Development of Immunoenzyme Assay of Herbicide Acetochlor and Its Application to Soil Testing with Comparison of Sample Preparation Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Components
2.2. Methods
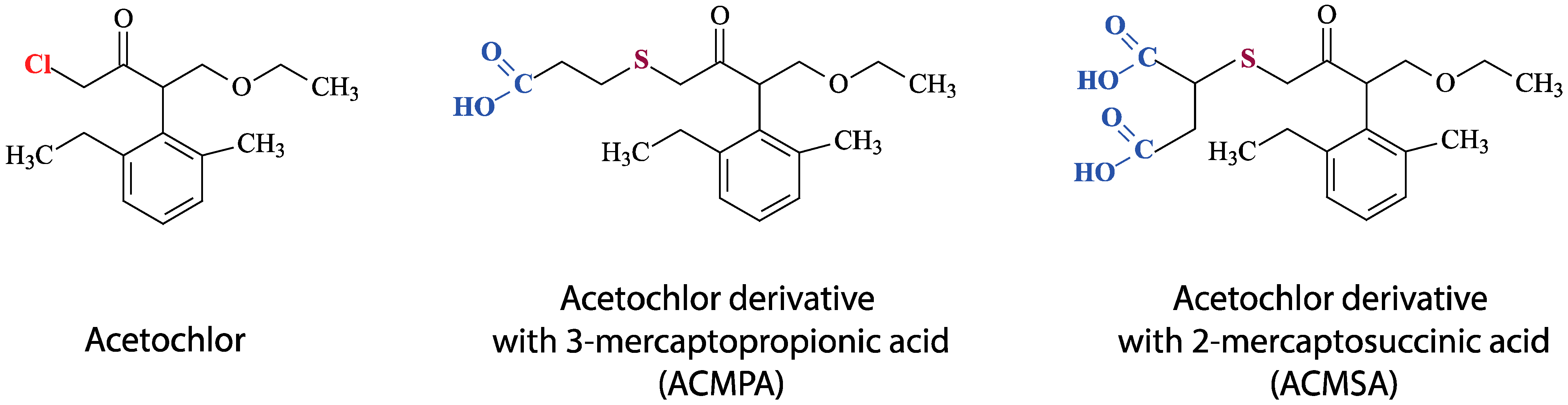
2.2.1. Preparation of a Conjugate of Acetochlor with a Carrier Protein
2.2.2. Rabbit Immunization and Preparation of Antisera
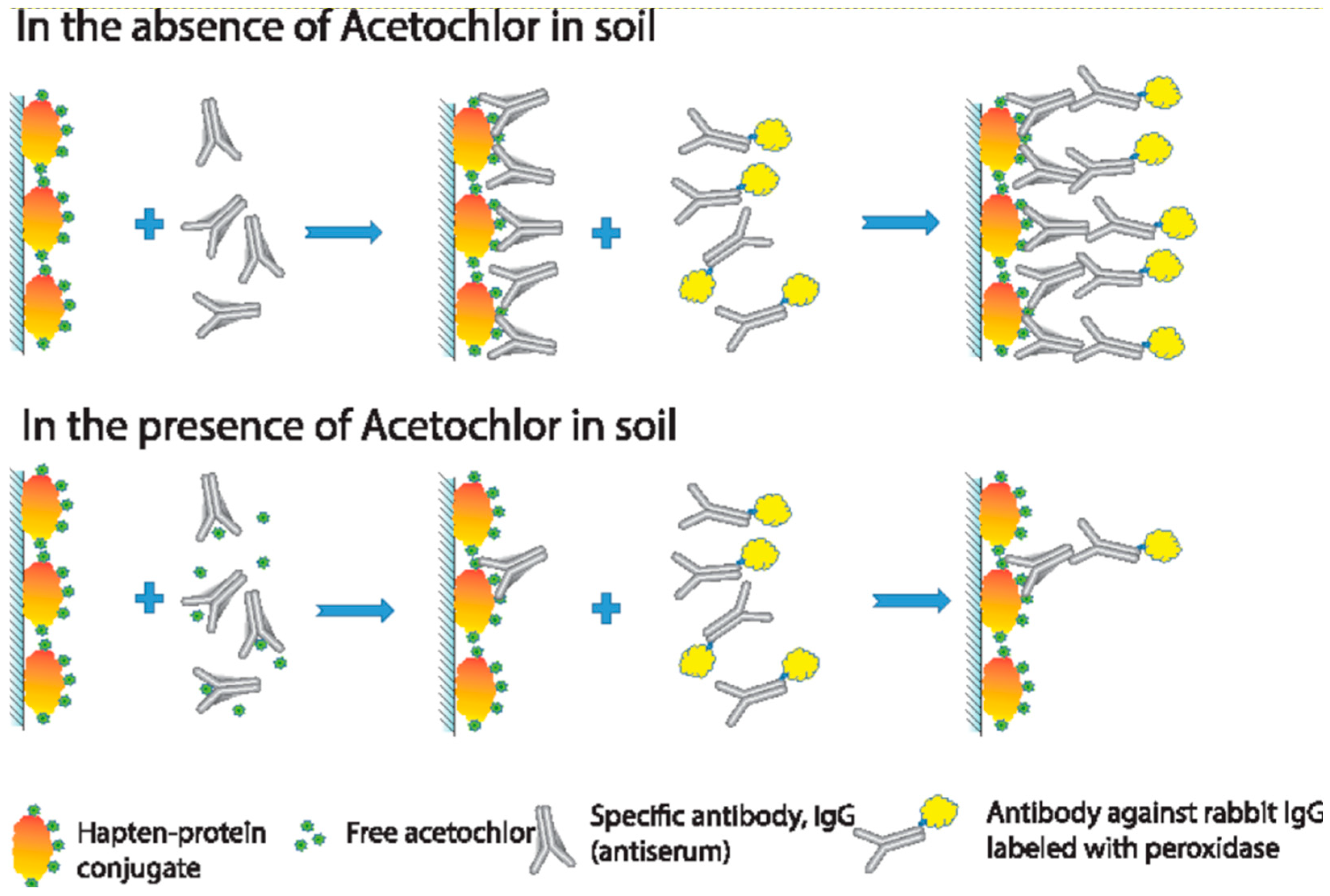
2.2.3. Competitive Enzyme Immunoassay of Acetochlor
2.2.4. Preparation of Samples for ELISA
2.2.5. Processing of Enzyme Immunoassay Results
3. Results and Discussion
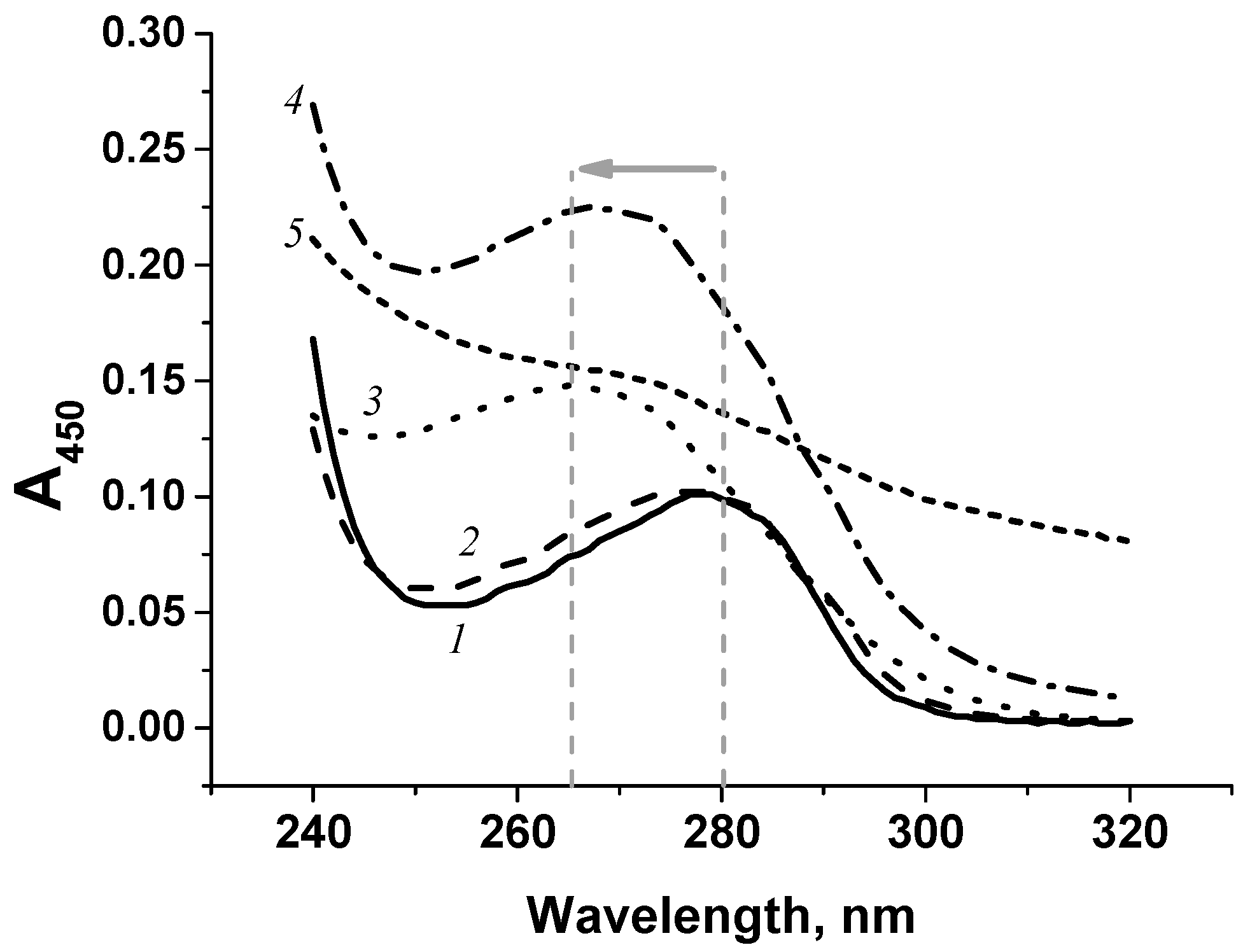
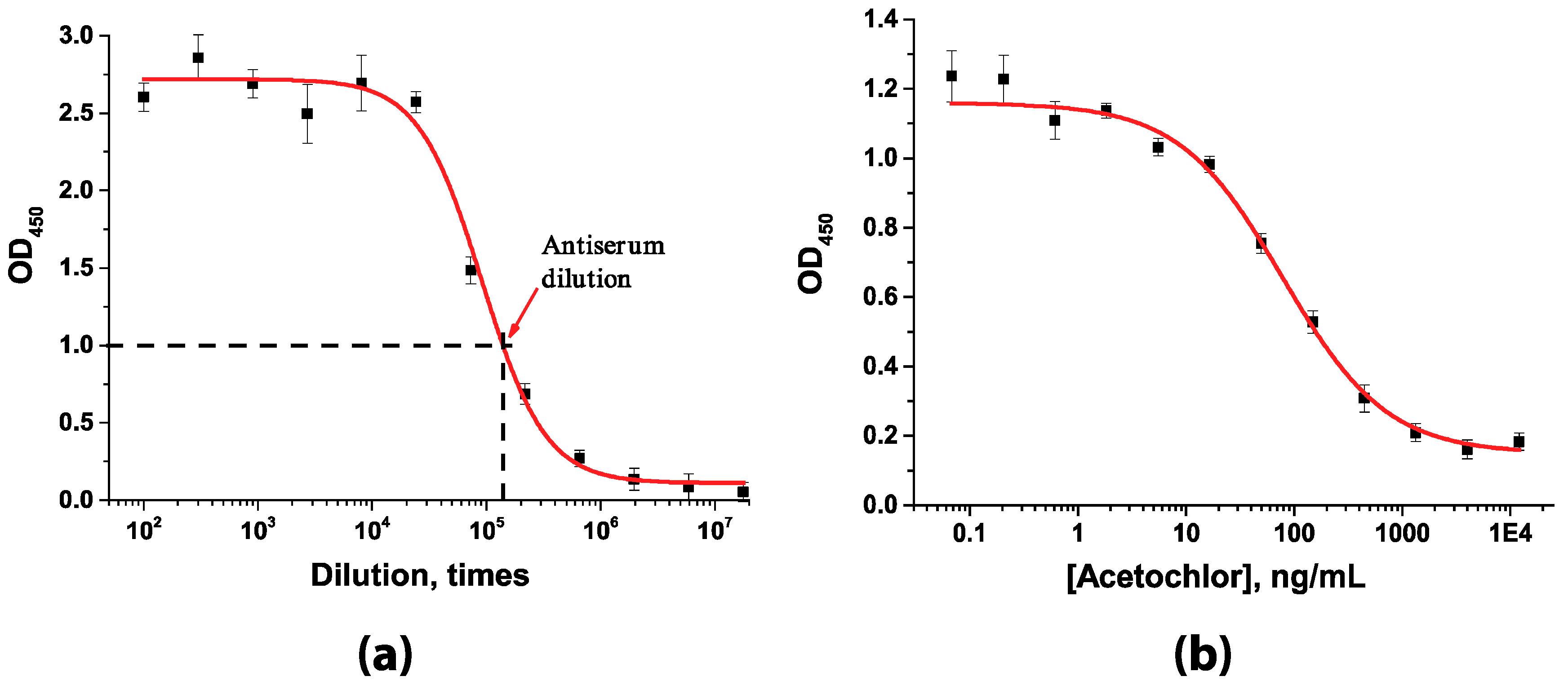
3.1. Production and Characterization of Immunoreagents
3.2. Application of Different Extraction Techniques of Acetochlor from Soil Samples
3.3. Selection of ELISA Conditions for Soil Extracts
3.4. Analysis of the Obtained Extracts by the ELISA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef]
- Panico, S.C.; van Gestel, C.A.; Verweij, R.A.; Rault, M.; Bertrand, C.; Barriga, C.A.M.; Coeurdassier, M.; Fritsch, C.; Gimbert, F.; Pelosi, C. Field mixtures of currently used pesticides in agricultural soil pose a risk to soil invertebrates. Envir. Pollut. 2022, 305, 119290. [Google Scholar] [CrossRef]
- Riedo, J.; Wettstein, F.E.; Rösch, A.; Herzog, C.; Banerjee, S.; Büchi, L.; Charles, R.; Wächter, D.; Martin-Laurent, F.; Bucheli, T.D. Widespread occurrence of pesticides in organically managed agricultural soils—The ghost of a conventional agricultural past? Environ. Sci. Technol. 2021, 55, 2919–2928. [Google Scholar] [CrossRef]
- Geissen, V.; Silva, V.; Lwanga, E.H.; Beriot, N.; Oostindie, K.; Bin, Z.; Pyne, E.; Busink, S.; Zomer, P.; Mol, H. Cocktails of pesticide residues in conventional and organic farming systems in Europe–Legacy of the past and turning point for the future. Environ. Pollut. 2021, 278, 116827. [Google Scholar] [CrossRef] [PubMed]
- Schleiffer, M.; Kretzschmar, U.; Speiser, B. Pestizidrückstände auf Biolebensmitteln–Untersuchungen in der Schweiz und Europa; 2021. Forschungsinstitut für biologischen Landbau FiBL, Frick, Aargau, Switzerland. Available online: https://orgprints.org/id/eprint/39911/1/Pestizidrueckstaende_Biolebensmittel_Mai_2021.pdf (accessed on 6 November 2025).
- Schleiffer, M.; Speiser, B. Presence of pesticides in the environment, transition into organic food, and implications for quality assurance along the European organic food chain–A review. Environ. Pollut. 2022, 313, 120116. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe; Publications Office of the European Union: Brussels, Belgium, 2018. [Google Scholar]
- Araya, G.; Perfetti-Bolaño, A.; Sandoval, M.; Araneda, A.; Barra, R.O. Groundwater Leaching Potential of Pesticides: A Historic Review and Critical Analysis: Groundwater leaching of pesticides. Environ. Toxicol. Chem. 2024, 43, 2478–2491. [Google Scholar] [CrossRef]
- Liu, X.; Bai, L.; Jin, C.; Li, J.; Deng, Y.; Liu, Q. Study on sensitivities of 16 rice varieties to Acetochlor. Agric. Sci. Technol. 2015, 16, 88. [Google Scholar]
- Bedmar, F.; Gimenez, D.; Costa, J.L.; Daniel, P.E. Persistence of acetochlor, atrazine, and s-metolachlor in surface and subsurface horizons of 2 typic argiudolls under no-tillage. Environ. Toxicol. Chem. 2017, 36, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Chen, S.-F.; Song, H.; Li, Z.; Luo, X.; Zhang, X.; Zhou, X. Current insights into environmental acetochlor toxicity and remediation strategies. Environ. Geochem. Health 2024, 46, 356. [Google Scholar] [CrossRef]
- E.F.S.A. Conclusion on the peer review of the pesticide risk assessment of the active substance acetochlor. EFSA J. 2011, 9, 2143. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Z.; Zhou, L.; Cao, Z.; Liao, X.; Ye, R.; Lu, H. Effects of acetochlor on neurogenesis and behaviour in zebrafish at early developmental stages. Chemosphere 2019, 220, 954–964. [Google Scholar] [CrossRef]
- Tomlin, C.D. The Pesticide Manual, 12th ed.; British Crop Protection Council: Farnham, UK, 2000; 1250p. [Google Scholar]
- Wang, X.; Li, S.; Zhang, C.; Xu, W.; Wu, M.; Cheng, J.; Li, Z.; Tao, L.; Zhang, Y. Stereoselective toxicity of acetochlor chiral isomers on the nervous system of zebrafish larvae. J. Hazard. Mater. 2024, 464, 133016. [Google Scholar] [CrossRef]
- Lu, A.; Ivantsova, E.; Martyniuk, C.J. A comparative review and computational assessment of acetochlor toxicity in fish: A novel endocrine disruptor? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109685. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Bahena-Ocampo, I.U.; González-Castañeda, G.; Bonilla, E.; Milić, M.; Bonassi, S.; Sánchez-Alarcón, J. miRNAs: A potentially valuable tool in pesticide toxicology assessment-current experimental and epidemiological data review. Chemosphere 2022, 295, 133792. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, X.; Qiu, C.; Qian, Y.; Chen, J.; Shao, C.; Xie, J.; Deng, G.; Peng, C. Effects of long-term herbicide application on the crops in soybean-peanut rotations in the red soil upland of Southern China. Field Crops Res. 2020, 248, 107723. [Google Scholar] [CrossRef]
- Liu, C.; Wen, S.; Li, S.; Tian, Y.; Wang, L.; Zhu, L.; Wang, J.; Kim, Y.M.; Wang, J. Enhanced remediation of chlorpyrifos-contaminated soil by immobilized strain Bacillus H27. J. Environ. Sci. 2024, 144, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Jr, R.S.; Koskinen, W.C.; Graff, C.D.; Anderson, J.L.; Mulla, D.J.; Nater, E.A.; Alonso, D.G. Acetochlor persistence in surface and subsurface soil samples. Water Air Soil Pollut. 2013, 224, 1747. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) no 1372/2011. Concerning the Non-Approval of the Active Substance Acetochlor, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending Commission Decision 2008/934/EC. Off. J. Eur. Union OJEU 2011, 341, 45. [Google Scholar]
- E.F.S.A. Reasoned opinion on the setting of import tolerances for acetochlor in soya beans and cotton seeds. EFSA J. 2015, 13, 4224. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Hu, Z.; Shi, Y. Effects of acetochlor on wheat growth characteristics and soil residue in dryland. Gesunde Pflanz. 2021, 73, 307–315. [Google Scholar] [CrossRef]
- Altiparmak, E.; Yilmaz, E.; Dadaser-Celik, F.; Ates, N. Sensitive quantification of acetochlor and metolachlor in water using Taguchi-optimized DLLME coupled with high-performance liquid chromatography. Microchem. J. 2024, 200, 110499. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Muratuly, A.; Bektassov, M.; Zhakupbekova, A.; Kenessov, B. Chromatographic determination of pesticides in soil: Current trends in analysis and sample preparation. Trends Environ. Anal. Chem. 2022, 35, e00174. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Jin, P.; Liu, L.; Xu, X.; Xu, C.; Xu, L.; Kuang, H. Greenness metrics of immunoassays for the detection of organophosphorus pesticide residues. TrAC Trends Anal. Chem. 2024, 178, 117828. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.; Eun, J.-B.; Shim, J.-H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J. Recent advances in rapid detection techniques for pesticide residue: A review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Jarukas, L.; Ivanauskas, L.; Kasparaviciene, G.; Baranauskaite, J.; Marksa, M.; Bernatoniene, J. Determination of organic compounds, fulvic acid, humic acid, and humin in peat and sapropel alkaline extracts. Molecules 2021, 26, 2995. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Amelung, W.; Don, A. Origin of carbon in agricultural soil profiles deduced from depth gradients of C: N ratios, carbon fractions, δ 13 C and δ 15 N values. Plant Soil 2021, 460, 123–148. [Google Scholar] [CrossRef]
- Antu, U.B.; Roy, T.K.; Kulsum, T.I.; Mitu, P.R.; Ismail, Z.; Arifin, M.; Datta, M.; Hossain, S.A.; Islam, M.S.; Mahiddin, N.A.; et al. Role of humic acid for climate change adaptation measures to boost up sustainable agriculture and soil health: A potential review. Int. J. Biol. Macromol. 2025, 313, 144043. [Google Scholar] [CrossRef]
- Cai, Y.; Li, L.; Zhang, J.; Li, Z.; Zhang, F.; Xu, Y.; Tai, Z. Development of a MOF-based SPE method combined with GC–MS for simultaneous determination of alachlor, acetochlor and pretilachlor in field soil. Envir. Monit. Assess. 2023, 195, 569. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Wang, L.; Shao, J.; Mei, W.; Wang, L. Development and application of a dispersive solid-phase extraction method for the simultaneous determination of chloroacetamide herbicide residues in soil by gas chromatography-tandem mass spectrometry (GC-MS/MS). Int. J. Environ. Anal. Chem. 2019, 99, 282–296. [Google Scholar] [CrossRef]
- Li, H.; Yin, J.; Liu, Y.; Shang, J. Effect of Protein on the Detection of Fluoroquinolone Residues in Fish Meat. J. Agric. Food Chem. 2012, 60, 1722–1727. [Google Scholar] [CrossRef]
- Zhang, S.; You, Q.; Zhuo, X.; Shi, Z.; Yao, W.; Lü, T.; Zhang, D. Rapid and simple determination of organophosphorus pesticides in urine using polydopamine-modified monolithic spin column extraction combined with liquid chromatography–mass spectrometry. J. Chromatogr. A 2023, 1696, 463959. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Veiga-del-Bano, J.M.; Andreo-Martinez, P.; Pérez-Lucas, G.; Navarro, S. Overview of the evolution and trends of the QuEChERS sample preparation procedure. Rev. Environ. Contam. Toxicol. 2024, 262, 22. [Google Scholar] [CrossRef]
- Ðurović-Pejčev, R.D.; Bursić, V.P.; Zeremski, T.M. Comparison of QuEChERS with traditional sample preparation methods in the determination of multiclass pesticides in soil. J. AOAC Int. 2019, 102, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, V.; Heris, M.-E.S.; Dastranj, M.; Farimani, M.M.; Eslami, Z.; Aboul-Enein, H.Y. Assessment of pesticide residues in soils using a QuEChERS extraction procedure and LC-MS/MS. Water Air Soil Pollut. 2021, 232, 159. [Google Scholar] [CrossRef]
- Li, L.; Yin, Y.; Zheng, G.; Liu, S.; Zhao, C.; Xie, W.; Ma, L.; Shan, Q.; Dai, X.; Wei, L. Determination of multiclass herbicides in sediments and aquatic products using QuECHERS combined with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) and its application to risk assessment of rice-fish co-culture system in China. Microchem. J. 2021, 170, 106628. [Google Scholar]
- Cebi, N.; Manav, O.G.; Olgun, E.O. Analysis of pesticide residues in hazelnuts using the QuEChERS method by liquid chromatography–tandem mass spectrometry. Microchem. J. 2021, 166, 106208. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Varela-Martínez, D.A.; Riaño-Herrera, D.A. Pesticide-residue analysis in soils by the QuEChERS method: A review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef]
- Wang, W.; Man, Y.; Xie, J.; Zhang, Z.; Wang, P.; Liu, X. Occurrence and risk assessment of three chloroamide herbicides in water and soil environment in northeastern, eastern and southern China. Environ. Res. 2023, 219, 115104. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.G.a.; Dıaz, A.N.; Dıaz, A.G.; Lovillo, J. Antibody production and development of a polarization fluoroimmunoassay for the herbicide triclopyr. Anal. Chim. Acta 2001, 439, 131–138. [Google Scholar] [CrossRef]
- Yakovleva, J.N.; Lobanova, A.I.; Panchenko, O.A.; Eremin, S.A. Production of antibodies and development of specific polarization fluoroimmunoassay for acetochlor. Int. J. Environ. Anal. Chem. 2002, 82, 851–863. [Google Scholar] [CrossRef]
- Farzana, F.; Roy, T.K.; Ali, M.M.; Hossain, S.A.; Ghosh, R.; Biswas, T.; Mazrin, M.; Jayoti, J.R.; Sarker, B.C.; Khan, A.S. Comprehensive assessment of marsh soil health index in beel aquaculture systems of coastal Bangladesh. Aquac. Int. 2025, 33, 409. [Google Scholar] [CrossRef]
- Zhang, B.; Lang, Y.; Guo, B.; Cao, Z.; Cheng, J.; Cai, D.; Shentu, X.; Yu, X. Indirect Competitive Enzyme-Linked Immunosorbent Assay Based on Broad-Spectrum Antibody for Simultaneous Determination of Thirteen Fluoroquinolone Antibiotics in Rana catesbeianus. Foods 2023, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Alsefri, S.; Balbaied, T.; Alatawi, H.; Albalawi, I.; Hogan, A.; Moore, E. Development of the QuEChERS extraction method for the determination of polychlorinated biphenyls (Aroclor 1254) in soil samples by using GC-MS. Separations 2023, 10, 250. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Q.; Wu, J.; He, K.; Feng, J.; Dong, S. Determination of Acetamiprid Residues in Vegetables by Indirect Competitive Chemiluminescence Enzyme Immunoassay. Foods 2022, 11, 2507. [Google Scholar] [CrossRef]
- Langlois, M.C.; Weavers, L.K.; Chin, Y.-P. Contaminant-mediated photobleaching of wetland chromophoric dissolved organic matter. Environ. Sci. Process. Impacts 2014, 16, 2098–2107. [Google Scholar] [CrossRef]
- Moshcheva, A.G.; Galvidis, I.A.; Zaslavskya, D.N.; Burkin, M.A. Development of heterologous immunoassay for the quantification of folic acid in dietary supplements, fortified crispbreads, infant formulas, and milk. J. Food Compos. Anal. 2025, 139, 107177. [Google Scholar] [CrossRef]
- Wang, J.; Shen, X.; Zhong, P.; Li, Z.; Tang, Q.; Huang, X.; Zherdev, A.V.; Dzantiev, B.B.; Eremin, S.A.; Xiao, Z. Heterologous immunoassay strategy for enhancing detection sensitivity of banned dye rhodamine B in fraudulent food. Chem. Biol. Technol. Agric. 2021, 8, 17. [Google Scholar] [CrossRef]
- Lyon, W.G.; Rhodes, D.E. Molecular size exclusion by soil organic materials estimated from their swelling in organic solvents. Environ. Toxicol. Chem. 1993, 12, 1405–1412. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Hurrass, J.; Müller, M.; Rotard, W. Swelling of organic matter in soil and peat samples: Insights from proton relaxation, water absorption and PAH extraction. In Humic Substances; Taylor & Francis: Abingdon, UK, 2003; pp. 78–88. [Google Scholar]
- Pszczolińska, K.; Michel, M. The QuEChERS approach for the determination of pesticide residues in soil samples: An overview. J. AOAC Int. 2016, 99, 1403–1414. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef]
- Gilevska, T.; Wiegert, C.; Droz, B.; Junginger, T.; Prieto-Espinoza, M.; Borreca, A.; Imfeld, G. Simple extraction methods for pesticide compound-specific isotope analysis from environmental samples. MethodsX 2022, 9, 101880. [Google Scholar] [CrossRef] [PubMed]
- Bodur, S.; Borahan, T.; Ates, N.; Bakırdere, S. Sensitive determination of acetochlor, alachlor, metolachlor and fenthion utilizing mechanical shaking assisted dispersive liquid–liquid microextraction prior to gas chromatography–mass spectrometry. Bull. Environ. Contam. Toxicol. 2020, 105, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Berlina, A.N.; Ragozina, M.Y.; Gusev, D.I.; Zherdev, A.V.; Dzantiev, B.B. Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms. Chemosensors 2023, 11, 393. [Google Scholar] [CrossRef]
- Berlina, A.; Smirnova, N.; Komova, N.; Serebrennikova, K.; Zherdev, A.; Dzantiev, B. Influence of Organic Solvents on the Results of Immunoenzyme Determination of Herbicide Butachlor: Selection of Sample Preparation Modes. Appl. Biochem. Microbiol. 2024, 60, 776–783. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Chen, F.; Yang, T. Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food Agric. Immunol. 2009, 20, 333–344. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Serebrennikova, K.V.; Zherdev, A.V.; Dzantiev, B.B. Determination of Nonylphenol in a Highly Sensitive Chemiluminescent Immunoenzyme Assay of Natural Waters. Appl. Sci. 2024, 14, 1685. [Google Scholar] [CrossRef]
- Okorkov, V.; Okorkova, L.; Lebedeva, A. Fertilization of Annual Grasses on Gray Forest Soils of the Vladimir Opole. Agrohimiâ 2023, 26–37. [Google Scholar] [CrossRef]
- Shein, E.; Kiryushin, V.; Korchagin, A.; Mazirov, M.; Dembovetskii, A.; Il’in, L. Assessment of agronomic homogeneity and compatibility of soils in the Vladimir Opolie region. Eurasian Soil Sci. 2017, 50, 1166–1172. [Google Scholar] [CrossRef]
- Lafay, F.; Daniele, G.; Fieu, M.; Pelosi, C.; Fritsch, C.; Vulliet, E. Ultrasound-assisted QuEChERS-based extraction using EDTA for determination of currently-used pesticides at trace levels in soil. Environ. Sci. Pollut. Res. 2022, 1–15. [Google Scholar] [CrossRef]


| Option No. | Title | Salt Composition per One Sample Extraction (50 mL Tube) |
|---|---|---|
| 1 | QuEChERS AOAC | 6 g MgSO4, 1.5 g sodium acetate |
| 2 | QuEChERS EN | 4 g MgSO4, 1 g NaCl, 1 g trisodium citrate dehydrate, 0.5 g disodium hydrogen citrate sesquihydrate |
| 3 | QuEChERS modified | 4 g MgSO4 anhydrous, 1 g NaCl |
| 4 | DCM extraction | Pure extractant without salts |
| Option | Soil Extraction Technique | Added, ng/mL | Detected, ng/mL | Detected, % |
|---|---|---|---|---|
| 1 | QuEChERS AOAC | 750 | 314.4 ± 39.9 | 41.9 ± 12.7 |
| 250 | 106.7 ± 6.9 | 42.7 ± 6.5 | ||
| 90 | 43.9 ± 4.5 | 48.8 ± 10.3 | ||
| 2 | QuEChERS EN | 750 | 664.2 ± 21.9 | 88.6 ± 3.3 |
| 250 | 273.6 ± 19.9 | 110.3 ± 7.3 | ||
| 90 | 105.7 ± 9.9 | 117.4 ± 9.4 | ||
| 3 | QuEChERS modified | 750 | 513.3 ± 12.3 | 81.7 ± 2.4 |
| 250 | 192.1 ± 14.2 | 76.8 ± 7.4 | ||
| 90 | 69.6 ± 0.7 | 77.33 ± 1.0 | ||
| 4 | DCM only | 750 | 652.5 ± 39.2 | 87 ± 6.0 |
| 250 | 158.0 ± 22.1 | 63.2 ± 14 | ||
| 90 | 74.7 ± 6.3 | 83 ± 8.5 |
| Soil Sample | Added, µg/g of Soil | Detected, µg/g | Detected, % |
|---|---|---|---|
| 1 | 4.5 | 3.9 ± 0.13 | 86.7 ± 3.3 |
| 1.5 | 1.59 ± 0.12 | 106 ± 7.5 | |
| 0.5 | 0.62 ± 0.06 | 124 ± 9.7 | |
| 2 | 4.5 | 3.35 ± 0.21 | 74.4 ± 6.3 |
| 1.5 | 1.24 ± 0.11 | 82.6 ± 8.9 | |
| 0.5 | 0.57 ± 0.07 | 114 ± 12.3 | |
| 3 | 4.5 | 5.2 ± 0.38 | 115.6 ± 7.3 |
| 1.5 | 1.4 ± 0.04 | 93.3 ± 2.9 | |
| 0.5 | 0.43 ± 0.04 | 86 ± 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Development of Immunoenzyme Assay of Herbicide Acetochlor and Its Application to Soil Testing with Comparison of Sample Preparation Techniques. Soil Syst. 2025, 9, 127. https://doi.org/10.3390/soilsystems9040127
Berlina AN, Zherdev AV, Dzantiev BB. Development of Immunoenzyme Assay of Herbicide Acetochlor and Its Application to Soil Testing with Comparison of Sample Preparation Techniques. Soil Systems. 2025; 9(4):127. https://doi.org/10.3390/soilsystems9040127
Chicago/Turabian StyleBerlina, Anna N., Anatoly V. Zherdev, and Boris B. Dzantiev. 2025. "Development of Immunoenzyme Assay of Herbicide Acetochlor and Its Application to Soil Testing with Comparison of Sample Preparation Techniques" Soil Systems 9, no. 4: 127. https://doi.org/10.3390/soilsystems9040127
APA StyleBerlina, A. N., Zherdev, A. V., & Dzantiev, B. B. (2025). Development of Immunoenzyme Assay of Herbicide Acetochlor and Its Application to Soil Testing with Comparison of Sample Preparation Techniques. Soil Systems, 9(4), 127. https://doi.org/10.3390/soilsystems9040127








