Jazz Colors: Pigment Identification in the Gouaches Used by Henri Matisse
Abstract
:1. Introduction
2. Methodology and Analytical Techniques
2.1. Infrared Spectroscopies (µ-FTIR and r-FTIR)
2.2. Raman Spectroscopies (Confocal Raman, p-Raman, SERS)
2.3. Reflectance Spectrophotometry (RS)
2.4. X-ray Fluorescence (p-XRF)
2.5. Microfadeometry (MFT)
2.6. PCA Analysis
3. Results and Discussion
3.1. Pigment Identification
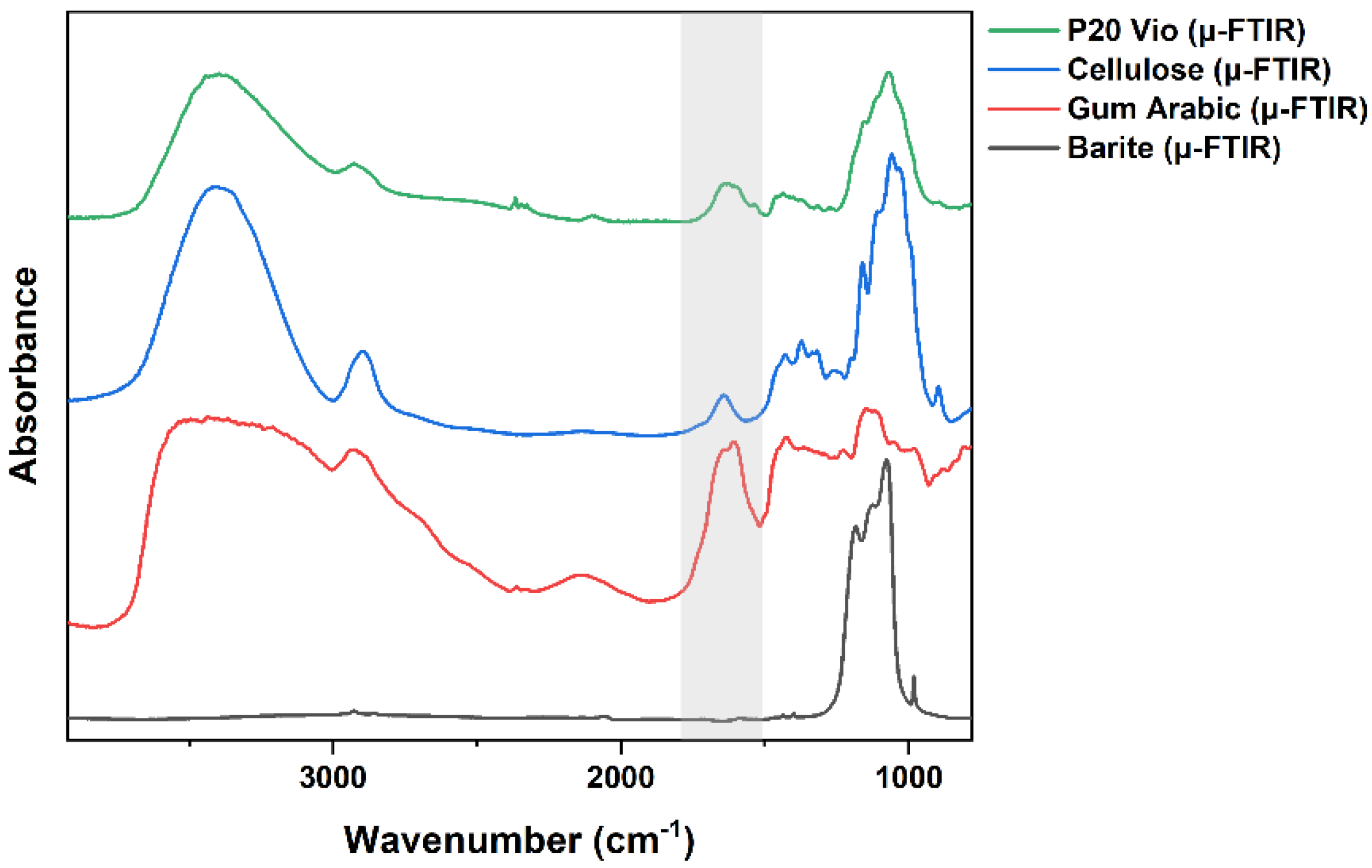
3.1.1. Medium, Extenders, Additives, or Impurities
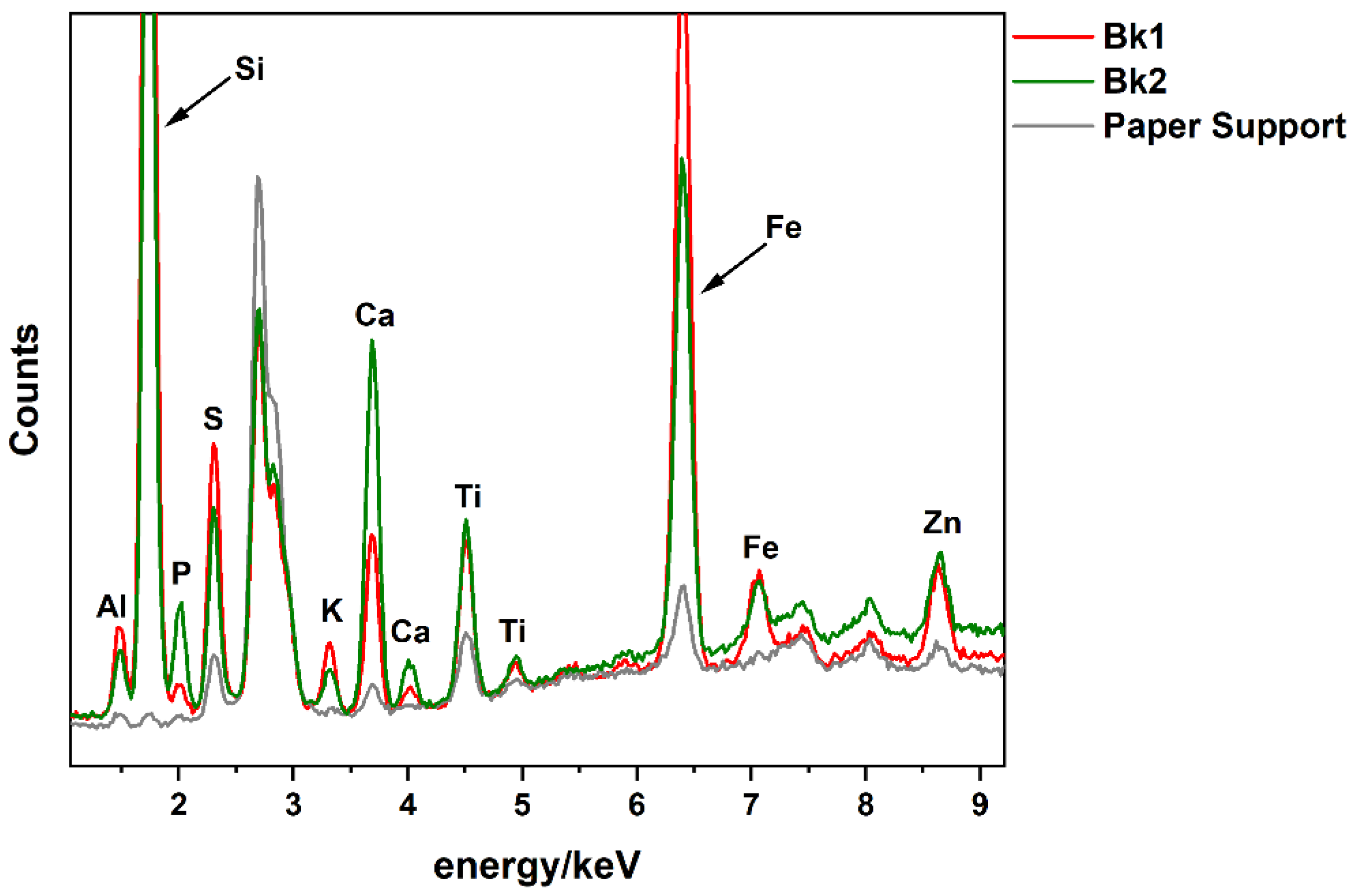
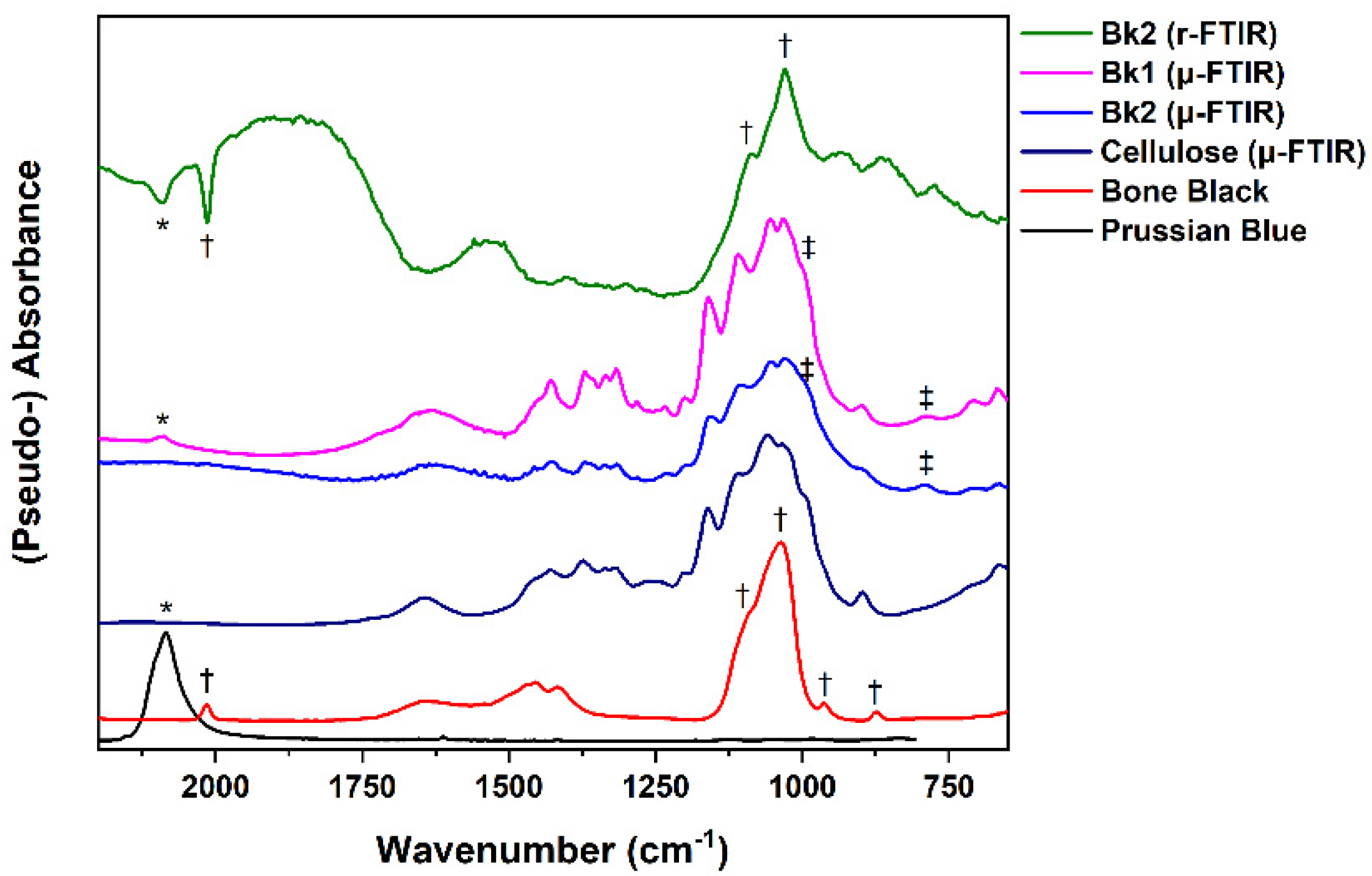
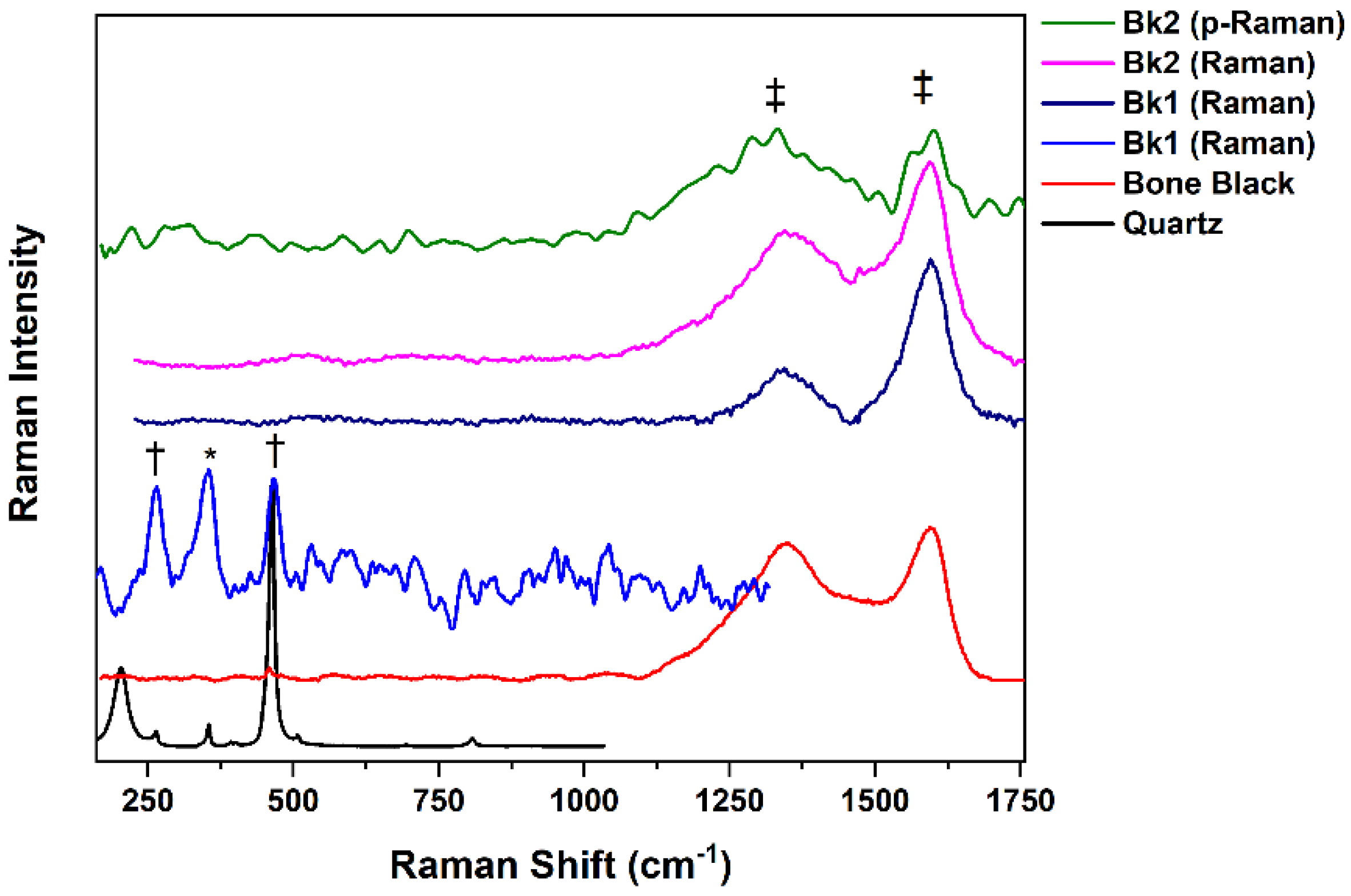
3.1.2. Black Gouaches
3.1.3. Violet Gouache
3.1.4. Magenta Gouache
3.1.5. Pink Gouaches
3.1.6. Blue Gouaches
3.1.7. Green Gouaches
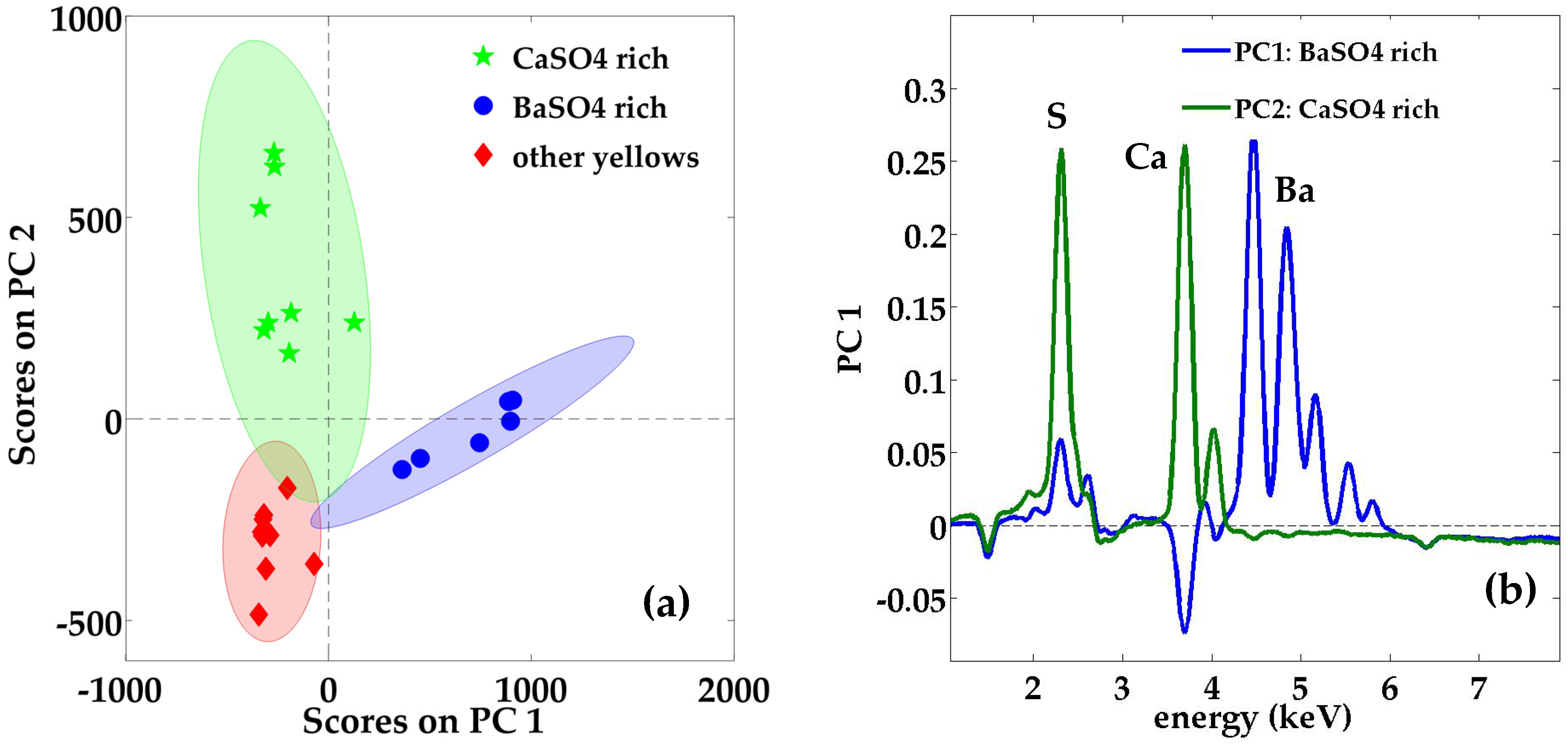
3.1.8. Yellow Gouaches
3.1.9. Orange Gouaches
3.1.10. Red Gouaches
3.1.11. Brown Gouache
3.1.12. An Gouaches
3.1.13. White Gouaches
3.1.14. Gray Gouaches
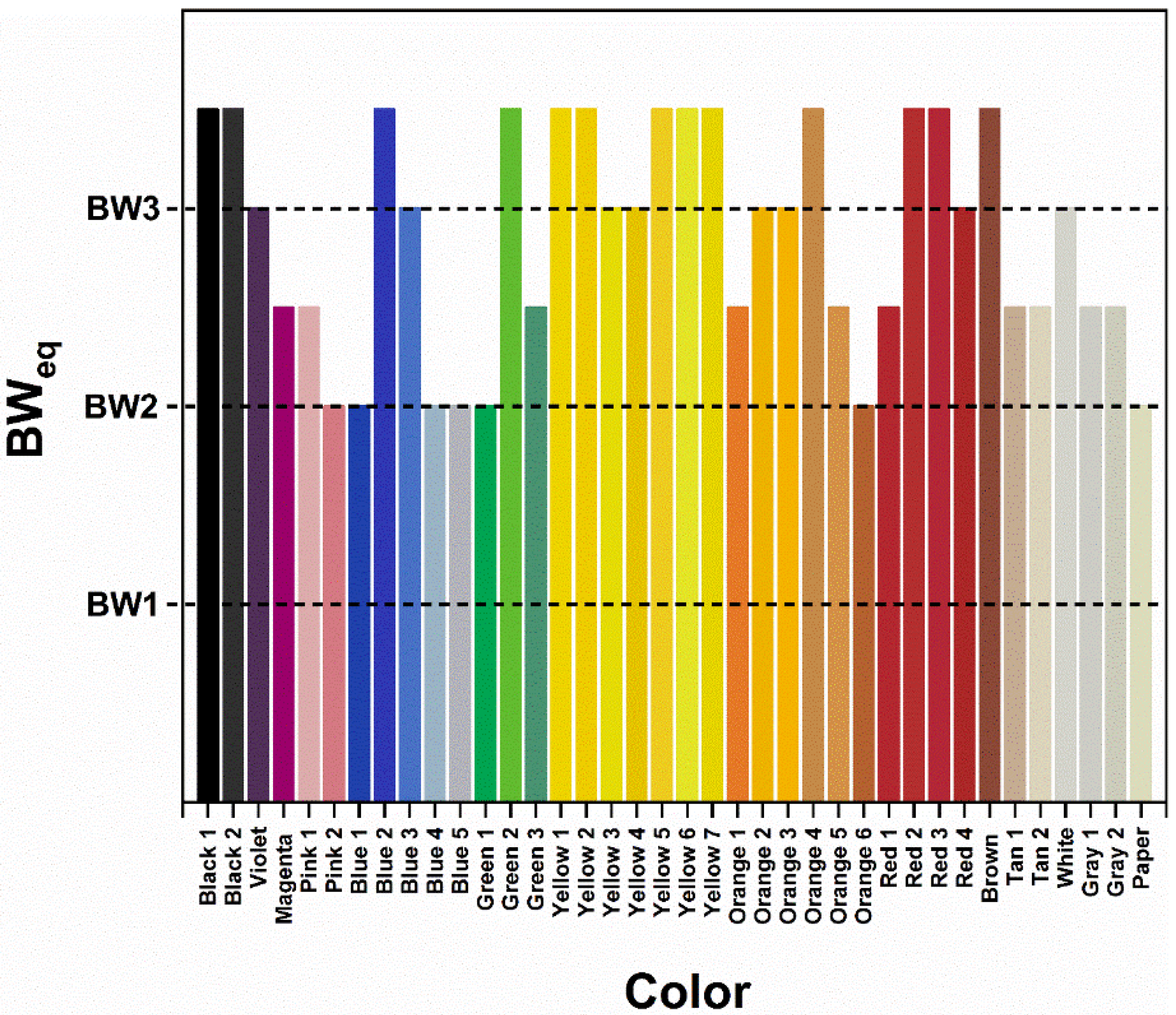
3.2. Color and Color Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullinan, N. Chromatic Composition. In Henri Matisse: The Cut-Outs; Buchberg, K., Cullinan, N., Hauptman, J., Serota, N., Eds.; Tate Publishing: London, UK, 2014. [Google Scholar]
- Rabinow, R.A. The Legacy of La Rue Férou: Livres D’Artiste Created for Tériade by Rouault, Bonnard, Matisse, Léger, Le Corbusier, Chagall, Giacometti, and Miró; New York University, Graduate School of Arts and Science: New York, NY, USA, 1995. [Google Scholar]
- Wheeler, M. The Last Works of Henri Matisse: Large Cut Gouaches; Museum of Modern Art: New York, NY, USA, 1962. [Google Scholar]
- Saudé, J. Traité D’enluminure D’art au Pochoir; Éditions de L’IBIS: Paris, France, 1925. [Google Scholar]
- Matisse, H.; Rouveyre, A.; Finsen, H. Matisse—Rouveyre: Correspondance; Flammarion: Paris, France, 2001. [Google Scholar]
- Lavarini, B. Henri Matisse: Jazz [1943–1947]: Ein Malerbuch als Selbstbekenntnis; Scaneg Verlag: München, Germany, 2000. [Google Scholar]
- Rogge, C.E.; Epley, B.A. Behind the Bocour Label: Identification of Pigments and Binders in Historic Bocour Oil and Acrylic Paints. J. Am. Inst. Conserv. 2017, 56, 15–42. [Google Scholar] [CrossRef]
- Izzo, F.C.; van den Berg, K.J.; van Keulen, H.; Ferriani, B.; Zendri, E. Modern oil paints—Formulations, organic additives and degradation: Some case studies. In Issues in Contemporary Oil Paint; Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., de Tagle, A., Heydenreich, G., van den Berg, K.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 75–104. [Google Scholar]
- Pause, R.; van der Werf, I.D.; van den Berg, K.J. Identification of Pre-1950 Synthetic Organic Pigments in Artists’ Paints. A Non-Invasive Approach Using Handheld Raman Spectroscopy. Heritage 2021, 4, 1348–1365. [Google Scholar] [CrossRef]
- Gautier, G.; Bezur, A.; Muir, K.; Casadio, F.; Fiedler, I. Chemical Fingerprinting of Ready-Mixed House Paints of Relevance to Artistic Production in the First Half of the Twentieth Century. Part I: Inorganic and Organic Pigments. Appl. Spectrosc. 2009, 63, 597–603. [Google Scholar]
- Buchberg, K.; Gross, M.; Lohrengel, S. Materials and Techniques. In Henri Matisse: The Cut-Outs; Buchberg, K., Cullinan, N., Hauptman, J., Serota, N., Eds.; Tate Publishing: London, UK, 2014. [Google Scholar]
- King, A. Henri Matisse: Paper Cut-Outs; St. Louis Art Museum: St. Louis, MO, USA, 1977. [Google Scholar]
- Mochon, R. Matisse’s “Swimming Pool”: A Microfading Study; Internal Report; Museum of Modern Art: New York, NY, USA, 2018. [Google Scholar]
- Liang, H.; Lange, R.; Andrei, L. Examination of the Light Sensitivity of Acanthes by Henri Matisse Using Microfade Spectroscopy; Internal Report; ISAAC Mobile Lab, Nottingham Trent University: Nottingham, UK, 2012. [Google Scholar]
- Townsend, J. Microfading Report on the Snail; Report T00540; Tate: London, UK, 2013. [Google Scholar]
- Martins, A. Microfading Report on the Matisse Gouaches Historical Set; The Museum of Modern Art: New York, NY, USA, 2018. [Google Scholar]
- Price, B.A.; Boris, P.; Lomax, S.Q. (Eds.) Infrared and Raman Users Group Spectral Database, 2007th ed.; IRUG: Philadelphia, PA, USA, 2009; Volumes 1–2. [Google Scholar]
- Lee, P.C.; Meisel, D.J. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Pozzi, F.; Lombardi, J.R.; Bruni, S.; Leona, M. Sample treatment considerations in the analysis of organic colorants by surface-enhanced Raman scattering. Anal. Chem. 2012, 84, 3751–3757. [Google Scholar] [CrossRef]
- Druzik, J. Oriel Microfading Tester (MFT): A Brief Description. Research and Technical Studies Specialty Group Postprints. In Proceedings of the 38th Annual Meeting of the American Institute for Conservation of Historic and Artistic Works, Milwaukee, WI, USA, 11–14 May 2010. [Google Scholar]
- Keene, L. Getty Spectral Viewer™—Program Written for Getty Conservation Institute [GCI] for Processing Newport-Oriel Microfade Tester SPEC32 Software Results. 2007. Available online: https://www.getty.edu/conservation/our_projects/science/lighting/partners.html (accessed on 19 October 2021).
- Ford, B. A microfading survey of the lightfastness of blue, black and red ballpoint pen inks in ambient and modified environments. AICCM Bull. 2018, 39, 26–34. [Google Scholar] [CrossRef]
- Mecklenburg, M.F.; del Hoyo-Meléndez, J.M. Development and application of a mathematical model to explain fading rate inconsistencies observed in light-sensitive materials. Color. Technol. 2012, 128, 139–146. [Google Scholar] [CrossRef]
- Prestel, T. A classification system to enhance lightfastness data interpretation based on microfading tests and rate of colour change. Color. Technol. 2017, 133, 506–512. [Google Scholar] [CrossRef]
- Daly, N.S.; Sullivan, M.; Lee, L.; Trentelman, K.J. Multivariate analysis of Raman spectra of carbonaceous black drawing media for the in situ identification of historic artist materials. Raman Spectrosc. 2018, 49, 1497–1506. [Google Scholar] [CrossRef]
- Kingma, K.J.; Hemley, R.J. Raman spectroscopics tudy of microcrystalline silica. Am. Mineral. 1994, 79, 269–273. [Google Scholar]
- Gettens, R.J.; Stout, G.L. Painting Materials: A Short Encyclopedia; Courier Corporation: North Chelmsford, MA, USA, 1966. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Feller, R.L. Barium Sulphate. In Artists’ Pigments: A Handbook of Their History and Characteristics; Feller, R.L., Ed.; National Gallery of Art: Washington, DC, USA, 1986; Volume 1. [Google Scholar]
- Vetter, W.; Schreiner, M. Characterization of pigment-binding media systems - comparison of non-invasive in-situ reflection FTIR with transmission technical FTIR microscopy E-Preserv. Sci 2011, 8, 10–22. [Google Scholar]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (Pre- ~ 1850 AD). Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: West Sussex, UK, 2004. [Google Scholar]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, L.D. Characterization of hydrated alumina by infrared spectroscopy. Application to study of bauxite ores. Anal. Chem. 1954, 26, 1883–1885. [Google Scholar] [CrossRef]
- Vila, A.; Ferrer, N.; García, J.F. Chemical composition of contemporary black printing inks based on infrared spectroscopy: Basic information for the characterization and discrimination of artistic prints. Anal. Chim. Acta 2007, 591, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Daveri, A.; Malagodi, M.; Vagnini, M.J. The Bone Black Pigment Identification by Noninvasive, In Situ Infrared Reflection Spectroscopy. J. Anal. Methods Chem. 2018, 2018, 6595643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccato, A.; Jehlicka, J.; Moens, L.; Vandenabeele, P.J. Raman spectroscopy for the investigation of carbon-based black pigments. Raman Spectrosc. 2015, 46, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Berrie, B. Prussian Blue. In Artists’ Pigments: A Handbook of Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3. [Google Scholar]
- Jensen, L.; Schatz, G.C.J. Resonance Raman Scattering of Rhodamine 6G as Calculated Using Time-Dependent Density Functional Theory. J. Phys. Chem. A 2006, 110, 5973–5977. [Google Scholar] [CrossRef] [PubMed]
- Cañamares, M.V.; Chenal, C.; Birke, R.L.; Lombardi, J.R.J. DFT, SERS, and Single-Molecule SERS of Crystal Violet. Phys. Chem. C 2008, 112, 20295–20300. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; John Wiley & Sons: Weinheim, Germany, 2006. [Google Scholar]
- Matisse, H.; Flam, J.D.; Tériade, E.; Szymusiak, D. Matisse et Tériade; Anthèse: Arcueil, France, 1996. [Google Scholar]
- Steger, S.; Stege, H.; Bretz, S.; Hahn, O.J. A complementary spectroscopic approach for the non-invasive in-situ identification of synthetic organic pigments in modern reverse paintings on glass (1913–1946). Cult. Herit. 2019, 38, 20–28. [Google Scholar] [CrossRef]
- Kennedy, A.; Stewart, H.; Stenger, J. Lithol Red: A Systematic Structural Study on Salts of a Sulfonated Azo Pigment. Chem. Eur. J. 2012, 18, 3064–3069. [Google Scholar] [CrossRef]
- Desnica, V.; Furić, K.; Schreiner, M. Notice “Multianalytical characterisation of a variety of ultramarine pigments”. E-Preserv. Sci. 2004, 1, 15–25. [Google Scholar]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; de Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.A. (Ed.) Pigment Handbook, Volume 1: Properties and Economics, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1988. [Google Scholar]
- Scherrer, N.C.; Stefan, Z.; Francoise, D.; Annette, F.; Renate, K. Synthetic organic pigments of the 20th and 21st century relevant to artist’s paints: Raman spectra reference collection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Buti, D.; Rosi, F.; Brunetti, B.G.; Miliani, C. In-situ identification of copper-based green pigments on paintings and manuscripts by reflection FTIR. Anal. Bioanal. Chem. 2013, 405, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Fremout, W.; Saverwyns, S.J. Identification of synthetic organic pigments: The role of a comprehensive digital Raman spectral library. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Helwig, K. Iron Ocide. In Artists’ Pigments: A Handbook of Their History and Characteristics; Berrie, B., Ed.; National Gallery of Art: Washington, DC, USA, 2007; Volume 4. [Google Scholar]
- Buxbaum, G.; Pfaff, G. (Eds.) Industrial Inorganic Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Capua, R.J. The obscure history of a ubiquitous pigment: Phosphorescent lithopone and its appearance on drawings by John la Farge. J. Am. Inst. Conserv. 2014, 53, 75–88. [Google Scholar] [CrossRef]
- de la Rie, E.R.; Michelin, A.; Ngako, M.; Del Federico, E.; Del Grosso, C. Photo-catalytic degradation of binding media of ultramarine blue containing paint layers: A new perspective on the phenomenon of “ultramarine disease” in paintings. Polym. Degrad. Stab. 2017, 144, 43–52. [Google Scholar] [CrossRef]
- de Keijzer, M.; van Bommel, M.R. Bright new colours: The history and analysis of fluorescein, eosin, erythrosine, Rhodamin B and some of their derivatives. In The Diversity of Dyes in History and Archaeology; Atkinson, J.K., Ed.; Archetype Books: London, UK, 2017. [Google Scholar]


| Color | Pigment(s) Identified | MFT | Color | Pigment(s) Identified | MFT | ||
|---|---|---|---|---|---|---|---|
| Black 1 (Bk1) |  | Bone black/carbon black Prussian blue Silicates and metal oxides | >3 | Green 3 (G3) |  | PG7 Synthetic ultramarine Barium sulfate | 2.5 |
| Black 2 (Bk2) |  | Bone black/carbon black Prussian blue Silicates and metal oxides | >3 | Yellow 1 (Y1) |  | PY5 | >3 |
| Violet (V) |  | Crystal violet/methyl violet Rhodamine 6G Copper ferrocyanide Barium sulfate | 3 | Yellow 2 (Y2) |  | PY5 PY10 | >3 |
| Magenta (M) |  | Rhodamine 6G Rhodamine 3B Copper ferrocyanide Phototungstic (acid) Barium sulfate | 2.5 | Yellow 3 (Y3) |  | PY5 PY6 Bariumsulfate/zinc white/ lithopone | 3 |
| Pink 1 (Pk1) |  | Crystal violet/methyl violet Rhodamine 6G Copper ferrocyanide Barium sulfate | 2.5 | Yellow 4 (Y4) |  | PY3 PY6 Barium sulfate | 3 |
| Pink 2 (Pk2) |  | PR49:2 | 2 | Yellow 5 (Y5) |  | PY3 PY10 Lead chromate Calcium sulfate | >3 |
| Blue 1 (B1) |  | Synthetic ultramarine | 2 | Yellow 6 (Y6) |  | PY3 Gypsum Barium sulfate | >3 |
| Blue 2 (B2) |  | Synthetic ultramarine Titanium white Calcium carbonate Zinc white | 3 | Yellow 7 (Y7) |  | PY5 PY3 Barium sulfate | >3 |
| Blue 3 (B3) |  | Synthetic ultramarine Zinc white/ Barium sulfate/lithopone | 3 | Orange 1 (O1) |  | PY5 PR4 | 2.5 |
| Blue 4 (B4) |  | Synthetic ultramarine | 2 | Orange 2 (O2) |  | PY5 PY10 Lead chromate Calcium sulfate | 3 |
| Blue 5 (B5) |  | Synthetic ultramarine | 2 | Orange 3 (O3) |  | PY5 PY10 | 3 |
| Green 1 (G1) |  | PG7 PY3 Barium sulfate Aluminum Hydrate | 2 | Orange 4 (O4) |  | Iron oxide (yellow ochre) Lithopone Calcium carbonate | >3 |
| Green 2 (G2) |  | PG7 PY3 Barium sulfate | >3 | Orange 5 (O5) |  | Iron oxide (yellow ochre) | 2.5 |
| Orange 6 (O6) |  | PR4 PY5 Iron oxide (Mars red) Iron oxide (ochre) Calcium sulfate Lead chromate | 2 | Tan 2 (T2) |  | Iron oxide | * |
| Red 1 (R1) |  | PR3 PR49:2 Vermilion Barium sulfate | 2.5 | White 1 (W1) |  | Barium sulfate/zinc white/ lithopone | ≈3 |
| Red 2 (R2) |  | PR3 PR49:2 Lead chromate Barium sulfate | >3 | White 2 (W2) |  | Zinc white Barium sulfate/lithopone | * |
| Red 3 (R3) |  | PR49:2 | >3 | Gray 1 (Gy1) |  | Zinc white Barium sulfate/zinc white/ lithopone Prussian blue | 2.5 |
| Red 4 (R4) |  | PR3 PR49:2 | 3 | Gray 2 (Gy2) |  | Titanium white (anatase) Calcium carbonate Ultramarine blue Prussian blue Barium sulfate/zinc white/ lithopone | 2.5 |
| Brown (Br) |  | Iron oxide (Sienna/Mars red) Lead chromate Calcium sulfate | >3 | Gray 3 (Gy3) |  | Titanium white (anatase) Calcium carbonate Ultramarine blue Prussian blue | * |
| Tan 1 (T1) |  | Possibly iron oxide Barium sulfate/zinc white/Lithopone | 2.5 | Paper |  | Low mineral content | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.; Prud’hom, A.C.; Duranton, M.; Haddad, A.; Daher, C.; Genachte-Le Bail, A.; Tang, T. Jazz Colors: Pigment Identification in the Gouaches Used by Henri Matisse. Heritage 2021, 4, 4205-4221. https://doi.org/10.3390/heritage4040231
Martins A, Prud’hom AC, Duranton M, Haddad A, Daher C, Genachte-Le Bail A, Tang T. Jazz Colors: Pigment Identification in the Gouaches Used by Henri Matisse. Heritage. 2021; 4(4):4205-4221. https://doi.org/10.3390/heritage4040231
Chicago/Turabian StyleMartins, Ana, Anne Catherine Prud’hom, Maroussia Duranton, Abed Haddad, Celine Daher, Anne Genachte-Le Bail, and Tiffany Tang. 2021. "Jazz Colors: Pigment Identification in the Gouaches Used by Henri Matisse" Heritage 4, no. 4: 4205-4221. https://doi.org/10.3390/heritage4040231






