Photocatalytic Activity for Hydrogen Evolution of Heteroatom-Doped SrTiO3 Prepared Using a Graphitic-Carbon Nitride Nanosheet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Photocatalyst
2.3. Characterization
2.4. Photocatalytic Reaction
3. Results and Discussion
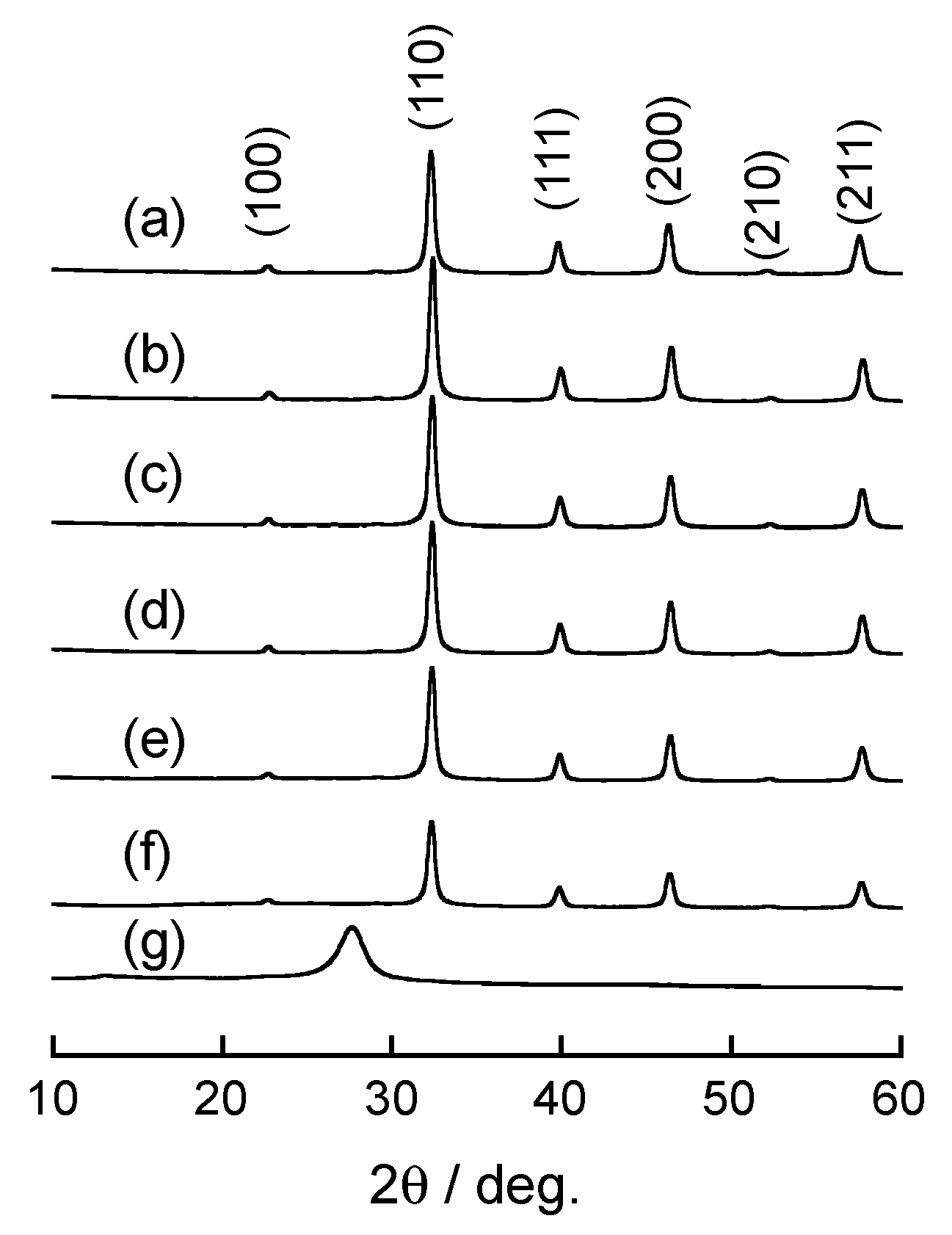
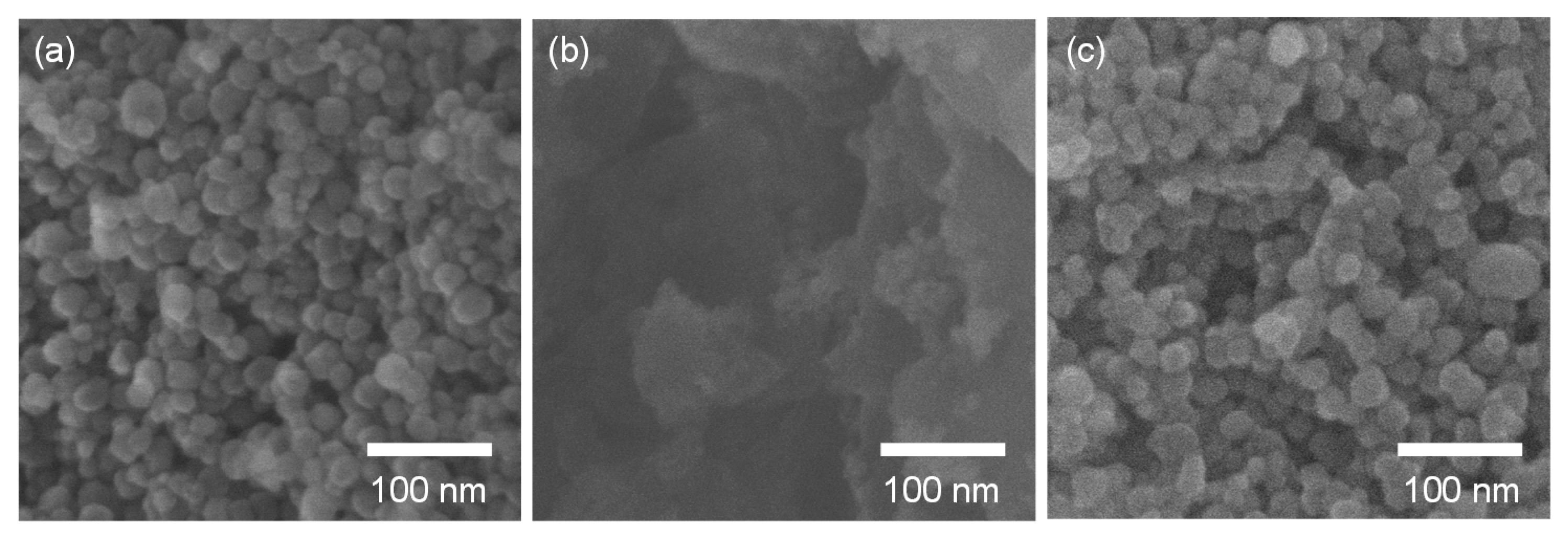
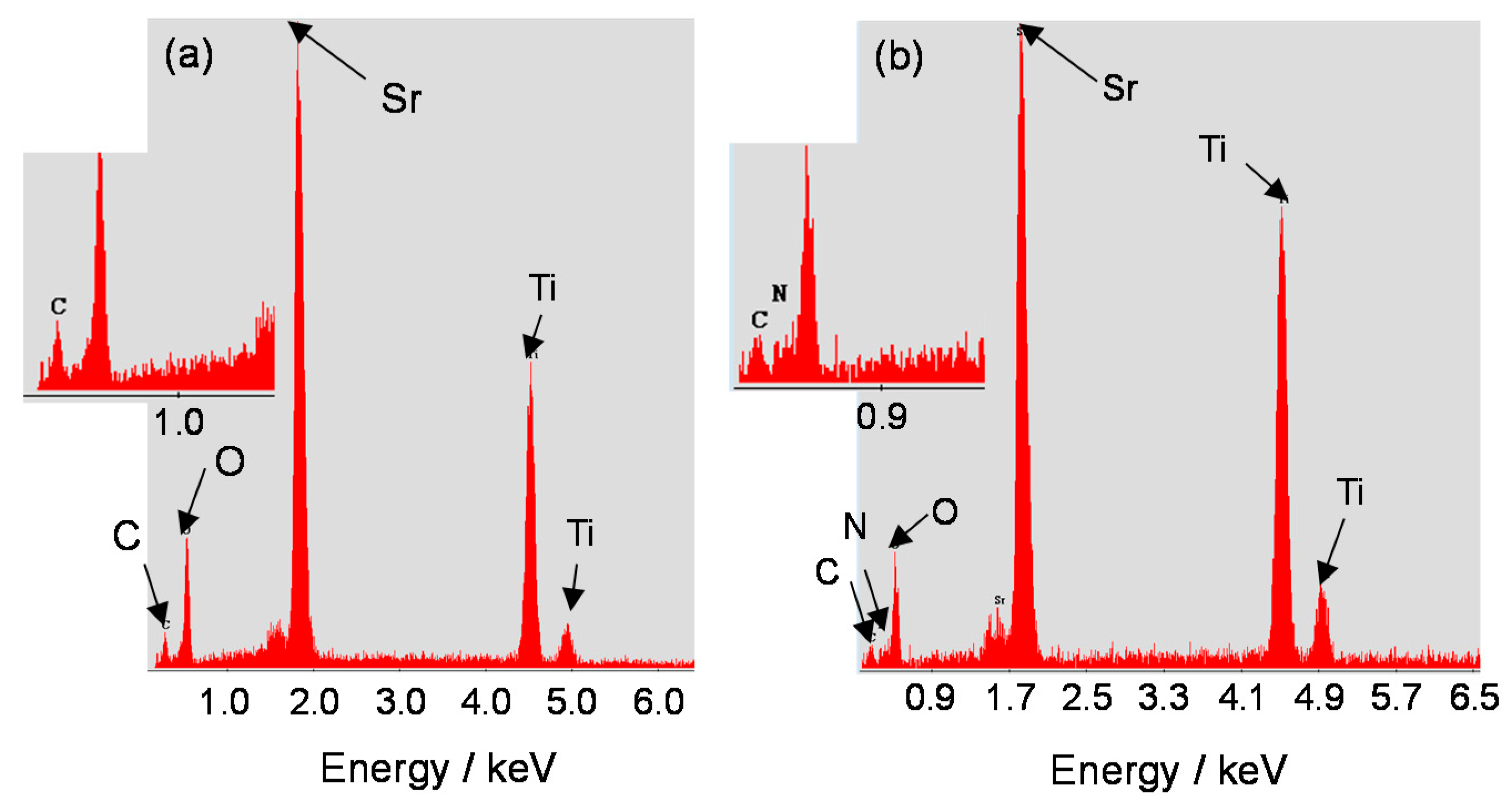
3.1. Characterization of Doped SrTiO3
3.2. The Photocatalytic Property of Doped SrTiO3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubo, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, Y.-J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.L.; Wang, C.F.; Ni, M.; Leung, M.K.H.; Wang, X.X.; Fu, X.Z. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Ishii, T.; Kato, H.; Kudo, A. H2 evolution from an aqueous methanol solution on SrTiO3 photocatalysts codoped with chromium and tantalum ions under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 181–186. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic activity of S-doped TiO2 photocatalyst under visible light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-doped anatase TiO2 powder as a visible-light sensitive photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Takata, T.; Hara, M.; Saito, N.; Toda, K.; Inoue, Y.; Kobayashi, H.; Domen, K. Overall water splitting on (Ga1-xZnx)(N1-xOx) solid solution photocatalyst: Relationship between physical properties and photocatalytic activity. J. Phys. Chem. B 2005, 109, 20504–20510. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Sun, S.; Sun, M.; Fang, Y.; Wang, Y.; Wang, H. One-step in situ calcination synthesis of g-C3N4/N-TiO2 hybrids with enhanced photoactivity. RSC Adv. 2016, 6, 13063–13071. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer semiconductors for artificial photosynthesis: Hydrogen evolution by mesoporous graphitic carbon nitride with visible light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesizedviadirect polymerization of ureafor efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–82. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Randorn, C.; Irvine, J.T.S. g-C3N4 coated SrTiO3 as an efficient photocatalyst for H2 production in aqueous solution under visible light irradiation. Int. J. Hydrogen Energy 2011, 36, 13501–13507. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, Q.; Yang, J. Enhanced visible light activity on direct contact Z-scheme g-C3N4-TiO2 photocatalyst. Appl. Surf. Sci. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Q.; Zhong, J.; Li, J.; Hu, C.; Deng, Z.; Duan, R. In-Situ construction of direct Z-scheme Bi2WO6/g-C3N4 composites with remarkably promoted solar-driven photocatalytic activity. Mater. Chem. Phys. 2018, 217, 207–215. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; Sittert, C.G.C.E.; Governder, P.P. Insights into the photocatalytic mechanism of mediator-free direct Z-scheme g-C3N4/Bi2MoO6(010) and g-C3N4/Bi2WO6(010) heterostructures: A hybrid density functional theory study. Appl. Surf. Sci. 2018, 427, 487–498. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, L.; Guo, Y.; Meng, J.; Guo, Y. Easy dispersion and excellent visible-light photocatalytic activity of the ultrathin urea-derived g-C3N4 nanosheets. Appl. Surf. Sci. 2017, 425, 535–546. [Google Scholar] [CrossRef]
- He, Y.M.; Wang, Y.; Zhang, L.H.; Teng, B.T.; Fan, M.H. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar]
- Sun, M.X.; Fang, Y.L.; Wang, Y.; Sun, S.F.; He, J.; Yan, Z. Synthesis of Cu2O/graphene/rutile TiO2 nanorod ternary composites with enhanced photocatalytic activity. J. Alloys Compd. 2015, 650, 520–527. [Google Scholar] [CrossRef]
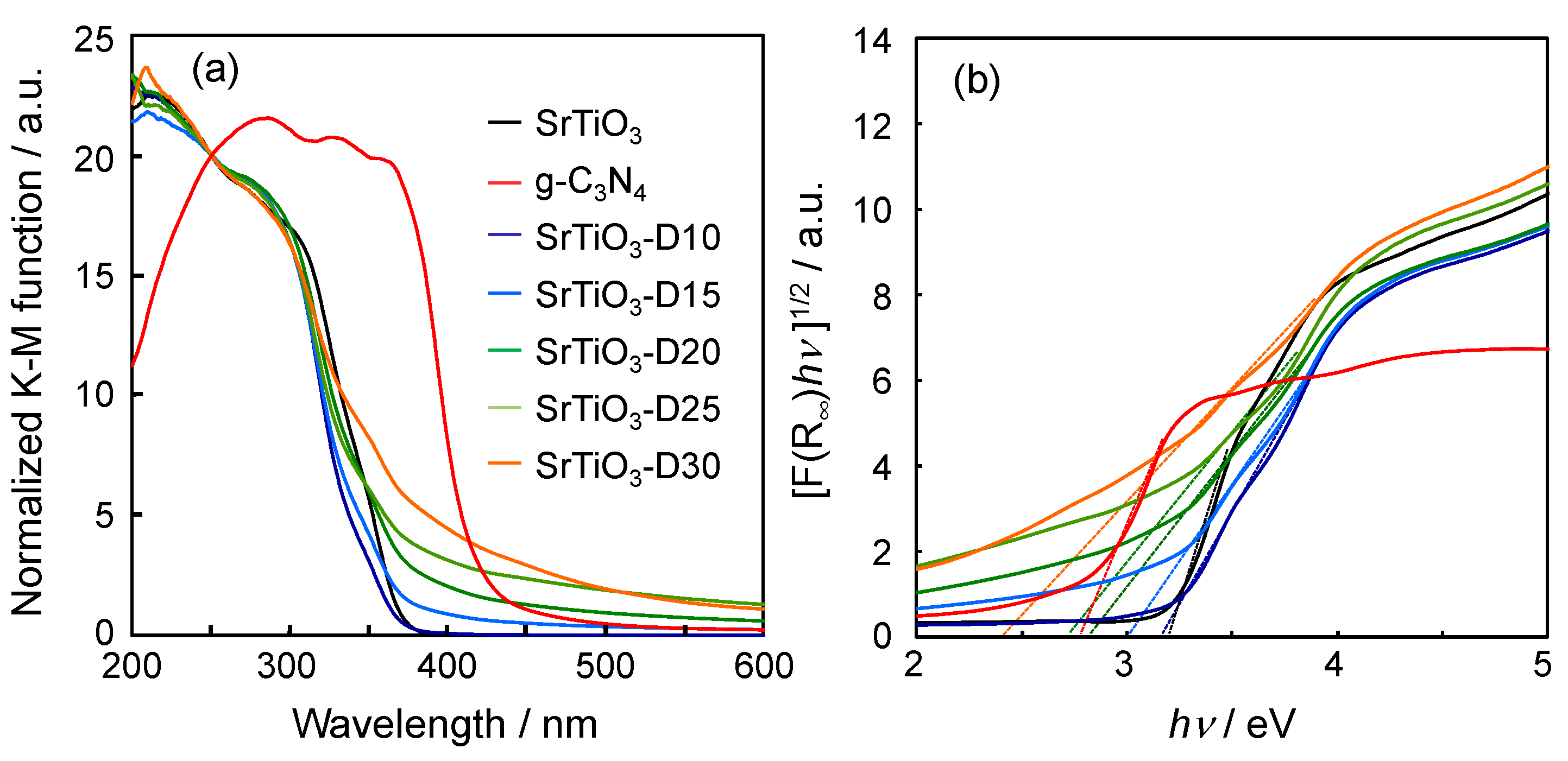
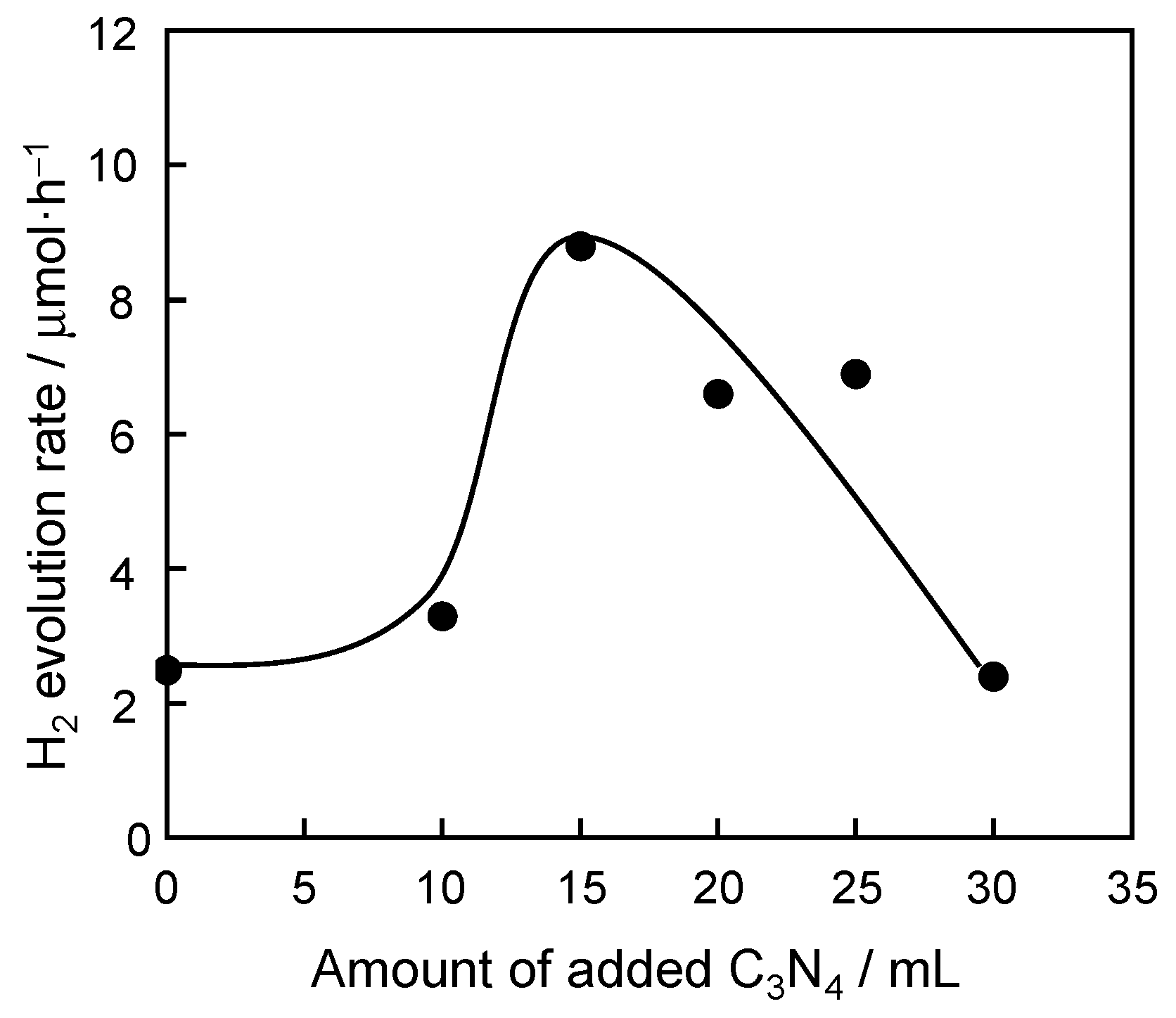
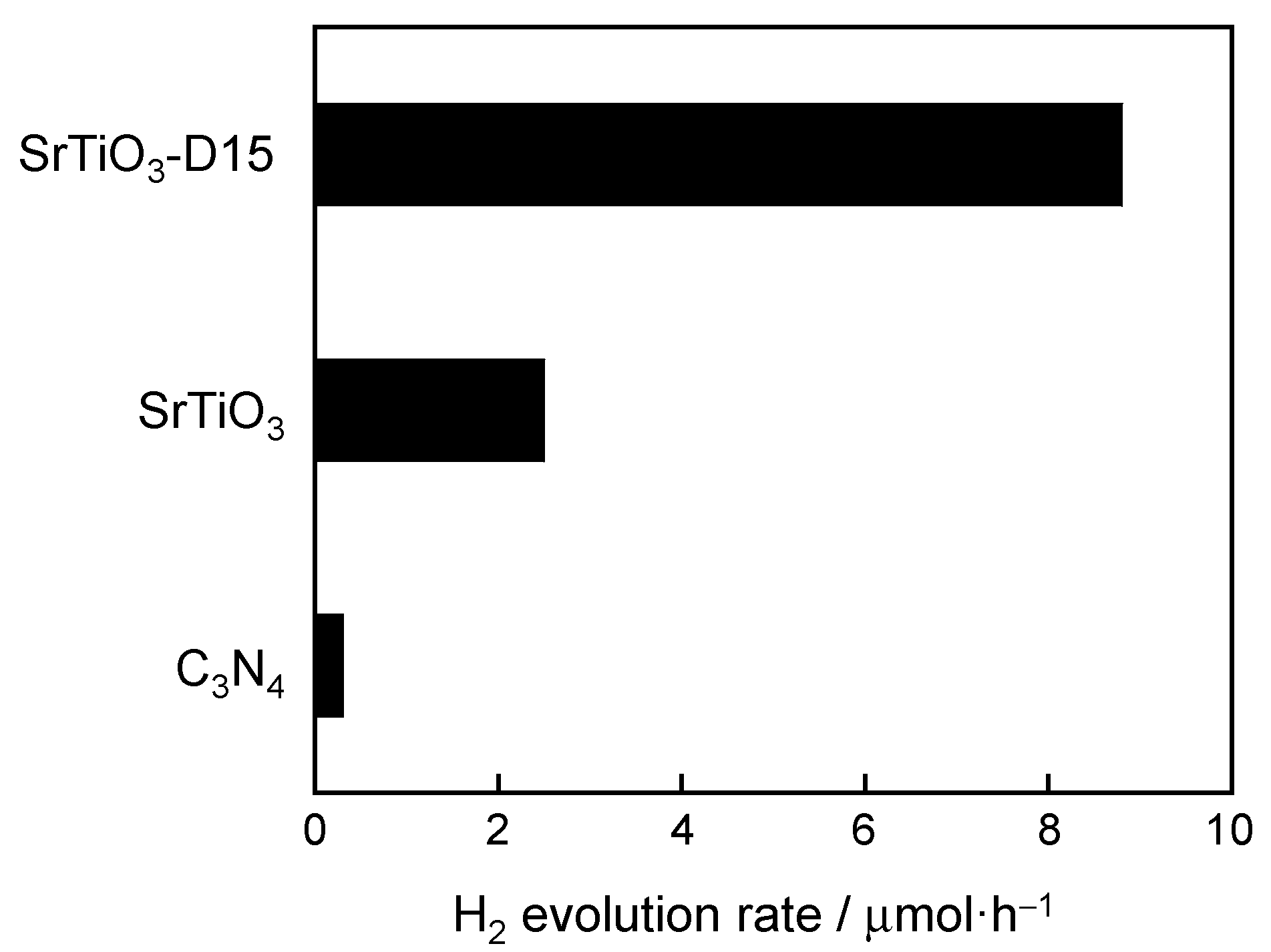

| Sample | Band Gap/eV |
|---|---|
| SrTiO3 | 3.21 |
| C3N4 nanosheet | 2.78 |
| SrTiO3-D10 | 3.17 |
| SrTiO3-D15 | 3.02 |
| SrTiO3-D20 | 2.81 |
| SrTiO3-D25 | 2.74 |
| SrTiO3-D30 | 2.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeue, K.; Yamamoto, Y.; Suzuki, M. Photocatalytic Activity for Hydrogen Evolution of Heteroatom-Doped SrTiO3 Prepared Using a Graphitic-Carbon Nitride Nanosheet. Ceramics 2020, 3, 22-30. https://doi.org/10.3390/ceramics3010003
Ikeue K, Yamamoto Y, Suzuki M. Photocatalytic Activity for Hydrogen Evolution of Heteroatom-Doped SrTiO3 Prepared Using a Graphitic-Carbon Nitride Nanosheet. Ceramics. 2020; 3(1):22-30. https://doi.org/10.3390/ceramics3010003
Chicago/Turabian StyleIkeue, Keita, Yuta Yamamoto, and Masashige Suzuki. 2020. "Photocatalytic Activity for Hydrogen Evolution of Heteroatom-Doped SrTiO3 Prepared Using a Graphitic-Carbon Nitride Nanosheet" Ceramics 3, no. 1: 22-30. https://doi.org/10.3390/ceramics3010003




