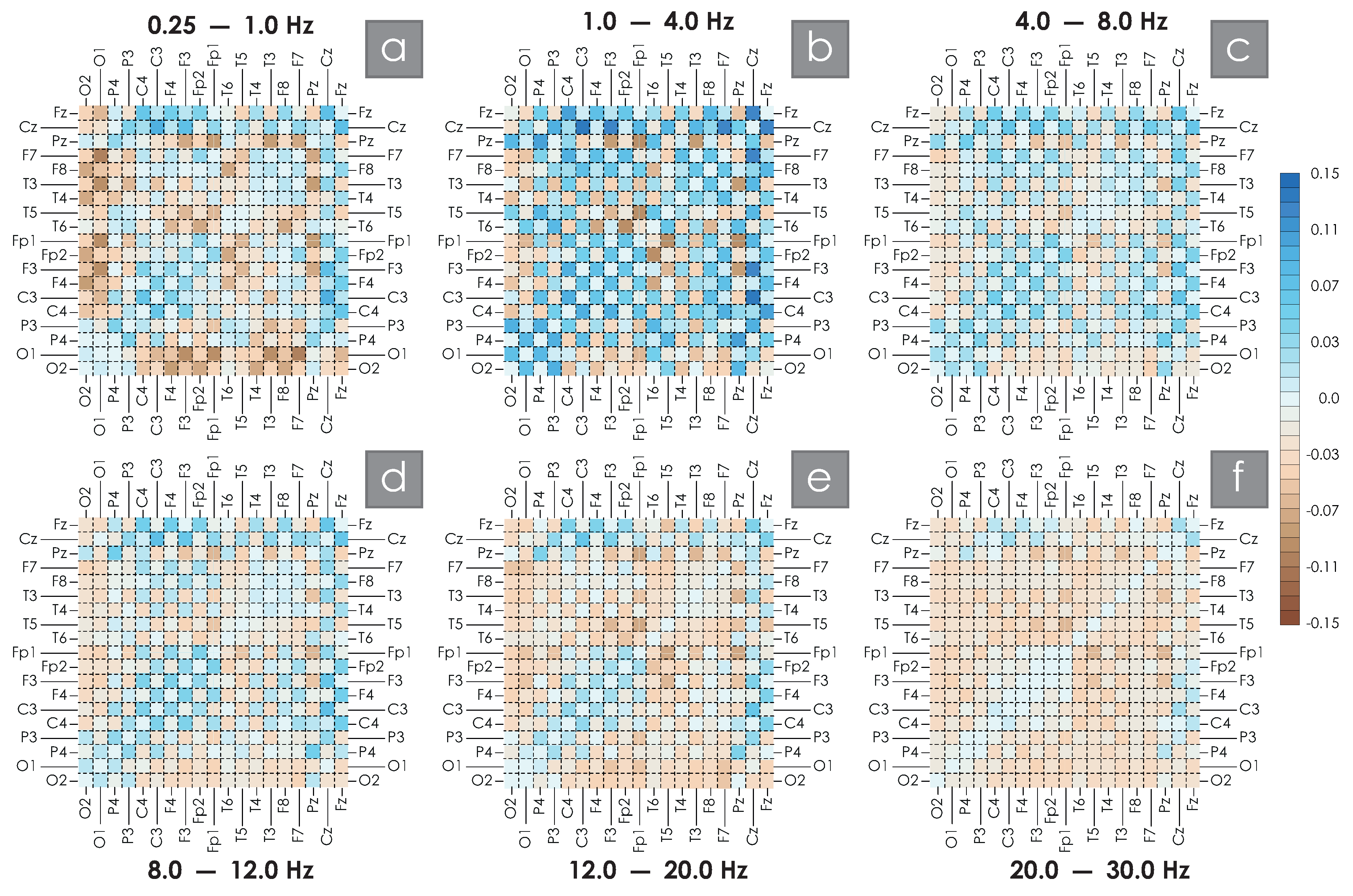
| C3C4 | C3Cz | F3Cz | F3F4 | F7Cz | F7F8 | O1O2 | P3P4 | |
|---|---|---|---|---|---|---|---|---|
| −0.73 | −0.62 | −0.55 | −0.57 | −0.31 | −0.45 | −0.32 | −0.63 | |
| −0.81 | −0.66 | −0.65 | −0.70 | −0.62 | −0.62 | −0.62 | −0.77 | |
| −0.71 | −0.65 | −0.66 | −0.65 | −0.63 | -0.53 | −0.53 | −0.65 | |
| −0.71 | −0.68 | −0.67 | −0.65 | −0.57 | −0.37 | −0.36 | −0.63 | |
| −0.70 | −0.68 | −0.64 | −0.60 | −0.33 | 0.11 | −0.03 | −0.57 | |
| −0.53 | −0.57 | −0.50 | −0.41 | −0.06 | 0.34 | 0.14 | 0.21 |
| BMI | 21 | 32.9 | Hypertension | 1 (4%) | 21 (53.8%) |
| SBP | 110 | 130 | Chronic coronary disease | 0 | 6 (15.4%) |
| DBP | 70 | 85 | Myocardial infarction | 0 | 3 (7.7%) |
| HR | 76 | 80 | Atrial fibrillation | 0 | 1 (2.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravlev, M.; Kiselev, A.; Orlova, A.; Egorov, E.; Drapkina, O.; Simonyan, M.; Drozhdeva, E.; Penzel, T.; Runnova, A. Changes in the Spatial Structure of Synchronization Connections in EEG During Nocturnal Sleep Apnea. Clocks & Sleep 2025, 7, 1. https://doi.org/10.3390/clockssleep7010001
Zhuravlev M, Kiselev A, Orlova A, Egorov E, Drapkina O, Simonyan M, Drozhdeva E, Penzel T, Runnova A. Changes in the Spatial Structure of Synchronization Connections in EEG During Nocturnal Sleep Apnea. Clocks & Sleep. 2025; 7(1):1. https://doi.org/10.3390/clockssleep7010001
Chicago/Turabian StyleZhuravlev, Maxim, Anton Kiselev, Anna Orlova, Evgeniy Egorov, Oxana Drapkina, Margarita Simonyan, Evgenia Drozhdeva, Thomas Penzel, and Anastasiya Runnova. 2025. "Changes in the Spatial Structure of Synchronization Connections in EEG During Nocturnal Sleep Apnea" Clocks & Sleep 7, no. 1: 1. https://doi.org/10.3390/clockssleep7010001
APA StyleZhuravlev, M., Kiselev, A., Orlova, A., Egorov, E., Drapkina, O., Simonyan, M., Drozhdeva, E., Penzel, T., & Runnova, A. (2025). Changes in the Spatial Structure of Synchronization Connections in EEG During Nocturnal Sleep Apnea. Clocks & Sleep, 7(1), 1. https://doi.org/10.3390/clockssleep7010001









