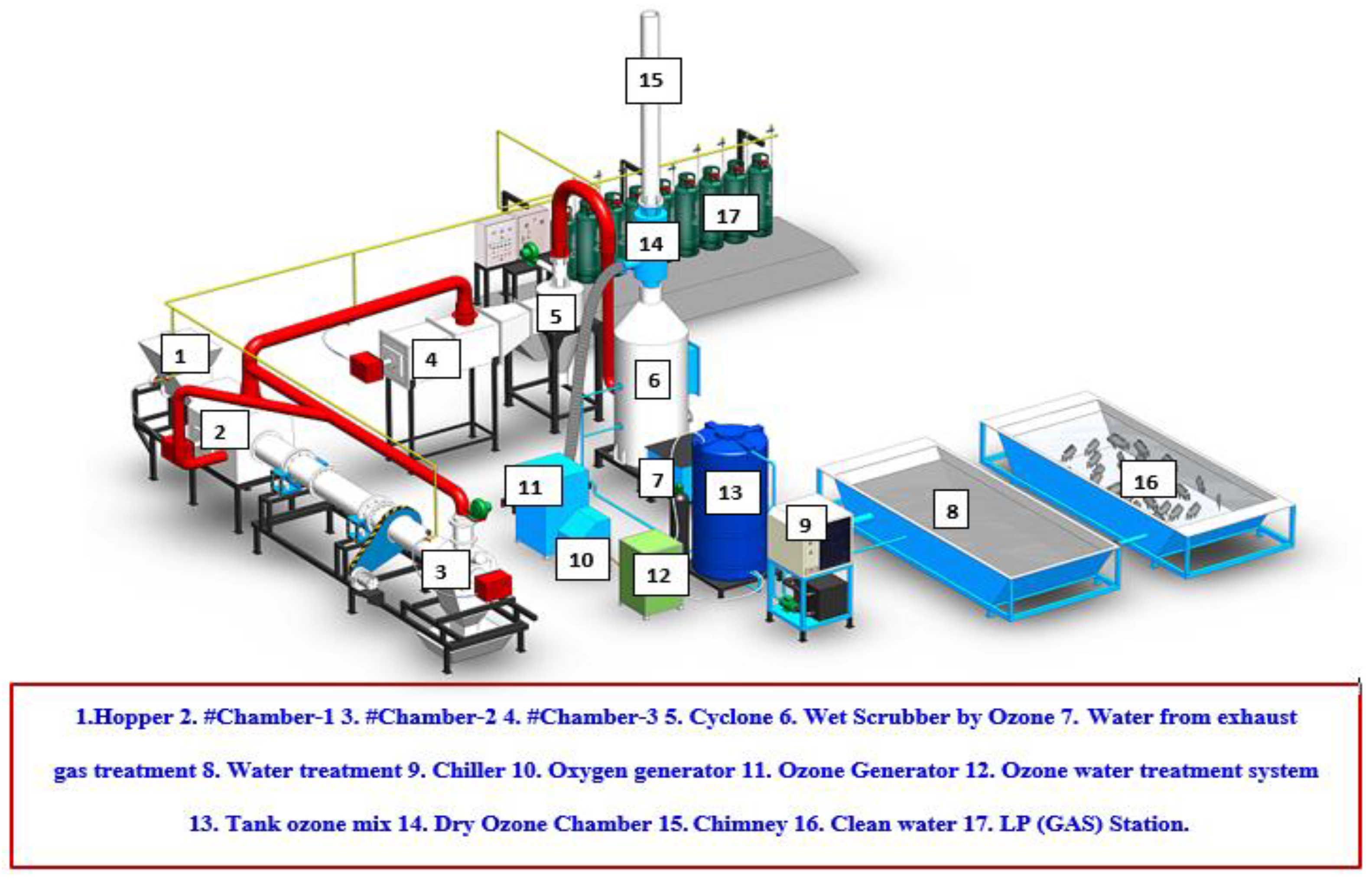
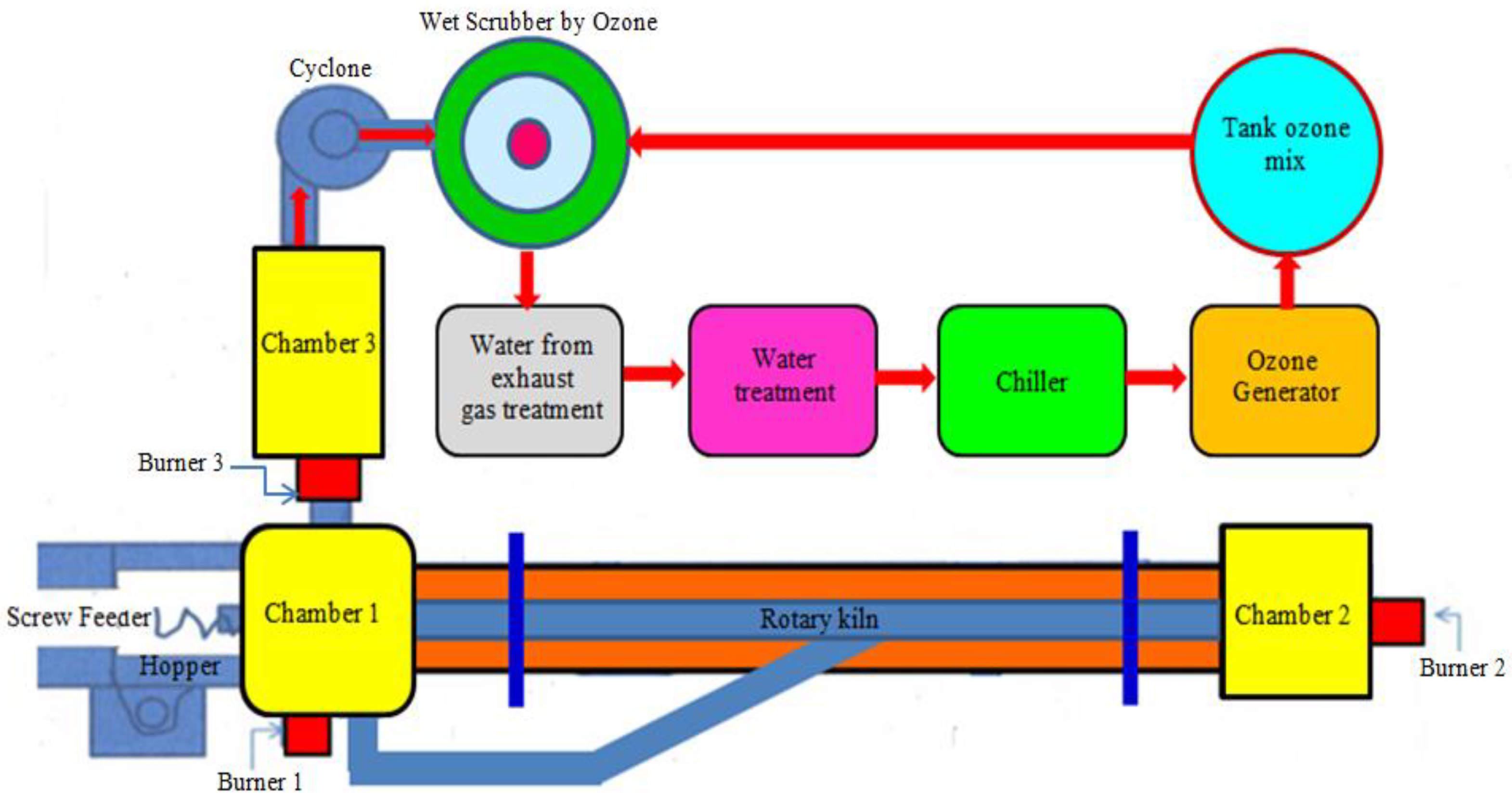
Treatment of Infectious Waste through the Application Rotary Kiln Incinerators and Ozone Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rotary Kiln Infectious Waste Incinerator
- (1)
- An aeration fan is used to inject and move air into the combustion chamber, which is designed to have air flow through the front and back pipes to equalize the air flow from both sides.
- (2)
- The secondary chamber is used for the second round of exhaust gas combustion for eliminating gas pollution (e.g., dioxins and furans). The chamber maintains the desired combustion temperatures above 850–1000 °C to enhance the efficiency of gas pollutant suppression.
- (3)
- The third chamber is used for the final round of exhaust gas combustion to eliminate gas pollution (e.g., dioxins and furans). This chamber also maintains the desired combustion temperatures above 850–1200 °C. Subsequently, the remaining gases flow to a cyclone separator, where dust and small particles are trapped.
- (4)
- The cyclone separator functions as a dust collector; it typically employs centrifugal force, a form of inertia, to force the air containing dirt and dust through a vertical cylinder with a cone-shaped bottom. As the air continues to spin, the heavier particles of dirt and dust begin to separate from the other debris and move outward toward the walls of the chamber. Thereafter, they slide to the bottom of the container and into the dust bin. Alternatively, the pollutant gases flow upward to the top of the cyclone pipe, and the air pressure is increased using an aeration fan.
- (5)
- The aeration fan uses a high-pressure 0.5 horsepower pump to increase the pressure of the remaining gases after combustion and move them from the cyclone separator toward the wet scrapper.
- (6)
- Gas scrubbing collects the dust and small particles that pass through the cyclone separator. The gas then flows through from the side of the tank above water onto the cooling pad in the opposite direction. Water at a temperature of 15–20 °C is sprayed from the top toward the steering wheel that directs the flow to the activated charcoal, which absorbs toxic gases and odors such as methyl sulfide.
- (7)
- In a gas treatment chamber with high oxidation reaction, most types of microbes are killed, in particular the most dangerous bacteria which pose the greatest threat to human health. Odors, chemical compounds, and toxic gases are also eliminated using the treatment in this chamber before the remaining gases are released into the atmosphere.
- (8)
- The combustion gas vent has a measurement point set at a height of 10 times that of the diameter of the vent. The measurement point is installed to check the quantity and quality of the treated gases before they are released out into the environment. As the system is heated via combustion, cold water is used to treat the polluting gases.
- (9)
- The cool water tank stores the cool water required for injection from a nozzle during gas scrubbing. The water gate adjusts and mixes the water containing heat and toxic gas before this water enters the cool water pump. A small portion of the warm water is subsequently released into the warm water tank.
- (10)
- The warm water tank stores warm water that is heated by the absorbed pollutants from the exhaust gases produced via combustion. The debris is dropped to the bottom of the tank and subsequently disposed of. Thereafter, clean water is fed to a chilling machine.
- (11)
- The chilling machine produces cold water that is to be stored in the coolant tank before being sprayed into the scrubbing tank. Once the water absorbs the heat, leading to an increase in its temperature, it is circulated back to the chiller to be cooled again as shown in Figure 1.
2.2. Energy Consumption and Operating Cost Analysis
2.3. O3 Add-On into the Infectious Waste Incineration System
2.4. Measuring the Concentrations of Pollutants Released into the Atmosphere
3. Results and Discussion
3.1. Treatment of Pollution Gases and the Efficiency of a Rotary Kiln Using a Three-Burner System and O3
3.2. Efficiency Comparison of a Rotary Kiln Using a Three-Burner System with and without an O3 System and the Standards of the U.S. EPA
3.3. Efficiency Comparison of Wastewater with and without an O3 System and the Standards of the U.S. EPA (1986)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Statistic of Solid Waste. Available online: https://www.pcd.go.th/garbage/ (accessed on 20 November 2019).
- Xu, X.-F.; Tan, Q.-Y.; Liu, L.-L.; Li, J.-H. Assessment of Medical Waste Disposal Technologies Based on the AHP. Huan Jing Ke Xue 2018, 39, 5717–5722. [Google Scholar]
- Zhan, M.-X.; Chen, T.; Fu, J.-Y.; Lin, X.-Q.; Lu, S.-Y.; Li, X.-D.; Yan, J.-H.; Buekens, A. High temperature suppression of dioxins. Chemosphere 2016, 146, 182–188. [Google Scholar] [CrossRef]
- Qian, L.; Chun, T.; Long, H.; Li, J.; Di, Z.; Meng, Q.; Wang, P. Emission reduction research and development of PCDD/Fs in the iron ore sintering. Process Saf. Environ. Prot. 2018, 117, 82–91. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Open burning as a source of dioxins. Crit. Rev. Environ. Sci. Technol. 2017, 47, 543–620. [Google Scholar] [CrossRef]
- Rathoure, A. Dioxins source origin and toxicity assessment. Biodivers. Int. J. 2018, 2, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Swetha, G.; Gopi, T.; Chandra Shekar, S.; Ramakrishna, C.; Saini, B.; Rao, P.V.L. Combination of adsorption followed by ozone oxidation with pressure swing adsorption technology for the removal of VOCs from contaminated air streams. Chem. Eng. Res. Des. 2017, 117, 725–732. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Zhang, Z.; He, Y.; Zhu, Y.; Shao, J.; Yuan, D.; Chen, G.; Cen, K. Flue gas treatment with ozone oxidation: An overview on NOx, organic pollutants, and mercury. Chem. Eng. J. 2020, 382, 123030. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, V. Toxic environmental releases from medical waste incineration: A review. Environ. Monit. Assess. 2007, 132, 67–81. [Google Scholar] [CrossRef]
- Hossain, M.S.; Santhanam, A.; Norulaini, N.N.; Omar, A.M. Clinical solid waste management practices and its impact on human health and environment–A review. Waste Manag. 2011, 31, 754–766. [Google Scholar] [CrossRef]
- Hospital, Medical, and Infectious Waste Incinerators (HMIWI) Fact Sheets. Available online: https://www.epa.gov/stationary-sources-air-pollution/hospital-medical-and-infectious-waste-incinerators-hmiwi-fact (accessed on 20 November 2019).
- Abd El-Salam, M.M. Hospital waste management in El-Beheira governorate, Egypt. J. Environ. Manag. 2010, 91, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Infection Waste Management Data. Available online: https://www.pcd.go.th/hazards/ (accessed on 20 November 2019).
- Langlais, B.; Reckhow, D.A.; Brink, D.R. Ozone in Water Treatment: Application and Engineering; Lewis Publishers: Chelsea, MI, USA, 1991. [Google Scholar]
- Wongsadee, T.; Trachoo, N.; Suttajit, M. Effects of ozone on the survival of Campylobacter jejuni (Thai). Asia-Pac. J. Sci. Technol. 2008, 13, 919–929. [Google Scholar]
- Dufresne, S.; Hewitt, A.; Robitaille, S. Ozone sterilization: Another option for healthcare in the 21st century. Am. J. Infect. Control 2004, 32, E26–E27. [Google Scholar] [CrossRef]
- Silva, L.M.d.; Jardim, W.F. Trends and strategies of ozone application in environmental problems. Química Nova 2006, 29, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Tichonovas, M.; Krugly, E.; Jankunaite, D.; Racys, V.; Martuzevicius, D. Ozone-UV-catalysis based advanced oxidation process for wastewater treatment. Environ. Sci. Pollut. Res. 2017, 24, 17584–17597. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Nabity, J.A.; Lee, J.M. Low temperature ozone oxidation of solid waste surrogates. Adv. Space Res. 2015, 56, 970–981. [Google Scholar] [CrossRef]
- Scandelai, A.P.J.; Filho, L.C.; Martins, D.C.C.; Freitas, T.K.F.D.S.; Garcia, J.C.; Tavares, C.R.G. Combined processes of ozonation and supercritical water oxidation for landfill leachate degradation. Waste Manag. 2018, 77, 466–476. [Google Scholar] [CrossRef]
- Nidoni, P.G. Incineration process for solid waste management and effective utilization of by products. Int. Res. J. Eng. Technol. 2017, 4, 378–382. [Google Scholar]
- Gupta, G.K.; Liu, H.; Shukla, P. Pulp and paper industry–based pollutants, their health hazards and environmental risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar]
- Jiang, X.; Li, Y.; Yan, J. Hazardous waste incineration in a rotary kiln: A review. Waste Dispos. Sustain. Energy. 2019, 1, 3–37. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Lombardi, F.; Lategano, E.; Cordiner, S.; Torretta, V. Waste incineration in rotary kilns: A new simulation combustion tool to support design and technical change. Waste Manag. Res. 2013, 31, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Vattanapuripakorn, W.; Khannam, K.; Sonsupap, S.; Tongsantia, U.; Sarasamkan, J.; Bubphachot, B. Treatment of Flue Gas from an Infectious Waste Incinerator using the Ozone System ARTICLE INFO ABSTRACT. Environ. Nat. Resour. J. 2021, 19, 348–357. [Google Scholar] [CrossRef]
- Ngessa, V. A study of basic costs for tracking total cost of ownership of information and communication technology in an academic institution: The case of the institute of accountancy arusha. Int. J. Adv. Res. 2019, 7, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Oleniacz, R. Impact of a large medical waste incinerator on air quality. Pol. J. Environ. Stud. Ser. Monogr. (Eds J. Bień L. Woln.) 2010, 2, 176–182. [Google Scholar]
- Nitrogen Oxides (NOx): Why and How They Are Controlled; EPA-456/F-99-006R. Available online: https://www3.epa.gov/ttn/catc/cica/other7_e.html (accessed on 20 November 2019).
- National air quality and emissions trends report. Available online: https://www.epa.gov/sites/default/files/2017-11/documents/trends_report_1999.pdf (accessed on 20 November 2019).
- Wu, H.; Wang, Q.; Yang, H. Promoting the Removal of Particulate Matter by Heterogeneous Vapor Condensation in a Double-Loop Wet Flue Gas Desulfurization System. Energy Fuels 2019, 33, 4632–4639. [Google Scholar] [CrossRef]
- Sarbassov, Y.; Duan, L.; Manovic, V.; Anthony, E.J. Sulfur trioxide formation/emissions in coal-fired air- and oxy-fuel combustion processes: A review. Greenh. Gases Sci. Technol. 2018, 8, 402–428. [Google Scholar] [CrossRef]
- Anderlohr, C.; Brachert, L.; Mertens, J.; Schaber, K. Collection and Generation of Sulfuric Acid Aerosols in a Wet Electrostatic Precipitator. Aerosol Sci. Technol. 2015, 49, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Simone, D. The Production and Characterisation of High Purity Ozone and Experimental and Modelling Studies of Anomalous Oxygen Isotope Effects in the Formation of Carbon Dioxide from Irradiated Mixtures of Carbon Monoxide and Ozone or Oxygen. Ph.D. Thesis, Université Pierre et Marie Curie, lle de France, Paris, France, July 2014. [Google Scholar]
- Magdziarz, A.; Wilk, M. Ozone Effects on the Emissions of Pollutants Coming from Natural Gas Combustion. Pol. J. Environ. Stud. 2010, 19, 1331–1336. [Google Scholar]
- Review of Literature to Determine the Uses for Ozone in the Treatment of Water and Wastewater. Available online: https://www.crew.ac.uk/publication/uses-ozone-treatment-water-and-wastewater (accessed on 24 September 2021).
- Tozihi, M.; Vahedpour, M.; Nazari, F.A. Theoretical Study on the Mechanism and Thermodynamics of Ozone-Water Gas Phase Reaction. J. Iran. Chem. Soc. 2010, 7, 585–596. [Google Scholar] [CrossRef]
- Ozon som Alternativ for Behandling av Ballastvann. Available online: https://hdl.handle.net/11250/2657574 (accessed on 21 November 2019).
- Spiliotopoulou, A.; Rojas-Tirado, P.; Chhetri, R.K.; Kaarsholm, K.M.S.; Martin, R.; Pedersen, P.B.; Pedersen, L.-F.; Andersen, H.R. Ozonation control and effects of ozone on water quality in recirculating aquaculture systems. Water Res. 2018, 133, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | Value (USD) |
|---|---|
| Energy Cost of Electricity (Unit) | 0.12 |
| Energy Cost of Water (Unit) | 0.48 |
| Energy Cost of Fuel (Lite/Unit) | 0.36 |
| Operation Cost for 4-Person Staff (Month/20,000) = (80,000) (THB) | 2447 |
| Cost of the Technology/(THB) | |
| Ozone Technology Cost (Size 100–160 g/Nm3) (Sets) = (2,000,000) | 61,180 |
| Rotary Kiln Technology Cost (Size 100 kg/h) (Sets) = (10,000,000) | 305,903 |
| Total Cost of the Technology (Sets) = (12,000,000) | 369,084 |
| Availability (Hours) | 24 |
| Parameters | Value |
|---|---|
| Combustion Chamber 1 | 700–1000 °C |
| Combustion Chamber 2 | 850–1000 °C |
| Combustion Chamber 3 | 850–1200 °C |
| Wet Scrubber | 15–20 °C |
| Fuel Consumption Rate (LPG) | 18–25 L/h |
| Waste Throughput Rate | 100 kg/h |
| Combustion Duration | 1.0 h |
| Airflow Throughput Rate | 0.1–0.2 m3/s |
| Speeds of the Rotary Kilns | 0.6–1 rpm/min |
| Parameters | Value |
|---|---|
| Temperature in the Wet Scrubber | 15–20 °C |
| Oxygen Consumption Rate | 10 L/min |
| Ozone Concentration | 100–160 g/Nm3 |
| Effect of Residence Time | 3–8 s |
| Flow Rate Mixing | 65 L/min |
| AC Voltage | 5–10 kV |
| Oxidation Duration Time | 0.5–5 min |
| No. | Parameters | Analysis Method | Standard Value [31] |
|---|---|---|---|
| 1 | Mercury (Hg) | Isokinetic, Cold Vapor—ASS | 0.05 mg/m3 |
| 2 | Lead (Pb) | Isokinetic, ICP-AES | 1.5 mg/m3 |
| 3 | Cadmium (Cd) | Isokinetic, ICP-AES | 0.5 mg/m3 |
| 4 | Hydrogen Fluoride (HF) | Ion Chromatography | 16.4 mg/m3 |
| 5 | Particulate (TSP) | Isokinetic, Gravimetric | 320 mg/m3 |
| 6 | Sulfur Dioxide (SO2) | Barium Thorin Titrimetric | 79 mg/m3 |
| 7 | Oxides of Nitrogen (NOx as NO2) | Chemical Absorption, Colorimetric | 470 mg/m3 |
| 8 | Carbon Monoxide (CO) | Bag, Non-Dispersive Infrared | 45.8 mg/m3 |
| 9 | Hydrogen Chlorine (HCl) | Ion Chromatography | 119 mg/m3 |
| Parameters | Rotary Kiln (3-Burner) | 15% O2 | 7.0% O2 | U.S. EPA (1) [31] |
|---|---|---|---|---|
| Mercury (Hg) | 0.006 | <0.001 | <0.001 | 0.05 |
| Lead (Pb) | ND (2) | ND (2) | <0.19 | 1.5 |
| Cadmium (Cd) | ND (2) | ND (2) | <0.02 | 0.5 |
| Hydrogen Fluoride (HF) | 0.680 | 0.013 | 0.034 | 16.4 |
| Particulate (TSP) | 21.900 | 3.4 | 7.4 | 320 |
| Sulfur Dioxide (SO2) | 5.600 | <3.4 | <3.4 | 79 |
| Oxides of Nitrogen (NOx as NO2) | 16.300 | <2.0 | <2.0 | 470 |
| Carbon Monoxide (CO) | 13.700 | 1.7 | 3.7 | 45.8 |
| Hydrogen Chlorine (HCl) | 0.022 | <0.015 | <0.015 | 119 |
| Opacity | 6% | 5% | 5% | 10% |
| Parameters | Non-Ozone | Ozone | U.S. EPA (1) STD [31] |
|---|---|---|---|
| TSP (mg/m3) | 21.9 ± 0.86 | 3.4 ± 0.13 | 120 |
| CO (mg/m3) | 13.7 ± 0.29 | 1.7 ± 0.28 | 45.80 |
| NO2 (mg/m3) | 16.3 ± 0.57 | 2.0 ± 0.39 | 470 |
| HCl (mg/m3) | 0.022 ± 0.012 | 0.015 ± 0.003 | 119 |
| Hg (mg/m3) | 0.0069 ± 0.0014 | 0.001 ± 0.0004 | 0.05 |
| SO2 (mg/m3) | 5.6 ± 1.65 | 3.4 ± 0.2 | 79 |
| HF (mg/m3) | 0.68 ± 0.17 | 0.013 ± 0.01 | 16.40 |
| Opacity (%) | 6.0 ± 0.96 | 5.0 ± 0.53 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khannam, K.; Vattanapuripakorn, W.; Sonsupap, S.; Sarasamkan, J.; Tongsantia, U.; Bubphachot, B. Treatment of Infectious Waste through the Application Rotary Kiln Incinerators and Ozone Technology. Appl. Syst. Innov. 2021, 4, 71. https://doi.org/10.3390/asi4040071
Khannam K, Vattanapuripakorn W, Sonsupap S, Sarasamkan J, Tongsantia U, Bubphachot B. Treatment of Infectious Waste through the Application Rotary Kiln Incinerators and Ozone Technology. Applied System Innovation. 2021; 4(4):71. https://doi.org/10.3390/asi4040071
Chicago/Turabian StyleKhannam, Khomson, Wenich Vattanapuripakorn, Sathapon Sonsupap, Jiradanai Sarasamkan, Umakorn Tongsantia, and Bopit Bubphachot. 2021. "Treatment of Infectious Waste through the Application Rotary Kiln Incinerators and Ozone Technology" Applied System Innovation 4, no. 4: 71. https://doi.org/10.3390/asi4040071
APA StyleKhannam, K., Vattanapuripakorn, W., Sonsupap, S., Sarasamkan, J., Tongsantia, U., & Bubphachot, B. (2021). Treatment of Infectious Waste through the Application Rotary Kiln Incinerators and Ozone Technology. Applied System Innovation, 4(4), 71. https://doi.org/10.3390/asi4040071