A Bayesian Adaptive Design in Cancer Phase I Trials Using Dose Combinations with Ordinal Toxicity Grades
Abstract
:1. Introduction
2. Method
2.1. Dose–Toxicity Model
2.2. Prior and Posterior Distributions
2.3. Trial Design
- Each patient in the first cohort of two patients receives the same dose combination .
- In the ith cohort of two patients:
- (a)
- If i is even, then patient receives dose and patient receives dose , wherefor EWOC criterion.for CRM principle.
- (b)
- If i is odd, then patient receives dose and patient receives dose , wherefor EWOC criterion.for CRM principle.
- Repeat Step 2 until n patients are enrolled to the trial subject to the following stopping rule.
3. Simulations
3.1. Set-Up and Scenarios
3.2. Operating Characteristics
3.2.1. Safety
3.2.2. Efficiency
3.3. Results
4. Discrete Approach
- (i)
- Let ,, and .
- (ii)
- Let .
4.1. Operating Characteristics
4.2. Illustration
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Institute of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0, 4th ed.; 2009. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 20 June 2020).
- Gordon, N.H.; Willson, J.K.V. Using Toxicity Grades in the Design and Analysis of Cancer Phase-I Clinical-Trials. Stat. Med. 1992, 11, 2063–2075. [Google Scholar] [CrossRef]
- Wang, C.; Chen, T.; Tyan, I. Designs for phase I cancer clinical trials with differentiation of graded toxicity. Commun. Stat. Theory Methods 2000, 29, 975–987. [Google Scholar] [CrossRef]
- Paul, R.K.; Rosenberger, W.F.; Flournoy, N. Quantile estimation following non-parametric phase I clinical trials with ordinal response. Stat. Med. 2004, 23, 2483–2495. [Google Scholar] [CrossRef]
- Ivanova, A. Escalation, group and A+ B designs for dose-finding trials. Stat. Med. 2006, 25, 3668–3678. [Google Scholar] [CrossRef]
- Van Meter, E.M.; Garrett-Mayer, E.; Bandyopadhyay, D. Proportional odds model for dose-finding clinical trial designs with ordinal toxicity grading. Stat. Med. 2011, 30, 2070–2080. [Google Scholar] [CrossRef] [Green Version]
- Iasonos, A.; Zohar, S.; O’Quigley, J. Incorporating lower grade toxicity information into dose finding designs. Clin. Trials 2011, 8, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Tighiouart, M.; Cook-Wiens, G.; Rogatko, A. Escalation with overdose control using ordinal toxicity grades for cancer phase i clinical trials. J. Probab. Stat. 2012, 2012, 317634. [Google Scholar] [CrossRef] [Green Version]
- Bekele, B.N.; Thall, P.F. Dose-Finding Based on Multiple Toxicities in a Soft Tissue Sarcoma Trial. J. Am. Stat. Assoc. 2004, 99, 26–35. [Google Scholar] [CrossRef]
- Yuan, Z.; Chappell, R.; Bailey, H. The Continual Reassessment Method for Multiple Toxicity Grades: A Bayesian Quasi-Likelihood Approach. Biometrics 2007, 63, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, R.; George, S. Flexible phase I clinical trials: Allowing for nonbinary toxicity response and removal of other common limitations. Stat. Biopharm. Res. 2009, 1, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Bekele, B.N.; Li, Y.; Ji, Y. Risk-group-specific dose finding based on an average toxicity score. Biometrics 2010, 66, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Krailo, M.D.; Azen, S.P.; Tighiouart, M. A novel toxicity scoring system treating toxicity response as a quasi-continuous variable in Phase I clinical trials. Contemp. Clin. Trials 2010, 31, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Tighiouart, M.; Kowalski, J. Dose escalation with overdose control using a quasi-continuous toxicity score in cancer Phase I clinical trials. Contemp. Clin. Trials 2012, 33, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzalfani, M.; Zohar, S.; Qin, R.; Mandrekar, S.J.; Deley, M.C.L. Dose-finding designs using a novel quasi-continuous endpoint for multiple toxicities. Stat. Med. 2013, 32, 2728–2746. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhu, C.; Zhang, F.; Yuan, Y.; Zhang, S.; Zhang, W.; Li, C.; Wang, L.; Xia, J. The continual reassessment method for multiple toxicity grades: A Bayesian model selection approach. PLoS ONE 2014, 9, e98147. [Google Scholar] [CrossRef]
- O’Quigley, J.; Pepe, M.; Fisher, L. Continual Reassessment Method: A Practical Design for Phase 1 Clinical Trials in Cancer Published. Biometrics 1990, 46, 33–48. [Google Scholar] [CrossRef]
- Babb, J.; Rogatko, A.; Zacks, S. Cancer phase I clinical trials: Efficient dose escalation with overdose control. Stat. Med. 1998, 17, 1103–1120. [Google Scholar] [CrossRef]
- Tighiouart, M.; Babb, J.S.; Rogatko, A. Flexible Bayesian methods for cancer phase I clinical trials. Dose escalation with overdose control. Stat. Med. 2005, 24, 2183–2196. [Google Scholar] [CrossRef]
- Tighiouart, M.; Rogatko, A. Dose findingwith escalation with overdose control (EWOC) in cancer clinical trials. Stat. Sci. 2010, 25, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Tighiouart, M.; Cook-Wiens, G.; Rogatko, A. A Bayesian adaptive design for cancer phase I trials using a flexible range of doses. J. Biopharm. Stat. 2018, 28, 562–574. [Google Scholar] [CrossRef]
- Thall, P.F.; Millikan, R.E.; Mueller, P.; Lee, S.J. Dose-finding with two agents in Phase I oncology trials. Biometrics 2003, 59, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ivanova, A. Two-dimensional dose finding in discrete dose space. Biometrics 2005, 61, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yin, G. Sequential continual reassessment method for two-dimensional dose finding. Stat. Med. 2008, 27, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Yuan, Y. A latent contingency table approach to dose finding for combinations of two agents. Biometrics 2009, 65, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Yuan, Y. Bayesian dose finding in oncology for drug combinations by copula regression. J. R. Stat. Soc. Ser. C Appl. Stat. 2009, 58, 211–224. [Google Scholar] [CrossRef]
- Braun, T.M.; Wang, S. A hierarchical Bayesian design for phase i trials of novel combinations of cancer therapeutic agents. Biometrics 2010, 66, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Wages, N.A.; Conaway, M.R.; O’Quigley, J. Continual reassessment method for partial ordering. Biometrics 2011, 67, 1555–1563. [Google Scholar] [CrossRef]
- Shi, Y.; Yin, G. Escalation with overdose control for phase I drug-combination trials. Stat. Med. 2013, 32, 4400–4412. [Google Scholar] [CrossRef]
- Gasparini, M. General classes of multiple binary regression models in dose finding problems for combination therapies. J. R. Stat. Soc. Ser. C Appl. Stat. 2013, 62, 115–133. [Google Scholar] [CrossRef]
- Riviere, M.K.; Yuan, Y.; Dubois, F.; Zohar, S. A Bayesian dose-finding design for drug combination clinical trials based on the logistic model. Pharm. Stat. 2014, 13, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Tighiouart, M.; Piantadosi, S.; Rogatko, A. Dose finding with drug combinations in cancer phase I clinical trials using conditional escalation with overdose control. Stat. Med. 2014, 33, 3815–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mander, A.P.; Sweeting, M.J. A product of independent beta probabilities dose escalation design for dual-agent phase I trials. Stat. Med. 2015, 34, 1261–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tighiouart, M.; Li, Q.; Rogatko, A. A Bayesian adaptive design for estimating the maximum tolerated dose curve using drug combinations in cancer phase I clinical trials. Stat. Med. 2017, 36, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Diniz, M.A.; Li, Q.; Tighiouart, M. Dose Finding for Drug Combination in Early Cancer Phase I Trials Using Conditional Continual Reassessment Method. J. Biom. Biostat. 2017, 8, 381. [Google Scholar] [PubMed]
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling; 2003; Available online: www.ci.tuwien.ac.at/Conferences/DSC-2003/Drafts/Plummer.pdf (accessed on 17 July 2020).
- Tighiouart, M.; Li, Q.; Piantadosi, S.; Rogatko, A. A Bayesian Adaptive Design for Combination of Three Drugs in Cancer Phase I Clinical Trials. Am. J. Biostat. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tighiouart, M. Two-stage design for phase I-II cancer clinical trials using continuous dose combinations of cytotoxic agents. J. R. Stat. Soc. Ser. C Appl. Stat. 2019, 68, 235–250. [Google Scholar] [CrossRef]
- Jiménez, J.; Kim, S.; Tighiouart, M. A Bayesian seamless phase I–II trial design with two stages for cancer clinical trials with drug combinations. Biom. J. 2020. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.L.; Tighiouart, M.; Gasparini, M. Cancer phase I trial design using drug combinations when a fraction of dose limiting toxicities is attributable to one or more agents. Biom. J. 2019, 61, 319–332. [Google Scholar] [CrossRef]
- Forastiere, A.; Goepfert, H.; Maor, M.; Pajak, T.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.; Chao, C.; et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Thanarajasingam, G.; Atherton, P.; Novotny, P.; Loprinzi, C.; Sloan, J.; Grothey, A. Longitudinal adverse event assessment in oncology clinical trials: The Toxicity over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016, 17, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Rogatko, A.; Babb, J.; Wang, H.; Slifker, M.; Hudes, G. Patient Characteristics Compete with Dose as Predictors of Acute Treatment Toxicity in Early Phase Clinical Trials. Clin. Cancer Res. 2004, 10, 4645–4651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresham, G.; Diniz, M.A.; Razaee, Z.S.; Luu, M.; Kim, S.; Hays, R.D.; Piantadosi, S.; Tighiouart, M.; Yothers, G.; Ganz, P.A.; et al. Evaluating Treatment Tolerability in Cancer Clinical Trials using the Toxicity Index. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.A.; Kim, S.; Tighiouart, M. A Bayesian Adaptive Design in Cancer Phase I Trials Using Dose Combinations with Ordinal Toxicity Grades; JSM Proceedings, Biopharmaceutical Section; American Statistical Association: Alexandria, VA, USA, 2017; pp. 3185–3195. [Google Scholar]
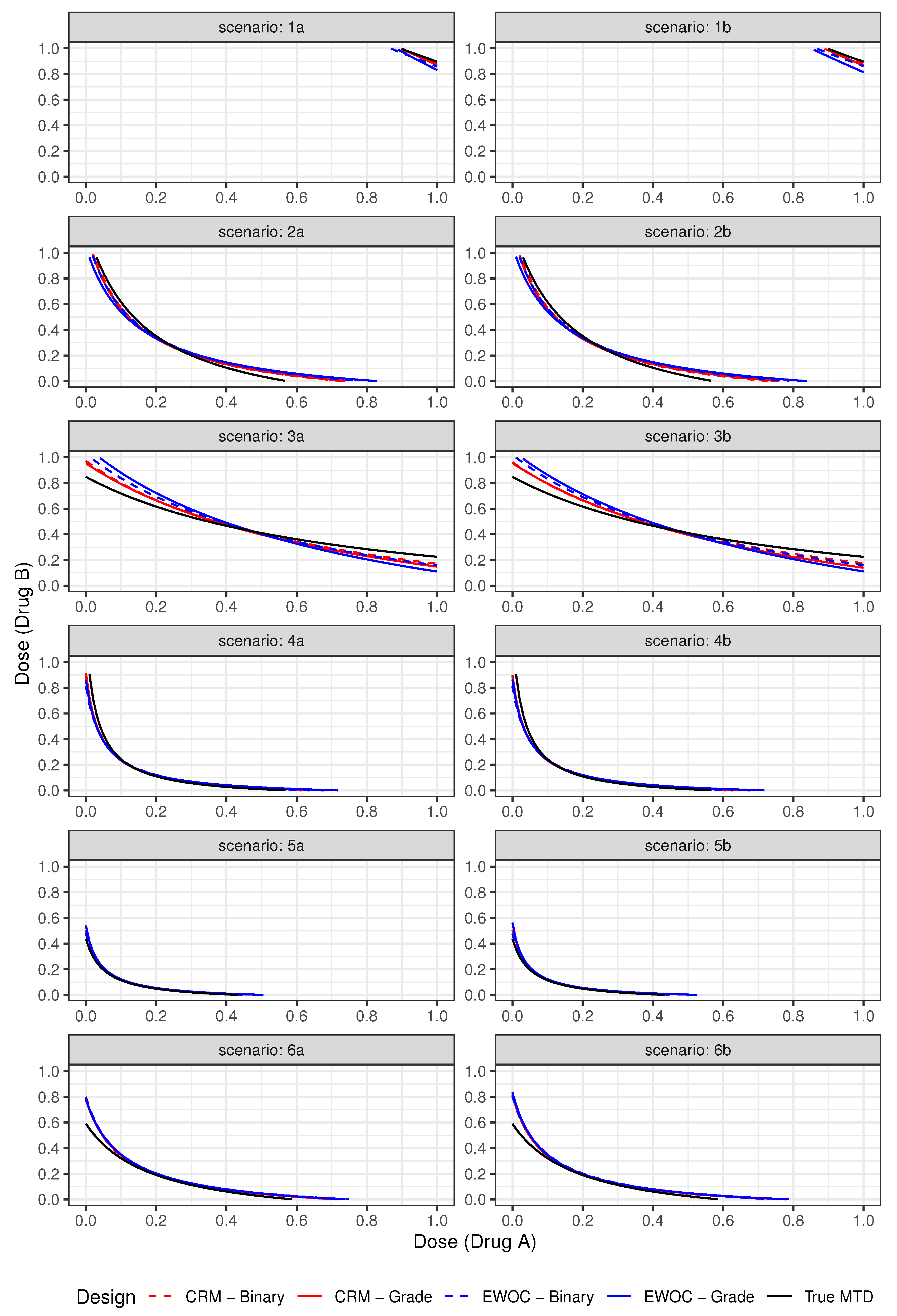
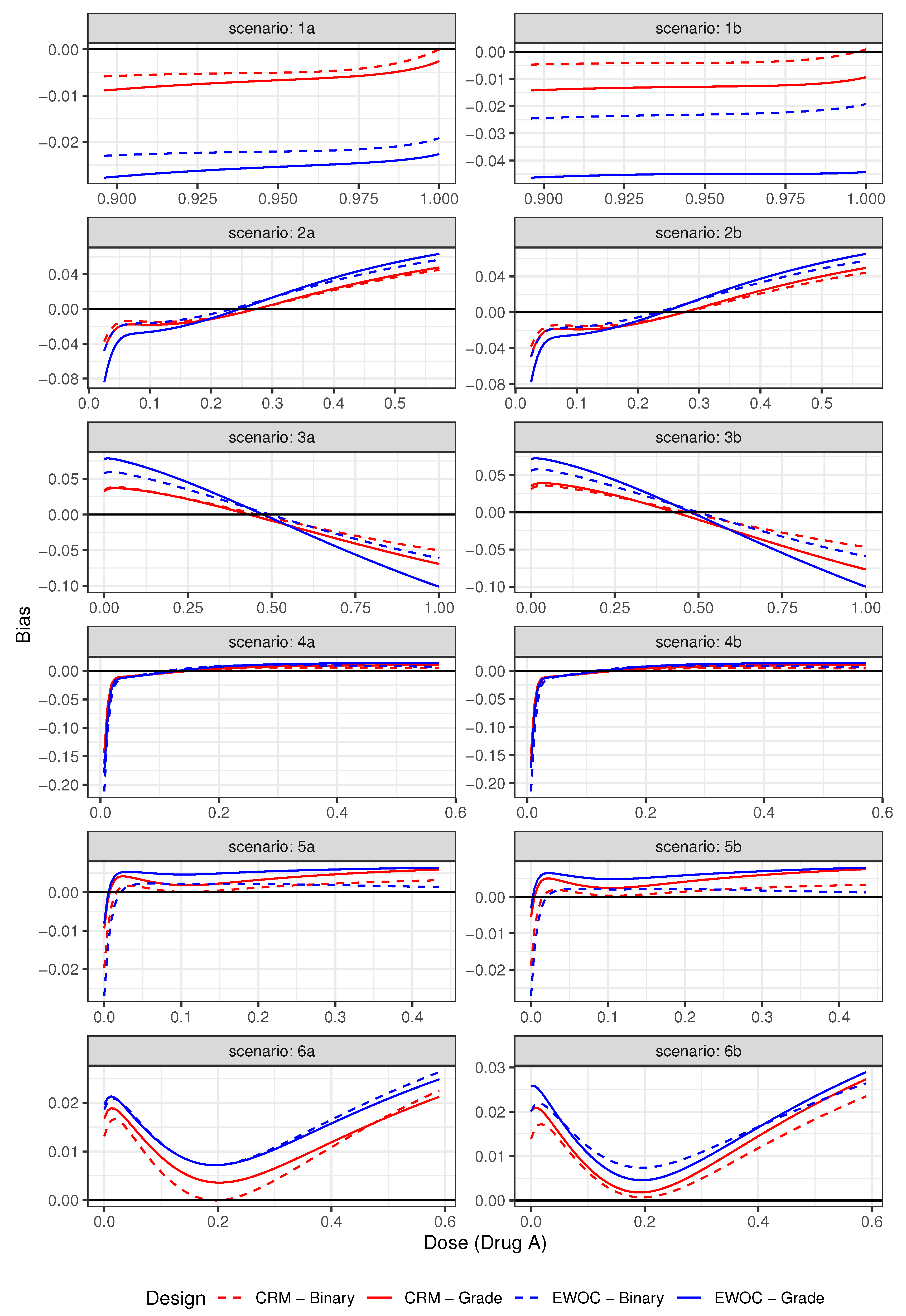
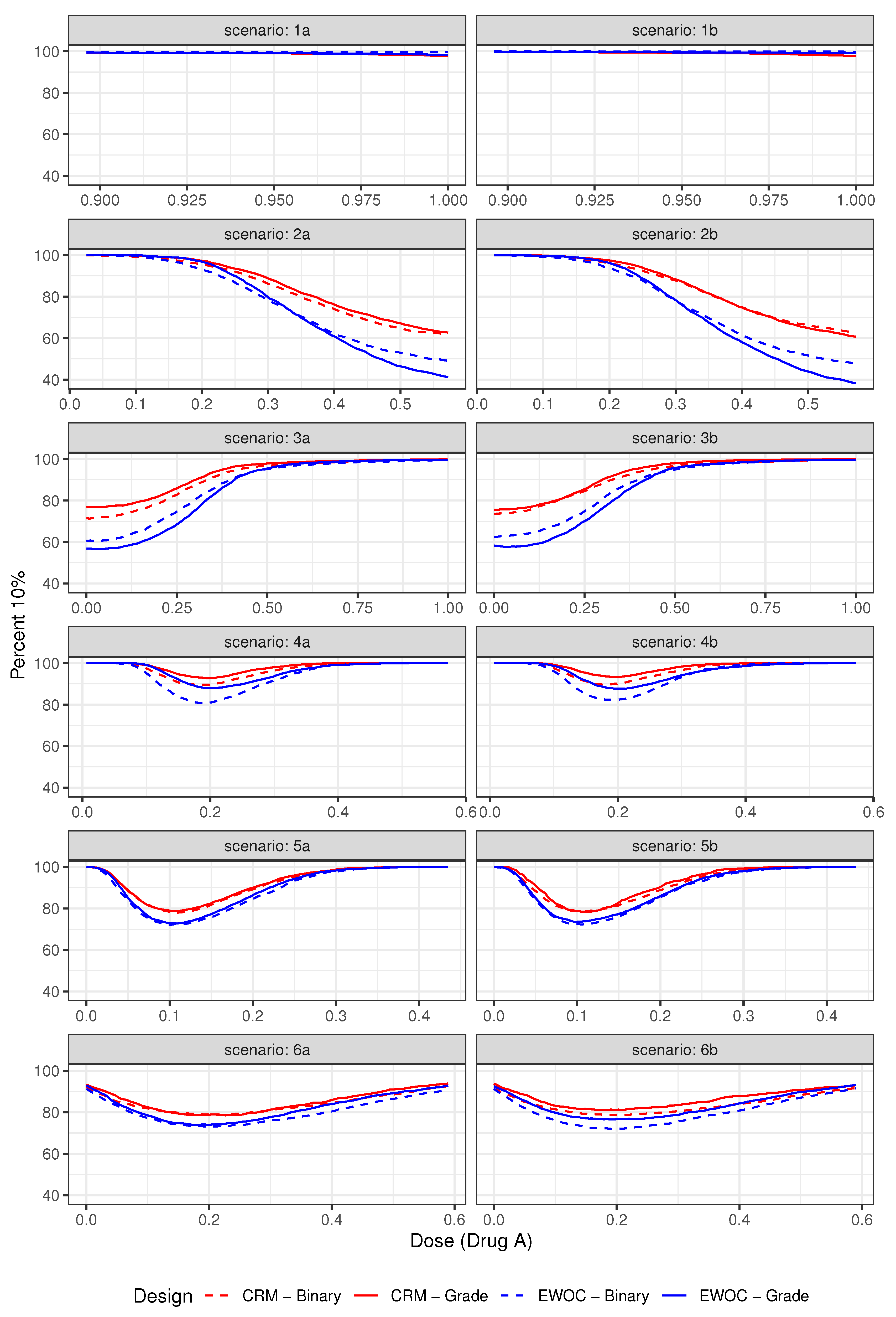
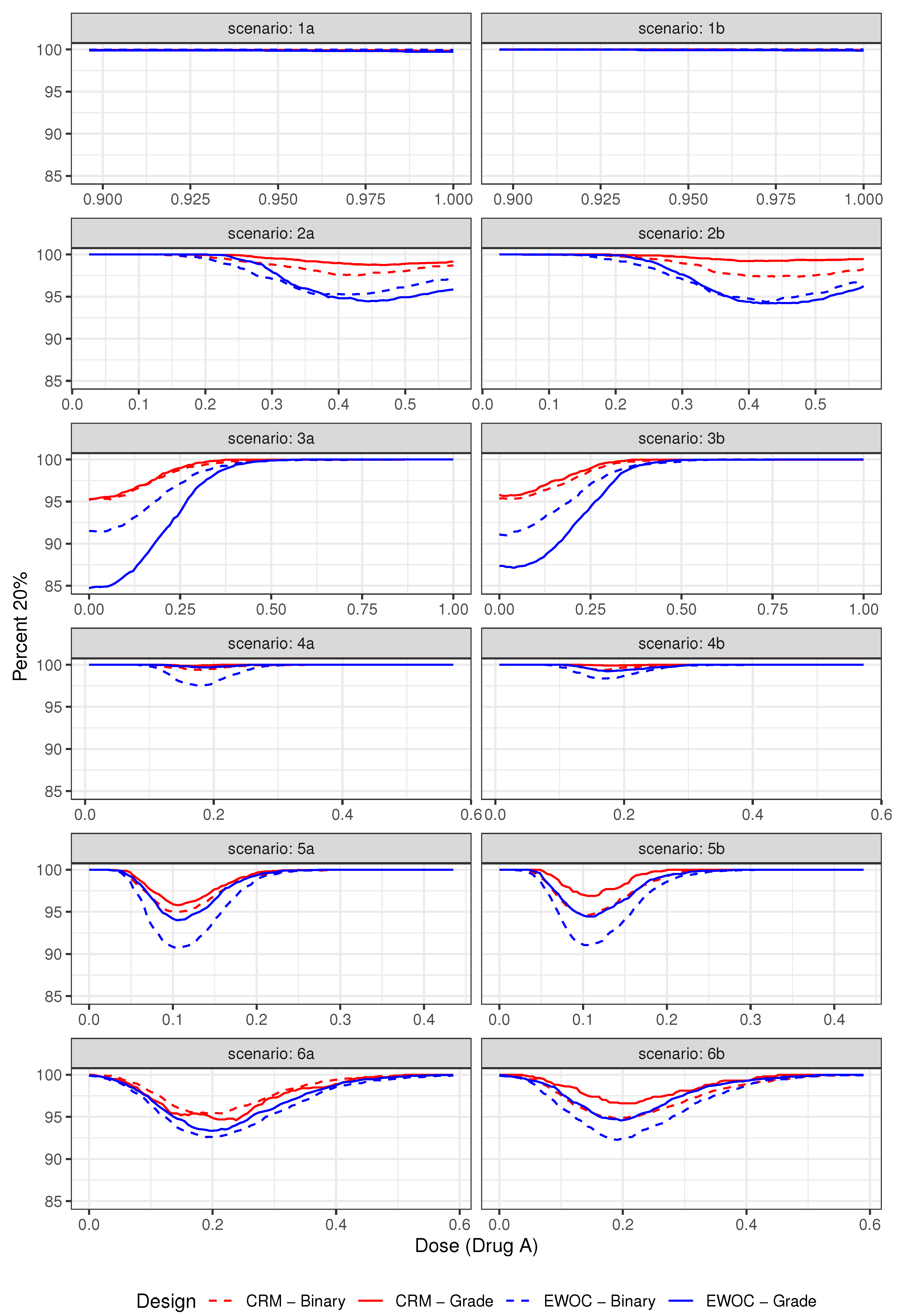
| Average % Grade 2 | Average % DLTs (Z = 3) | ||||
|---|---|---|---|---|---|
| Scenario | Design | (Z = 2) | (%Trials: DLT Rate (Z = 3) > 0.43) | ||
| Binary | Ordinal | Binary | Ordinal | ||
| 1a | EWOC | 76.12 | 81.41 | 16.31 (0.0) | 10.86 (0.0) |
| CRM | 76.03 | 81.32 | 16.47 (0.0) | 11.14 (0.0) | |
| 1b | EWOC | 78.47 | 83.33 | 16.35 (0.0) | 11.48 (0.0) |
| CRM | 78.76 | 84.34 | 16.16 (0.0) | 10.48 (0.0) | |
| 2a | EWOC | 61.22 | 61.77 | 30.31 (0.0) | 29.37 (0.0) |
| CRM | 59.36 | 60.39 | 32.33 (0.23) | 31.26 (0.47) | |
| 2b | EWOC | 64.34 | 65.23 | 30.44 (0.07) | 29.45 (0.40) |
| CRM | 62.32 | 67.80 | 32.45 (0.43) | 31.67 (0.70) | |
| 3a | EWOC | 67.65 | 69.63 | 25.29 (0.0) | 22.96 (0.0) |
| CRM | 65.29 | 67.46 | 27.36 (0.0) | 25.53 (0.0) | |
| 3b | EWOC | 69.67 | 71.48 | 25.25 (0.0) | 23.30 (0.0) |
| CRM | 67.40 | 69.27 | 27.53 (0.0) | 25.64 (0.0) | |
| 4a | EWOC | 58.98 | 58.47 | 32.64 (0.07) | 33.06 (1.10) |
| CRM | 57.74 | 57.79 | 33.95 (0.20) | 34.11 (1.57) | |
| 4b | EWOC | 61.90 | 61.51 | 32.90 (0.03) | 33.17 (0.97) |
| CRM | 60.73 | 60.37 | 34.06 (0.20) | 34.45 (1.57) | |
| 5a | EWOC | 49.11 | 48.44 | 36.73 (2.63) | 37.66 (6.30) |
| CRM | 48.38 | 47.73 | 37.00 (2.17) | 38.55 (10.20) | |
| 5b | EWOC | 57.39 | 55.96 | 36.71 (2.33) | 38.14 (8.97) |
| CRM | 57.06 | 55.10 | 36.96 (2.13) | 38.98 (13.40) | |
| 6a | EWOC | 52.04 | 52.19 | 32.83 (1.00) | 32.67 (1.47) |
| CRM | 50.45 | 50.98 | 34.98 (2.77) | 34.65 (3.50) | |
| 6b | EWOC | 61.04 | 61.17 | 32.85 (1.20) | 32.70 (2.00) |
| CRM | 59.11 | 59.32 | 34.91 (2.20) | 34.63 (4.40) | |
| Scenario 01 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose Level | Z = 1 | Z = 2 | ||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 5 | 0.50 | 0.44 | 0.38 | 0.31 | 0.24 | 0.45 | 0.53 | 0.60 | 0.68 | 0.75 |
| 4 | 0.53 | 0.49 | 0.43 | 0.35 | 0.26 | 0.40 | 0.46 | 0.53 | 0.63 | 0.70 |
| 3 | 0.58 | 0.57 | 0.51 | 0.43 | 0.36 | 0.33 | 0.36 | 0.44 | 0.53 | 0.59 |
| 2 | 0.65 | 0.57 | 0.55 | 0.47 | 0.44 | 0.20 | 0.33 | 0.38 | 0.48 | 0.53 |
| 1 | 0.68 | 0.65 | 0.58 | 0.53 | 0.48 | 0.15 | 0.20 | 0.33 | 0.40 | 0.47 |
| Scenario 02 | ||||||||||
| Dose Level | Z = 1 | Z = 2 | ||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 5 | 0.36 | 0.44 | 0.48 | 0.41 | 0.34 | 0.28 | 0.35 | 0.42 | 0.52 | 0.60 |
| 4 | 0.30 | 0.39 | 0.43 | 0.45 | 0.38 | 0.22 | 0.23 | 0.33 | 0.43 | 0.45 |
| 3 | 0.24 | 0.27 | 0.31 | 0.43 | 0.49 | 0.17 | 0.20 | 0.21 | 0.33 | 0.39 |
| 2 | 0.15 | 0.27 | 0.28 | 0.37 | 0.44 | 0.11 | 0.14 | 0.19 | 0.25 | 0.30 |
| 1 | 0.10 | 0.25 | 0.28 | 0.30 | 0.38 | 0.08 | 0.13 | 0.15 | 0.21 | 0.27 |
| Scenario 01 | ||||||||
| Criterion | Model | PS | S-3 | S-2 | S-1 | AV | % Grade 2 | Average DLT Rate |
| (% Excessive DLT) | ||||||||
| EWOC | Binary | 57.2 | 9.5 | 29.0 | 64.6 | 84.8 | 54.33 | 31.91 (1.27) |
| Ordinal | 71.7 | 26.7 | 54.7 | 92.2 | 82.4 | 54.81 | 31.59 (1.63) | |
| CRM | Binary | 54.2 | 8.2 | 25.3 | 62.7 | 82.4 | 53.74 | 32.92 (1.66) |
| Ordinal | 70.5 | 26.2 | 61.9 | 91.6 | 81.1 | 53.86 | 32.87 (2.43) | |
| Scenario 02 | ||||||||
| Criterion | Model | PS | S-3 | S-2 | S-1 | AV | % Grade 2 | Average DLT Rate |
| (% Excessive DLT) | ||||||||
| EWOC | Binary | 26.6 | 3.3 | 12.5 | 40.1 | 64.2 | 30.14 | 21.11 (0.00) |
| Ordinal | 38.4 | 38.2 | 58.4 | 84.9 | 63.9 | 30.95 | 21.30 (0.00) | |
| CRM | Binary | 29.2 | 3.7 | 12.6 | 40.0 | 68.8 | 31.57 | 23.24 (0.04) |
| Ordinal | 44.7 | 49.9 | 69.4 | 90.5 | 71.3 | 32.77 | 23.33 (0.00) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, M.A.; Kim, S.; Tighiouart, M. A Bayesian Adaptive Design in Cancer Phase I Trials Using Dose Combinations with Ordinal Toxicity Grades. Stats 2020, 3, 221-238. https://doi.org/10.3390/stats3030017
Diniz MA, Kim S, Tighiouart M. A Bayesian Adaptive Design in Cancer Phase I Trials Using Dose Combinations with Ordinal Toxicity Grades. Stats. 2020; 3(3):221-238. https://doi.org/10.3390/stats3030017
Chicago/Turabian StyleDiniz, Márcio A., Sungjin Kim, and Mourad Tighiouart. 2020. "A Bayesian Adaptive Design in Cancer Phase I Trials Using Dose Combinations with Ordinal Toxicity Grades" Stats 3, no. 3: 221-238. https://doi.org/10.3390/stats3030017





