Mathematical Description of Rooting Profiles of Agricultural Crops and its Effect on Transpiration Prediction by a Hydrological Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Description of Root Density Profiles
2.2. Mean Half-Distance between Roots
2.3. Experimental Data
2.4. Parameter Estimation and Model Selection
2.5. Sensitivity of Agro-Hydrological Model Output to Root Profile Shape
3. Results
3.1. Function Selection and Parameter Values
3.2. Sensitivity of Agro-Hydrological Model Output to Root Profile Shape
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Agr. Ecosyst. Environ. 108, 135 144
- Agron. J. 67, 519 523; 77, 1015 1017; 79, 434 438; 80, 271 275; 82, 606 612; 85, 1058 1060; 87, 1210 1216; 90, 511 518; 91, 426 431; 94, 136 145; 72, 981 986
- Aust. J. Exp. Agr. 28, 249 252; 39, 709 720
- Aust. J. Plant Physiol. 5, 169 177
- Can. J. Plant Sci. 44, 240 248
- Crop Sci. 34, 810 812; 42, 773 780
- Eur. J. Agron. 19, 225 237; 8, 117 125.
- Field Crops Res. 11, 325 333; 21, 215 226; 22, 45 57; 37, 205 213; 66, 81 99; 93, 223 236
- Hydrol. Process. 18, 2275 2287
- Irrig. Sci. 12, 45 51; 12, 135 140; 12, 141 144; 12, 145 152; 12, 153 159; 12, 161 168; 17, 69 75
- J. Agr. Sci. (Cambridge) 137, 251 270
- JARQ Jpn. Agr. Res. Q. 34, 81 86
- Plant Soil 200, 107 112; 201, 149 155; 206, 123 136; 207, 87 96; 253, 301 309; 255, 169 177; 255, 387 397; 267, 309 318
- Soil Sci. Soc. Am. J. 68, 529 537; 69, 197 205
- Soil Till. Res. 23, 41 59; 33, 91 108; 41, 25 42; 55, 99 106; 68, 153–161; 80, 103 114
- Z. Pflanz. Bodenkunde 163, 481 489
References
- Hartmann, A.; Šimůnek, J.; Aidoo, M.K.; Seidel, S.J.; Lazarovitch, N. Implementation and application of a root growth module in Hydrus. Vadose Zone J. 2018, 17, 170040. [Google Scholar] [CrossRef]
- Schnepf, A.; Leitner, D.; Landl, M.; Lobet, G.; Mai, T.H.; Morandage, S.; Sheng, C.; Zörner, M.; Vanderborght, J.; Vereecken, H. CRootBox: A structural-functional modelling framework for root systems. Ann. Bot. 2018, 121, 1033–1053. [Google Scholar] [CrossRef] [PubMed]
- Sellers, P.J.; Dickinson, R.E.; Randall, D.A.; Betts, A.K.; Hall, F.G.; Berry, J.A.; Collatz, G.J.; Denning, A.S.; Mooney, H.A.; Nobre, C.A.; et al. Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science 1997, 275, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Pitman, A.J. The evolution of, and revolution in, land surface schemes designed for climate models. Int. J. Climatol. 2003, 23, 479–510. [Google Scholar] [CrossRef]
- Ferguson, I.M.; Jefferson, J.L.; Maxwell, R.M.; Kollet, S.J. Effects of root water uptake formulation on simulated water and energy budgets at local and basin scales. Environ. Earth Sci. 2016, 75, 316. [Google Scholar] [CrossRef]
- Zeng, X.; Dai, Y.J.; Dickinson, R.E.; Shaikh, M. The role of root distribution for climate simulation over land. Geophys. Res. Let. 1998, 25, 4533–4536. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Guidelines for Predicting Crop Water Requirements; Irrigation and Drainage Paper 24; Food and Agriculture Organization of the United Nations: Rome, Italy, 1975; p. 179. [Google Scholar]
- Bouman, B.A.M.; Van Keulen, H.; Van Laar, H.H.; Rabbinge, R. The ‘School of de Wit’ crop growth simulation models: A pedigree and historical overview. Agric. Syst. 1996, 52, 171–198. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef]
- Kroes, J.G.; Van Dam, J.C.; Bartholomeus, R.P.; Groenendijk, P.; Heinen, M.; Hendriks, R.F.A.; Mulder, H.M.; Supit, I.; Van Walsum, P.E.V. SWAP Version 4: Theory and Description of User Manual; Report 2780; Wageningen Environmental Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Bonfante, A.; Terribile, F.; Bouma, J. Refining physical aspects of soil quality and soil health when exploring the effects of soil degradation and climate change on biomass production: An Italian case study. Soil 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Ganmore-Neumann, R.; Ben-Hayyim, G. Root architecture effects on nutrient uptake. In Basic Life Sciences: Biology of Root Formation and Development; Altman, A., Waisel, Y., Eds.; Springer: Boston, MA, USA, 1997; Volume 65. [Google Scholar]
- Doussan, C.; Pages, L.; Pierret, A. Soil exploration and resource acquisition by plant roots: An architectural and modelling point of view. In Sustainable Agriculture, 1st ed.; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 419–431. [Google Scholar]
- Wu, L.; McGechan, M.B.; Watson, C.A.; Baddeley, J.A. Developing existing plant root system architecture models to meet future agricultural challenges. Adv. Agron. 2005, 41, 91–145. [Google Scholar]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Darrah, P.R.; Jones, D.L.; Kirk, G.J.D.; Roose, T. Modelling the rhizosphere: A review of methods for ‘upscaling’ to the whole-plant scale. Eur. J. Soil Sci. 2006, 57, 13–25. [Google Scholar] [CrossRef]
- Schneider, C.L.; Attinger, S.; Delfs, J.O.; Hildebrandt, A. Implementing small scale processes at the soil-plant interface—The role of root architectures for calculating root water uptake profiles. Hydrol. Earth Syst. Sci. 2010, 14, 279–289. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, I.S.; Dresbøll, D.B.; Thorup-Kristensen, K. Winter wheat cultivars and nitrogen (N) fertilization—Effects on root growth, N uptake efficiency and N use efficiency. Eur. J. Agron. 2015, 68, 38–49. [Google Scholar] [CrossRef]
- Shahzad, Z.; Amtmann, A. Food for thought: How nutrients regulate root system architecture. Curr. Opin. Plant Biol. 2017, 39, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Feddes, R.A.; Raats, P.A.C. Parameterizing the soil-water-plant root system. In Unsaturated-Zone Modeling: Progress, Challenges and Applications; Feddes, R.A., de Rooij, G.H., van Dam, J.C., Eds.; Wageningen UR Frontis Series: Dordrecht, The Netherlands, 2004; pp. 95–141. [Google Scholar]
- De Willigen, P.; Heinen, M.; Kirkham, M.B. Transpiration and root water uptake. In Encyclopedia of Hydrological Sciences; Anderson, M.H., Ed.; John Wiley and Sons: London, UK, 2005; Chapter 70. [Google Scholar]
- De Jong van Lier, Q.; Metselaar, K.; Van Dam, J.C. Root water extraction and limiting soil hydraulic conditions estimated by numerical simulation. Vadose Zone J. 2006, 5, 1264–1277. [Google Scholar] [CrossRef]
- De Jong van Lier, Q.; Van Dam, J.C.; Durigon, A.; Dos Santos, M.A.; Metselaar, K. Modeling water potentials and flows in the soil–plant system comparing hydraulic resistances and transpiration reduction functions. Vadose Zone J. 2013, 12, 1–20. [Google Scholar] [CrossRef]
- Raats, P.A.C. Uptake of water from soils by plant roots. Transp. Porous Med. 2007, 68, 5–28. [Google Scholar] [CrossRef] [Green Version]
- Diggle, A.J. ROOTMAP—A model in three-dimensional coordinates of the growth and structure of fibrous root systems. Plant Soil 1998, 105, 169–178. [Google Scholar] [CrossRef]
- Couvreur, V.; Vanderborght, J.; Javaux, M. A simple three-dimensional macroscopic root water uptake model based on the hydraulic architecture approach. Hydrol. Earth Syst. Sci. 2012, 16, 2957–2971. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M.; Van de Geijn, S.C. Root, shoot and soil parameters required for process-oriented models of crop growth limited by water or nutrients. Plant Soil 1996, 183, 1–25. [Google Scholar] [CrossRef]
- De Jong van Lier, Q.; Van Dam, J.C.; Metselaar, K.; de Jong, R.; Duijnisveld, W.H.M. Macroscopic root water uptake distribution using a matric flux potential approach. Vadose Zone J. 2008, 7, 1065–1078. [Google Scholar] [CrossRef]
- Gerwitz, A.; Page, E.R. An empirical mathematical model to describe plant root systems. J. Appl. Ecol. 1974, 11, 773–781. [Google Scholar] [CrossRef]
- O’Toole, J.C.; Bland, W.L. Genotypic variation in crop plant root systems. Adv. Agron. 1987, 85, 181–219. [Google Scholar]
- Zuo, Q.; Jie, F.; Zhang, R.; Meng, L. A generalized function of wheat’s root length density distributions. Vadose Zone J. 2004, 3, 271–277. [Google Scholar] [CrossRef]
- Hodgkinson, L.; Dodd, I.C.; Binley, A.; Ashton, R.W.; White, R.P.; Watts, C.M.; Whalley, W.R. Root growth in field-grown winter wheat: Some effects of soil conditions, season and genotype. Eur. J. Agron. 2017, 91, 74–83. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Van Wijk, M.T. Understanding plant rooting patterns in semi-arid systems: An integrated model analysis of climate, soil type and plant biomass. Glob. Ecol. Biogeogr. 2011, 20, 331–342. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Ober, E.S.; Sharp, R.E. Regulation of root growth responses to water deficit. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 33–53. [Google Scholar]
- Saidi, A.; Ookawa, T.; Hirasawa, T. Responses of root growth to moderate soil water deficit in wheat seedlings. Plant Prod. Sci. 2010, 13, 261–268. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 2005, 126, 129–140. [Google Scholar] [CrossRef]
- Stalham, M.A.; Allen, E.J. Effect of variety, irrigation regime and planting date on depth, rate, duration and density of root growth in the potato (Solanum tuberosum) Crop. J. Agric. Sci. 2001, 137, 251–270. [Google Scholar] [CrossRef]
- France, J.; Thornley, J.H.M. Mathematical Models in Agriculture: A Quantitative Approach to Problems in Agriculture and Related Sciences; University of Chicago Press: Chicago, IL, USA, 1985; p. 335. [Google Scholar]
- Richards, J.R. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Verduin, J. Baule-Mitscherlich limiting factor equation. Science 1953, 117, 392. [Google Scholar] [CrossRef] [PubMed]
- De Willigen, P.; Heinen, M.; Mollier, A.; Van Noordwijk, M. Two-dimensional growth of a root system modelled as a diffusion process. I. Analytical solutions. Plant Soil 2002, 240, 225–234. [Google Scholar] [CrossRef]
- Gardner, W.R. Dynamic aspects of water availability to plants. Soil Sci. 1960, 89, 63–73. [Google Scholar] [CrossRef]
- Jin, K.; Shen, J.; Ashton, R.W.; Dodd, I.C.; Parry, M.A.J.; Whalley, W.R. How do roots elongate in a structured soil? J. Exp. Bot. 2013, 64, 4761–4777. [Google Scholar] [CrossRef]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. 2004, 18. [Google Scholar] [CrossRef]
- GenStat Committee. GenStat® Release 7.1 Reference Manual Part 2: Directives; VSN Int.: Oxford, UK, 2003. [Google Scholar]
- Mian, M.A.R.; Nafziger, E.D.; Kolb, F.L.; Teyker, R.H. Root size and distribution of field-grown wheat genotypes. Crop Sci. 1994, 34, 810–812. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–897. [Google Scholar] [CrossRef]
- Mualem, Y. A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use and Crop Yield; Simulation Monograph Series; PUDOC: Wageningen, The Netherlands, 1978. [Google Scholar]
- Taylor, S.A.; Ashcroft, G.M. Physical Edaphology; Freeman and Co.: San Francisco, CA, USA, 1972; pp. 434–435. [Google Scholar]
- Boons-Prins, E.R.; De Koning, G.H.J.; Van Diepen, C.A.; Penning de Vries, F.W.T. Crop-Specific Parameters for Yield Forecasting across the European Community; Simulation Reports CABO-TT, No. 32; Wageningen University & Research: Wageningen, The Netherlands, 1993; p. 160. [Google Scholar]
- Pinto, V.M.; van Dam, J.C.; de Jong van Lier, Q.; Reichardt, K. Intercropping simulation using the SWAP model: Development of a 2x1D Algorithm. Agriculture 2019, 9, 126. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; FAO Irrigation and Drainage Paper 66; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; p. 500. [Google Scholar]
- Greaves, G.E.; Wang, Y. Yield response, water productivity, and seasonal water production functions for maize under deficit irrigation water management in southern Taiwan. Plant Prod. Sci. 2017, 20, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root distribution by depth for temperate agricultural crops. Field Crops Res. 2016, 189, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, N.J. Simple physics-based models of compensatory plant water uptake: Concepts and eco-hydrological consequences. Hydrol. Earth Syst. Sci. 2011, 15, 3431–3446. [Google Scholar] [CrossRef]
- Dos Santos, M.A.; De Jong van Lier, Q.; Van Dam, J.C.; Bezerra, A.H.F. Benchmarking test of empirical root water uptake models. Hydrol. Earth Syst. Sci. 2017, 21, 473–493. [Google Scholar] [CrossRef] [Green Version]
- Moraes, M.T.; Bengough, A.G.; Debiasi, H.; Franchini, J.C.; Levien, R.; Schnepf, A.; Leitner, D. Mechanistic framework to link root growth models with weather and soil physical properties, including example applications to soybean growth in Brazil. Plant Soil 2018, 428, 67–92. [Google Scholar] [CrossRef] [Green Version]
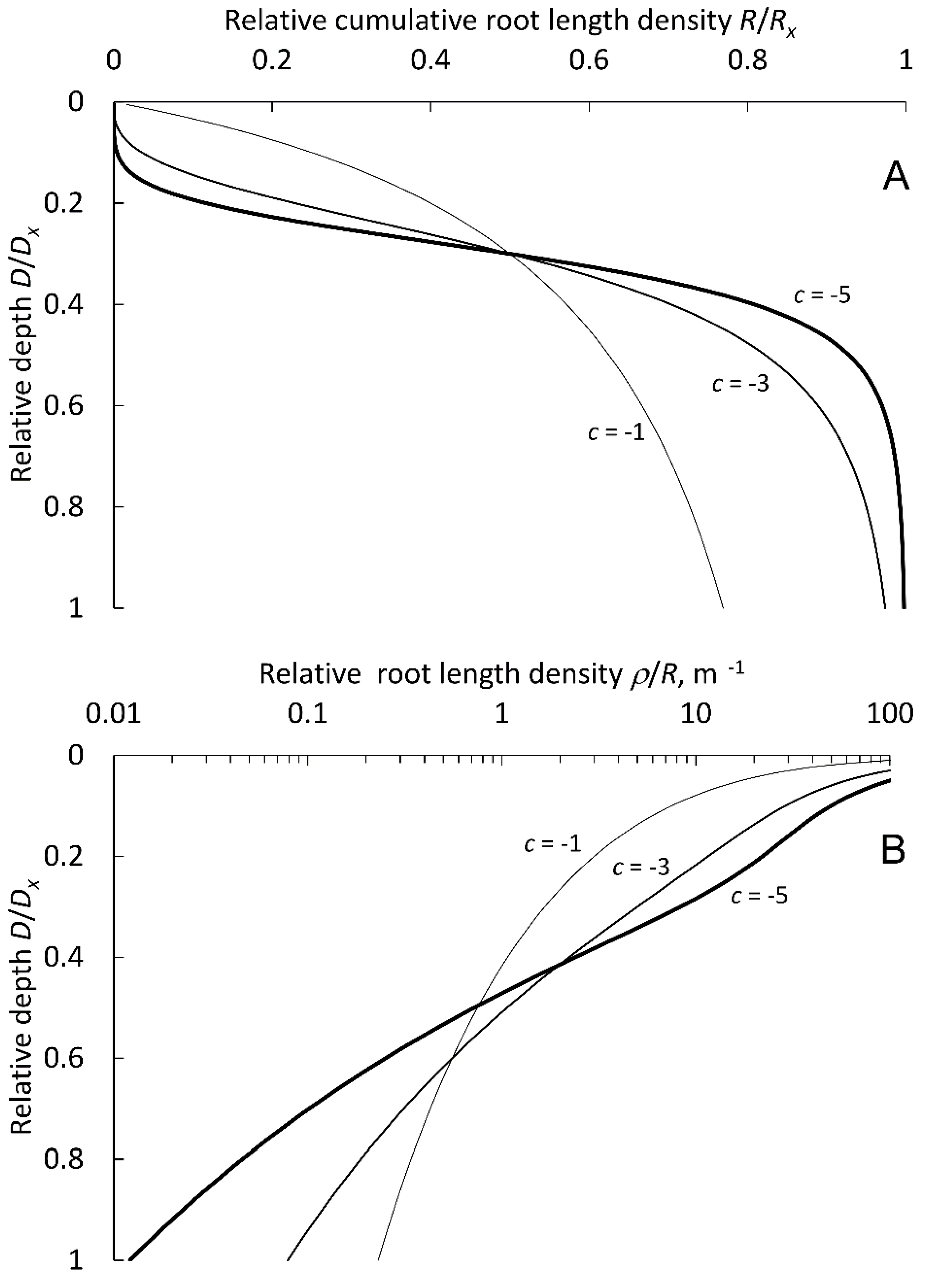
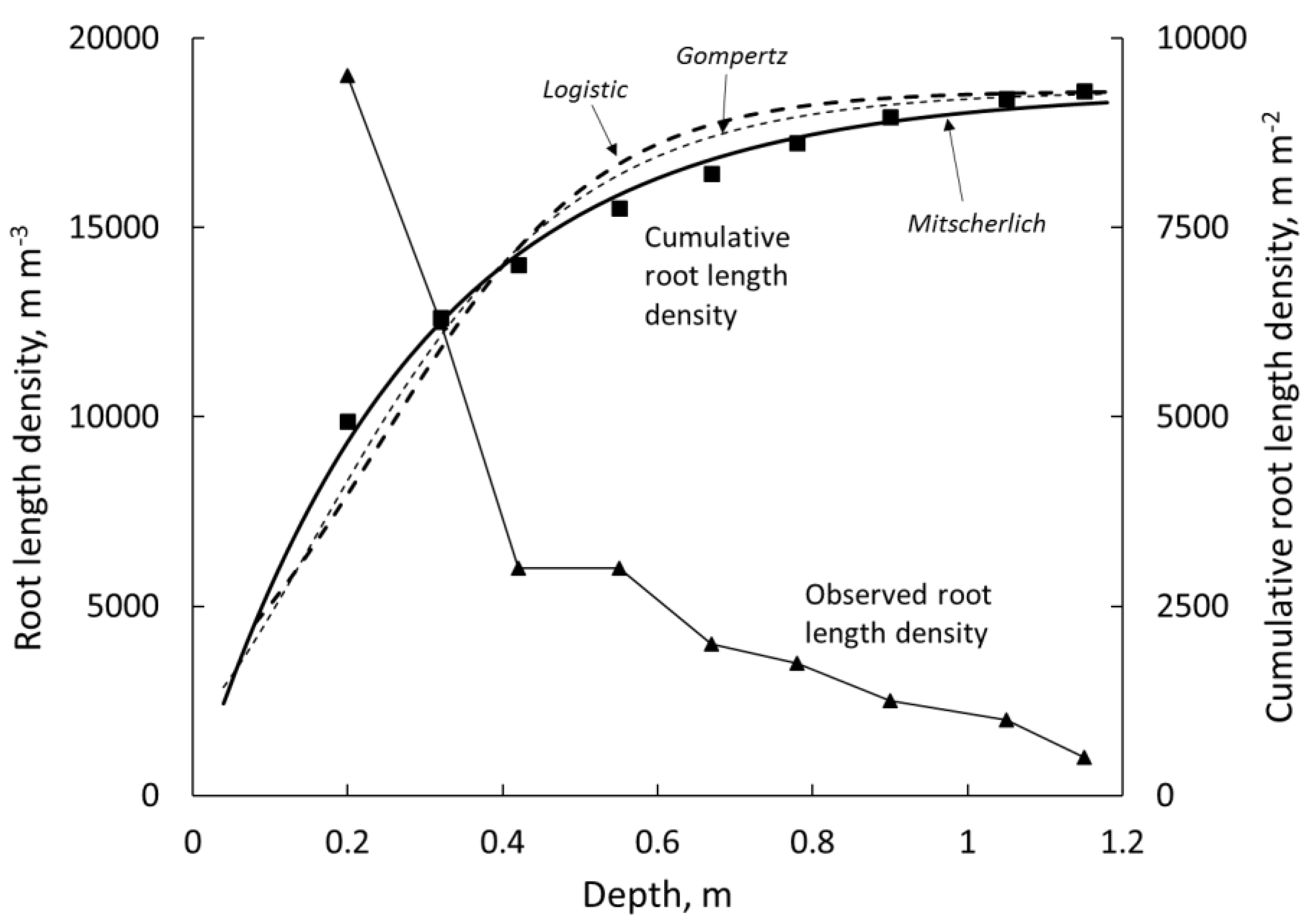
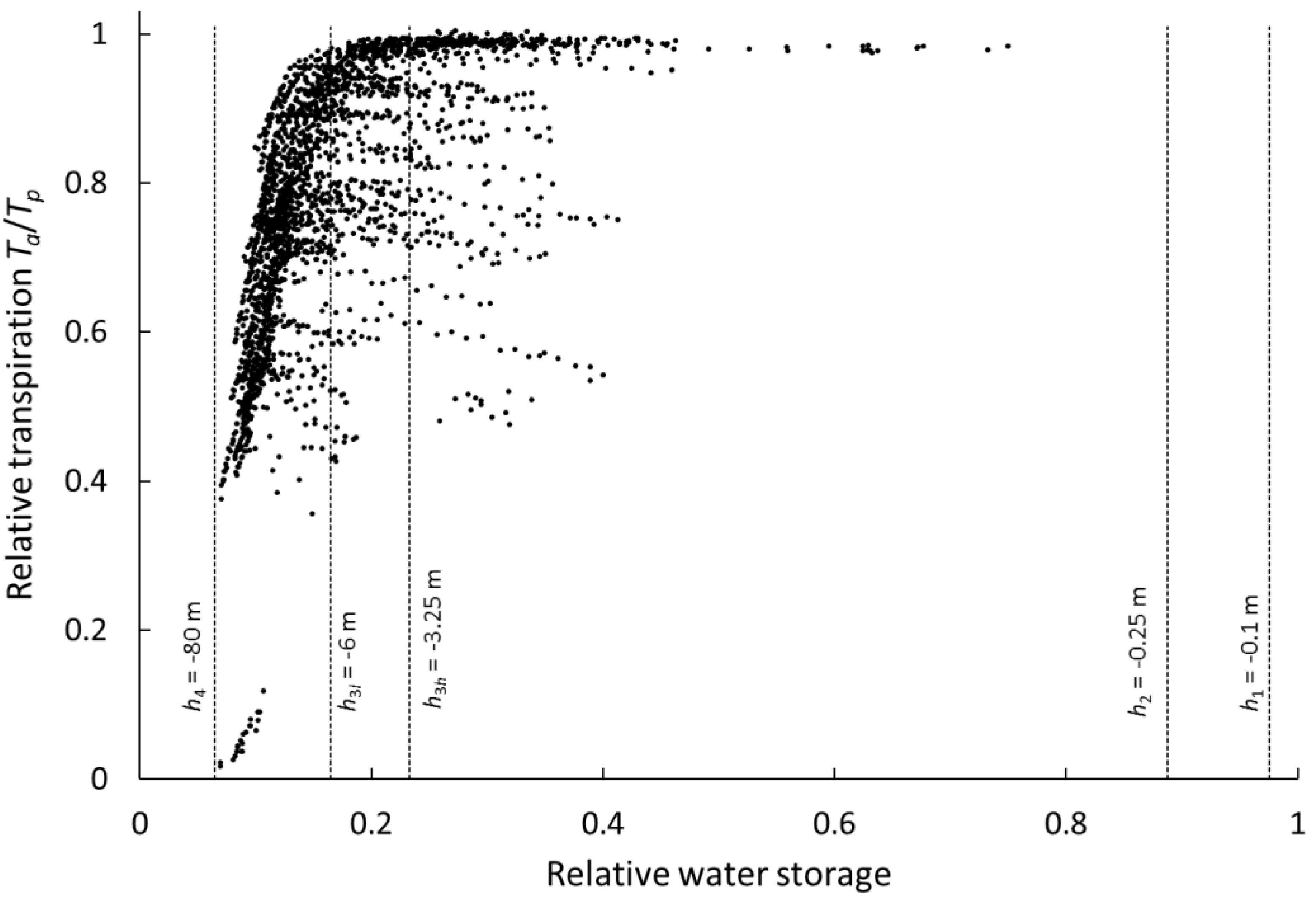
| Equation # | Df | D50 | D95 | |
|---|---|---|---|---|
| Generalized logistic | (2) | |||
| Logistic | (4) | m | ||
| Exponential (Mitscherlich) | (12) | |||
| Gompertz | (14) |
| Crop Group | Contains Species | Species Local Name | Relative Proportion of the Area | Number of Sources in Database |
|---|---|---|---|---|
| Wheat | Triticum aestivum Triticum turgidum xTriticosecale | Bread wheat Durum wheat Triticale | 22 | 12 |
| Maize | Zea mays | 13 | 9 | |
| Rice | Oryza sativa Oryza glaberrima | 11 | 7 | |
| Barley | Hordeum vulgare | 9 | 2 | |
| Soybean | Glycine max | 5 | 6 | |
| Pulses | Cajanus cajan Phaseolus aureus Pisum sativum Vicia faba Vigna unguiculata (= Vigna sinensis) | Pigeon pea Mung bean Pea Faba bean Cowpea | 4 | 6 |
| Cotton | Gossypium hirsutum | 3 | 6 | |
| Potato | Solanum tuberosum | 3 | 3 | |
| Sunflower | Helinathus annuus | 2 | 4 | |
| Rye | Secale cereale | 2 | 3 | |
| Rapeseed | Brassica napus Brassica rapa | 2 | 4 | |
| Sugarbeet | Beta vulgaris saccharifera | 1 | 3 | |
| Other | Arachis hypogea Avena sativa Lolium multiflorum Pennisetum glaucum Raphanus sativus oleiformis Sorghum bicolor Trifolium incarnataum Vicia villosa | Peanut Oats Italian ryegrass Pearl millet Fodder radish Sorghum Crimson clover Hairy vetch | 11 |
| Depth (m) | θr | θs | α (m−1) | n | λ | Ks (m·d−1) |
|---|---|---|---|---|---|---|
| 0.00–0.30 | 0.01 | 0.43 | 2.27 | 1.548 | −1.983 | 0.1965 |
| 0.30–2.00 | 0.02 | 0.38 | 2.14 | 2.075 | −1.039 | 0.2556 |
| Development Stage | Leaf Area Index (m2 m−2) | Crop Height (m) | Rooting Depth (m) |
|---|---|---|---|
| 0 | 0.05 | 0.01 | 0.05 |
| 0.30 | 0.14 | 0.15 | 0.2 |
| 0.50 | 0.61 | 0.40 | 0.5 |
| 0.70 | 4.1 | 1.40 | 0.8 |
| 1.0 | 5.0 | 1.70 | 0.9 |
| 1.4 | 5.8 | 1.80 | 0.9 |
| 2.0 | 5.2 | 1.75 | 0.9 |
| Description | Parameter | Value | Unit |
|---|---|---|---|
| Plant maximum height | Hmax | 175 | cm |
| Reflection coefficient, Albedo | Cref | 0.20 | ‒ |
| Minimum canopy resistance | RSC | 131 | s m−1 |
| Extinction coefficient for diffuse visible light | Kdif | 0.60 | ‒ |
| Extinction coefficient for direct visible light | Kdir | 0.75 | ‒ |
| Length of crop cycle - fixed | LCC | 120 | d |
| Interception coefficient Von Hoyningen-Hune and Braden | COFAB | 0.25 | cm |
| Sigmoid Function | Convergence (n = 570) | D95 > Dmax | R2adj | Mean D50 (m) | Mean D95 (m) |
|---|---|---|---|---|---|
| Generalized logistic (Equation (2)) | 54 | 5 | 1.00 | 0.322 | 0.644 |
| Logistic (Equation (4)) | 568 | 48 | 0.98 | 0.267 | 0.560 |
| Exponential (Equation (12)) | 568 | 422 | 0.98 | 0.460 | 1.942 |
| Gompertz (Equation (14)) | 568 | 174 | 0.99 | 0.272 | 0.699 |
| Crop | D50 (± Standard Error) (m) | D95 (± Standard Error) (m) | n | rn (± Standard Error) (mm) | nr | c | Rx (mm−1) |
|---|---|---|---|---|---|---|---|
| All crops | 0.27 (±0.24) | 0.56 (±0.48) | 568 | 5.6 (±5.7) | 494 | −3.969 | 10.2 |
| Wheat | 0.22 (±0.10) | 0.49 (±0.25) | 80 | 6.4 (±6.1) | 50 | −3.677 | 8.5 |
| Maize | 0.39 (±0.20) | 0.80 (±0.40) | 48 | 6.5 (±4.7) | 40 | −4.071 | 7.4 |
| Rice | 0.13 (±0.07) | 0.27 (±0.13) | 87 | 5.2 (±5.3) | 87 | −4.048 | 11.6 |
| Barley | 0.17 (±0.06) | 0.33 (±0.09) | 10 | 4.8 (±3.7) | 7 | −4.286 | 12.9 |
| Soybean | 0.27 (±0.17) | 0.60 (±0.33) | 42 | 7.1 (±8.0) | 40 | −3.716 | 6.8 |
| Pulses | 0.28 (±0.12) | 0.59 (±0.25) | 39 | 4.7 (±0.7) | 37 | −3.942 | 14.6 |
| Cotton | 0.32 (±0.10) | 0.67 (±0.22) | 97 | 5.4 (±1.3) | 97 | −3.989 | 10.9 |
| Potato | 0.32 (±0.06) | 0.62 (±0.13) | 40 | 3.2 (±0.6) | 40 | −4.482 | 27.7 |
| Sunflower | 0.35 (±0.19) | 0.79 (±0.46) | 26 | 4.3 (±1.2) | 23 | −3.596 | 19.1 |
| Rye | 0.22 (±0.06) | 0.47 (±0.13) | 7 | 4.6 (±2.1) | 6 | −3.955 | 15.2 |
| Rapeseed | 0.18 (±0.04) | 0.39 (±0.09) | 30 | 2.7 (±0.9) | 21 | −3.858 | 45.3 |
| Sugar beet | 0.48 (±0.15) | 0.95 (±0.28) | 13 | 3.9 (±1.8) | 7 | −4.349 | 19.2 |
| Other | 0.28 (±0.18) | 0.56 (±0.37) | 49 | 10.3 (±13.3) | 39 | −4.194 | 2.9 |
| Autumn Maize (February–June) | Summer Maize (November–February) | |||||
|---|---|---|---|---|---|---|
| D50 (m) | 0.15 | 0.30 | 0.45 | 0.15 | 0.30 | 0.45 |
| c = −1 | 0.017 (23%) | 0.006 (23%) | 0 | 0.010 (53%) | 0.004 (65%) | 0 |
| c = −3 | 0.049 (17%) | 0.035 (18%) | 0.019 (22%) | 0.030 (48%) | 0.018 (56%) | 0.008 (68%) |
| c = −5 | 0.053 (16%) | 0.041 (18%) | 0.026 (21%) | 0.033 (47%) | 0.021 (56%) | 0.011 (68%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metselaar, K.; Pinheiro, E.A.R.; de Jong van Lier, Q. Mathematical Description of Rooting Profiles of Agricultural Crops and its Effect on Transpiration Prediction by a Hydrological Model. Soil Syst. 2019, 3, 44. https://doi.org/10.3390/soilsystems3030044
Metselaar K, Pinheiro EAR, de Jong van Lier Q. Mathematical Description of Rooting Profiles of Agricultural Crops and its Effect on Transpiration Prediction by a Hydrological Model. Soil Systems. 2019; 3(3):44. https://doi.org/10.3390/soilsystems3030044
Chicago/Turabian StyleMetselaar, Klaas, Everton Alves Rodrigues Pinheiro, and Quirijn de Jong van Lier. 2019. "Mathematical Description of Rooting Profiles of Agricultural Crops and its Effect on Transpiration Prediction by a Hydrological Model" Soil Systems 3, no. 3: 44. https://doi.org/10.3390/soilsystems3030044





