Bacillus cereus Induced Necrotizing Fasciitis Mimicking Gastroenteritis: A Case Report
Abstract
:1. Introduction
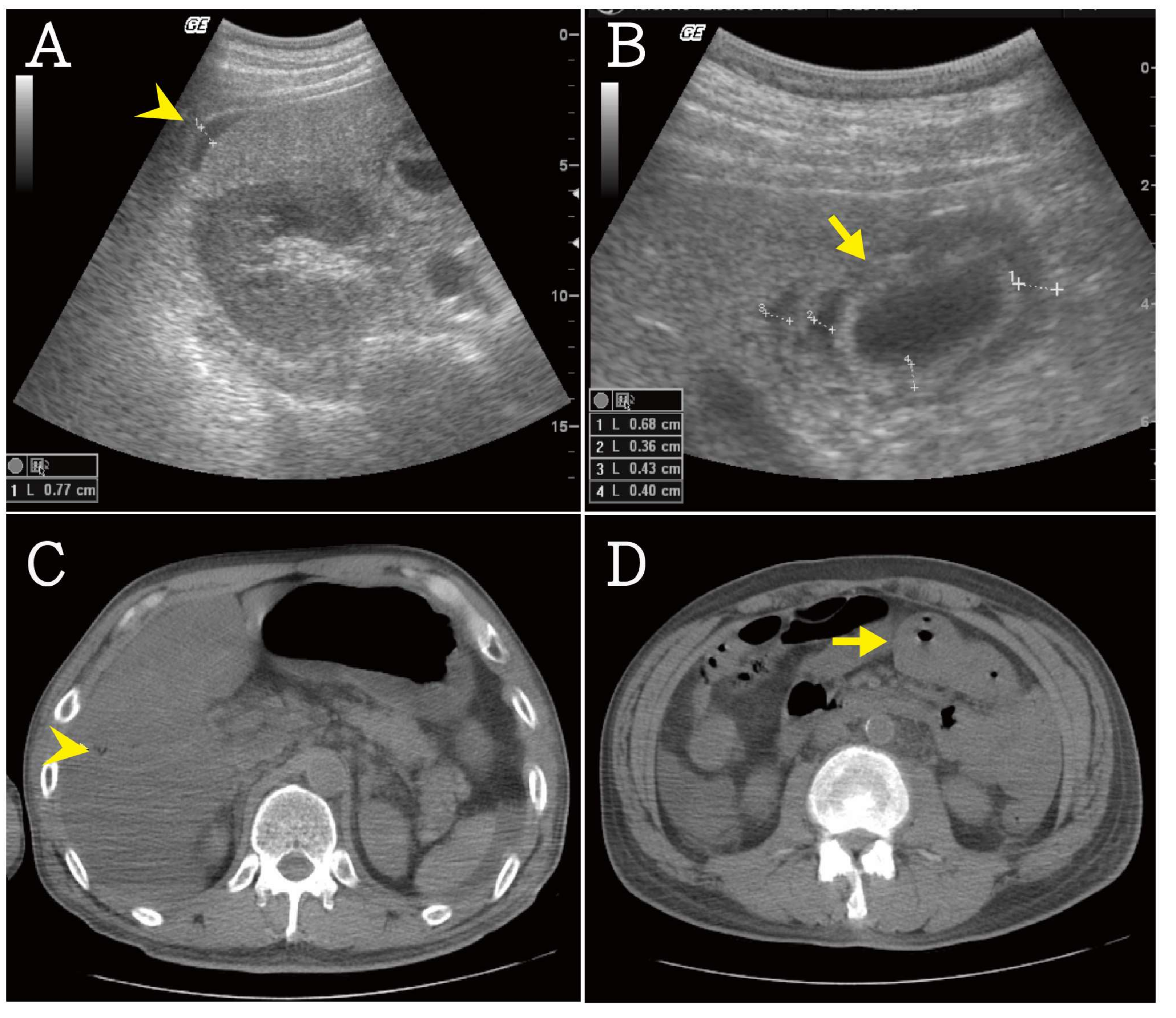
2. Case Presentation Section
3. Discussion
Author Contributions
Conflicts of Interest
References
- Hutchens, A.; Gupte, A.; McAuliffe, P.F.; Schain, D.; Soldevila-Pico, C.; Knapik, J.A.; Fujita, S.; Mozingo, D.W.; Richards, W.T. Bacillus cereus necrotizing fasciitis in a patient with end-stage liver disease. Surg. Infect. 2010, 11, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Minnaganti, V.R.; Patel, P.J.; Iancu, D.; Schoch, P.E.; Cunha, B.A. Necrotizing fasciitis caused by Aeromonas hydrophila. Heart Lung J. Crit. Care 2000, 29, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Puvanendran, R.; Huey, J.C.M.; Pasupathy, S. Necrotizing fasciitis. Can. Fam. Phys. 2009, 55, 981–987. [Google Scholar]
- Wong, C.H.; Chang, H.C.; Pasupathy, S.; Khin, L.W.; Tan, J.L.; Low, C.O. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J. Bone Jt. Surg. Am. Vol. 2003, 85, 1454–1460. [Google Scholar] [CrossRef]
- Hefny, A.F.; Eid, H.O.; Al-Hussona, M.; Idris, K.M.; Abu-Zidan, F.M. Necrotizing fasciitis: A challenging diagnosis. Eur. J. Emerg. Med. 2007, 14, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Frazee, B.W.; Fee, C.; Lynn, J.; Wang, R.; Bostrom, A.; Hargis, C.; Moore, P. Community-acquired necrotizing soft tissue infections: A review of 122 cases presenting to a single emergency department over 12 years. J. Emerg. Med. 2008, 34, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.T.; Saadai, P.; Greenstein, A.; Divino, C.M. Postprocedural necrotizing fasciitis: A 10-year retrospective review. Am. Surg. 2008, 74, 405–409. [Google Scholar] [PubMed]
- Voros, D.; Pissiotis, C.; Georgantas, D.; Katsaragakis, S.; Antoniou, S.; Papadimitriou, J. Role of early and extensive surgery in the treatment of severe necrotizing soft tissue infection. Br. J. Surg. 1993, 80, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Lewis, F., Jr.; Hadley, K.; Blaisdell, F.W. Bacteriology of necrotizing fasciitis. Am. J. Surg. 1977, 134, 52–57. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Sada, A.; Misago, N.; Okawa, T.; Narisawa, Y.; Ide, S.; Nagata, M.; Mitsumizo, S. Necrotizing fasciitis and myonecrosis “synergistic necrotizing cellulitis” caused by Bacillus cereus. J. Dermatol. 2009, 36, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Boulinguez, S.; Viraben, R. Cutaneous Bacillus cereus infection in an immunocompetent patient. J. Am. Acad. Dermatol. 2002, 47, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, F.; Bodansky, H.J. Bacillus cereus causing widespread necrotising skin infection in a diabetic person. Pract. Diabetes 2015, 32, 169–170. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.-C.; Chen, Y.-L.; Yiang, G.-T.; Chen, T.-Y.; Wu, M.-Y.; Ni, B.-Y.; Lin, C.-H. Bacillus cereus Induced Necrotizing Fasciitis Mimicking Gastroenteritis: A Case Report. Reports 2018, 1, 9. https://doi.org/10.3390/reports1010009
Lee L-C, Chen Y-L, Yiang G-T, Chen T-Y, Wu M-Y, Ni B-Y, Lin C-H. Bacillus cereus Induced Necrotizing Fasciitis Mimicking Gastroenteritis: A Case Report. Reports. 2018; 1(1):9. https://doi.org/10.3390/reports1010009
Chicago/Turabian StyleLee, Ling-Chi, Yu-Long Chen, Giou-Teng Yiang, Tsu-Yi Chen, Meng-Yu Wu, Bo-Yang Ni, and Ching-Hsiang Lin. 2018. "Bacillus cereus Induced Necrotizing Fasciitis Mimicking Gastroenteritis: A Case Report" Reports 1, no. 1: 9. https://doi.org/10.3390/reports1010009




