Stable and Efficient Photoinduced Charge Transfer of MnFe2O4/Polyaniline Photoelectrode in Highly Acidic Solution
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of MnFe2O4 and Polyaniline
2.2. Preparation of Polyaniline@ MnFe2O4 Catalyst
3. Characterization
Photoelectrocatalytic Activity
4. Results and Discussion
Characterization of Photocatalytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somorjai, G.A.; Frei, H.; Park, J.Y. Advancing the Frontiers in Nanocatalysis, Biointerfaces, and Renewable Energy Conversion by Innovations of Surface Techniques. J. Am. Chem. Soc. 2009, 131, 16589–16605. [Google Scholar] [CrossRef] [Green Version]
- Fushimi, C. Valorization of Biomass Power Generation System: Noble Use of Combustion and Integration with Energy Storage. Energy Fuels 2021, 35, 3715–3730. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [PubMed]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Han, N.; Liu, P.; Jiang, J.; Ai, L.; Shao, Z.; Liu, S. Recent advances in nanostructured metal nitrides for water splitting. J. Mater. Chem. A 2018, 6, 19912–19933. [Google Scholar] [CrossRef]
- Zhu, C.; Li, C.; Zheng, M.; Delaunay, J.-J. Plasma-Induced Oxygen Vacancies in Ultrathin Hematite Nanoflakes Promoting Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22355–22363. [Google Scholar] [CrossRef]
- Lim, H.; Kim, J.Y.; Evans, E.J.; Rai, A.; Kim, J.-H.; Wygant, B.R.; Mullins, C.B. Activation of a Nickel-Based Oxygen Evolution Reaction Catalyst on a Hematite Photoanode via Incorporation of Cerium for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 30654–30661. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Vo, D.-V.N.; Cornejo-Ponce, L.J.C. Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 2021, 274, 129734. [Google Scholar] [CrossRef]
- Jun, B.-M.; Elanchezhiyan, S.S.; Yoon, Y.; Wang, D.; Kim, S.; Prabhu, S.M.; Park, C.M.J. Accelerated photocatalytic degradation of organic pollutants over carbonate-rich lanthanum-substituted zinc spinel ferrite assembled reduced graphene oxide by ultraviolet (UV)-activated persulfate. Chem. Eng. J. 2020, 393, 124733. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.; Wu, Y.; Li, X.; Zhao, Q.; Hou, Y.; Teng, W. Facile solvothermal synthesis of MnFe2O4 hollow nanospheres and their photocatalytic degradation of benzene investigated by in situ FTIR. Catal. Commun. 2015, 68, 11–14. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Gautam, S.; Shandilya, P.; Priya, B.; Singh, V.P.; Raizada, P.; Rai, R.; Valente, M.A.; Singh, P. Superparamagnetic MnFe2O4 dispersed over graphitic carbon sand composite and bentonite as magnetically recoverable photocatalyst for antibiotic mineralization. Sep. Purif. Technol. 2017, 172, 498–511. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Hu, J.; Yan, L. A spray pyrolysis synthesis of MnFe2O4/SnO2 yolk/shell composites for magnetically recyclable photocatalyst. Mater. Lett. 2017, 199, 135–138. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum 2020, 173, 109150. [Google Scholar] [CrossRef]
- Mohamed, M.; Ibrahim, I.; Salama, T.M. Rational design of manganese ferrite-graphene hybrid photocatalysts: Efficient water splitting and effective elimination of organic pollutants. Appl. Catal. A Gen. 2016, 524, 182–191. [Google Scholar] [CrossRef]
- Seralessandri, L.; Varsano, F.; La Barbera, A.; Padella, F. On the oxygen-releasing step in the water-splitting thermochemical cycle by MnFe2O4–Na2CO3 system. Scr. Mater. 2006, 55, 875–877. [Google Scholar] [CrossRef]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. Int. J. Hydrogen Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Mahdi, R.; Alsultan, M.; Al-Keisy, A.; Swiegers, G.F. Photocatalytic Hydrogen Generation from pH-Neutral Water by a Flexible Tri-Component Composite. Catal. Lett. 2021, 151, 1700–1706. [Google Scholar] [CrossRef]
- Alsultan, M.; Choi, J.; Jalili, R.; Wagner, P.; Swiegers, G.F. Synergistic Amplification of Oxygen Generation in (Photo)Catalytic Water Splitting by a PEDOT/Nano-Co3O4/MWCNT Thin Film Composite. ChemCatChem 2020, 12, 1580–1584. [Google Scholar] [CrossRef]
- Alsultan, M.; Choi, J.; Jalili, R.; Wagner, P.; Swiegers, G.F. Synergistic amplification of (photo)catalytic oxygen and hydrogen generation from water by thin-film polypyrrole composites. Mol. Catal. 2020, 490, 110955. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Abd-Rabboh, H.S.M.; Benaissa, M.; Al-Metwaly, M.G.; Galal, A.H.; Ahmed, M.A. Fabrication of novel polyaniline/ZnO heterojunction for exceptional photocatalytic hydrogen production and degradation of fluorescein dye through direct Z-scheme mechanism. Opt. Mater. 2021, 117, 111198. [Google Scholar] [CrossRef]
- Hidalgo, D.; Bocchini, S.; Fontana, M.; Saracco, G.; Hernández, S. Green and low-cost synthesis of PANI–TiO2 nanocomposite mesoporous films for photoelectrochemical water splitting. RSC Adv. 2015, 5, 49429–49438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, Q.Y.; Jing, D.W.; Wang, Y.H.; Guo, L.J. Visible photoactivity and antiphotocorrosion performance of PdS-CdS photocatalysts modified by polyaniline. Int. J. Hydrogen Energy 2012, 37, 791–796. [Google Scholar] [CrossRef]
- Nsib, M.F.; Saafi, S.; Rayes, A.; Moussa, N.; Houas, A. Enhanced photocatalytic performance of Ni–ZnO/Polyaniline composite for the visible-light driven hydrogen generation. J. Energy Inst. 2016, 89, 694–703. [Google Scholar] [CrossRef]
- Makimizu, Y.; Nguyen, N.T.; Tucek, J.; Ahn, H.-J.; Yoo, J.; Poornajar, M.; Hwang, I.; Kment, S.; Schmuki, P. Activation of α-Fe2O3 for Photoelectrochemical Water Splitting Strongly Enhanced by Low Temperature Annealing in Low Oxygen Containing Ambient. Chem. A Eur. J. 2020, 26, 2685–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damian, A.; Omanovic, S. Ni and NiMo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J. Power Sources 2006, 158, 464–476. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Liu, X.; Wang, J.; Lv, J. The vital role of PANI for the enhanced photocatalytic activity of magnetically recyclable N–K2Ti4O9/MnFe2O4/PANI composites. Appl. Surf. Sci. 2014, 311, 230–238. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, S.; Khare, N. Enhanced photosensitization of zinc oxide nanorods using polyaniline for efficient photocatalytic and photoelectrochemical water splitting. Int. J. Hydrogen Energy 2016, 41, 21088–21098. [Google Scholar] [CrossRef]
- Kurtinaitiene, M.; Mazeika, K.; Ramanavicius, S.; Pakstas, V.; Jagminas, A. Effect of additives on the hydrothermal synthesis of manganese ferrite nanoparticles. Adv. Nano Res. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Han, A.; Liao, J.; Ye, M.; Li, Y.; Peng, X. Preparation of Nano-MnFe2O4 and Its Catalytic Performance of Thermal Decomposition of Ammonium Perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar] [CrossRef]
- Xu, C.; Sun, W.; Dong, Y.; Dong, C.; Hu, Q.; Ma, B.; Ding, Y. A graphene oxide–molecular Cu porphyrin-integrated BiVO4 photoanode for improved photoelectrochemical water oxidation performance. J. Mater. Chem. A 2020, 8, 4062–4072. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.-S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef] [Green Version]
- Mary Jacintha, A.; Umapathy, V.; Neeraja, P.; Rex Jeya Rajkumar, S. Synthesis and comparative studies of MnFe2O4 nanoparticles with different natural polymers by sol–gel method: Structural, morphological, optical, magnetic, catalytic and biological activities. J. Nanostructure Chem. 2017, 7, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, R.; Wen, Z.; Zhao, L. Synthesis and Characterization of Single-Crystalline MnFe2O4 Ferrite Nanocrystals and Their Possible Application in Water Treatment. Eur. J. Inorg. Chem. 2011, 2011, 2942–2947. [Google Scholar] [CrossRef]
- Sharifi, S.; Rahimi, K.; Yazdani, A. Highly improved supercapacitance properties of MnFe2O4 nanoparticles by MoS2 nanosheets. Sci. Rep. 2021, 11, 8378. [Google Scholar] [CrossRef]
- Gusain, M.; Nagarajan, R.; Singh, S.K. Highly ordered polyaniline: Synthesis, characterization and electrochemical properties. Polym. Bull. 2020, 77, 3277–3286. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Abroshan, E.; Farhadi, S.; Zabardasti, A. Novel magnetically separable Ag3PO4/MnFe2O4 nanocomposite and its high photocatalytic degradation performance for organic dyes under solar-light irradiation. Sol. Energy Mater. Sol. Cells 2018, 178, 154–163. [Google Scholar] [CrossRef]
- Liu, X.; Cai, L. A novel double Z-scheme BiOBr-GO-polyaniline photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Surf. Sci. 2019, 483, 875–887. [Google Scholar] [CrossRef]
- Reddy, K.R.; Karthik, K.V.; Prasad, S.B.B.; Soni, S.K.; Jeong, H.M.; Raghu, A.V. Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, H.; Sun, H.; Zeng, R. Constructing ternary polyaniline-graphene-TiO2 hybrids with enhanced photoelectrochemical performance in photo-generated cathodic protection. Appl. Surf. Sci. 2017, 410, 547–556. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymer-silver composites. Chem. Pap. 2013, 67, 814–848. [Google Scholar] [CrossRef]
- Al-Keisy, A.; Mahdi, R.; Ahmed, D.; Al-Attafi, K.; Abd Majid, W.H.J.C. Enhanced Photoreduction Activity in BiOI1-xFx Nanosheet for Efficient Removal of Pollutants from Aqueous Solution. ChemistrySelect 2020, 5, 9758–9764. [Google Scholar] [CrossRef]
- Lee, K.; Yu, H.; Lee, J.W.; Oh, J.; Bae, S.; Kim, S.K.; Jang, J. Efficient and moisture-resistant hole transport layer for inverted perovskite solar cells using solution-processed polyaniline. J. Mater. Chem. C 2018, 6, 6250–6256. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.A.; Bourdo, S.E.; Saini, V.; Biris, A.S. Acid-free polyaniline:graphene-oxide hole transport layer in organic solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 21640–21650. [Google Scholar] [CrossRef]
- Bera, S.; Khan, H.; Biswas, I.; Jana, S. Polyaniline hybridized surface defective ZnO nanorods with long-term stable photoelectrochemical activity. Appl. Surf. Sci. 2016, 383, 165–176. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Sefidi, P.Y.; Aydin, Z.; Kinayyigit, S. Toward enhancing the photoelectrochemical water splitting efficiency of organic acid doped polyaniline-WO3 photoanode by photo-assisted electrochemically reduced graphene oxide. Electrochim. Acta 2020, 333, 135475. [Google Scholar] [CrossRef]
- Hursán, D.; Kormányos, A.; Rajeshwar, K.; Janáky, C. Polyaniline films photoelectrochemically reduce CO2 to alcohols. Chem. Commun. 2016, 52, 8858–8861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhou, Y.; Wan, W.; Wang, F.; Zhang, Q.; Lin, Y. Nanoporous TiO2/polyaniline composite films with enhanced photoelectrochemical properties. Mater. Lett. 2014, 130, 150–153. [Google Scholar] [CrossRef]
- Alsultan, M.; Ranjbar, A.; Swiegers, G.F.; Wallace, G.G.; Balakrishnan, S.; Huang, J. Industrial Applications for Intelligent Polymers and Coatings; Makhlouf, A.S., Hosseini, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 223–251. [Google Scholar]


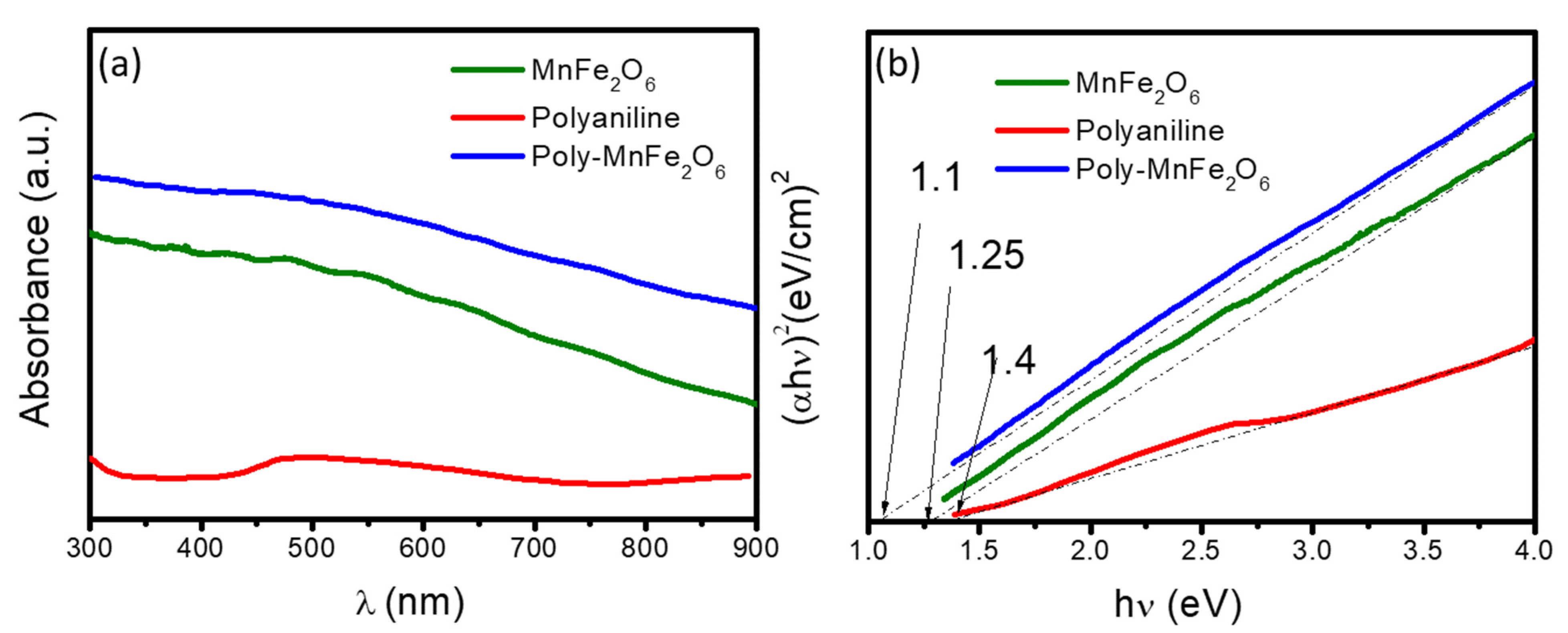
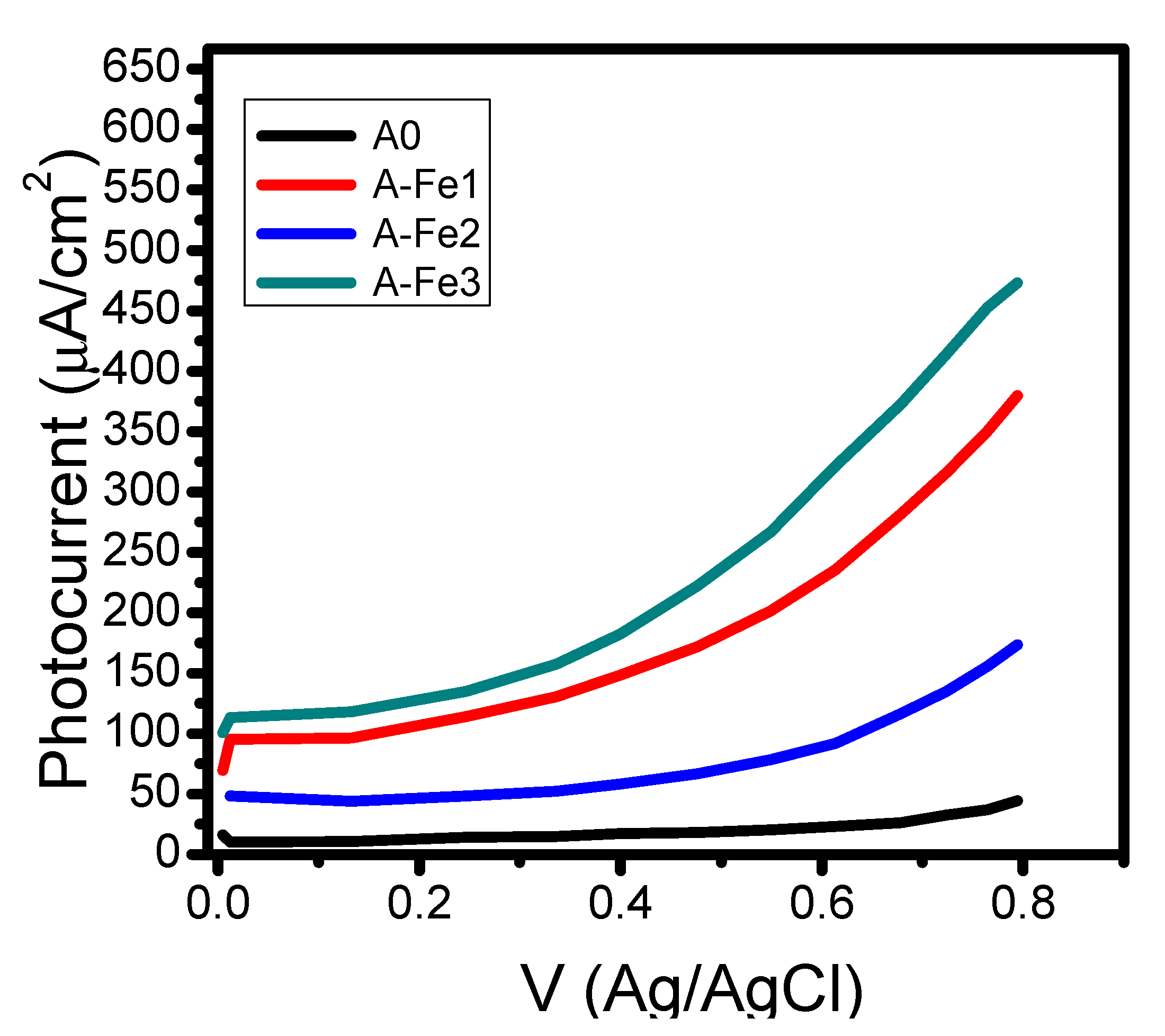
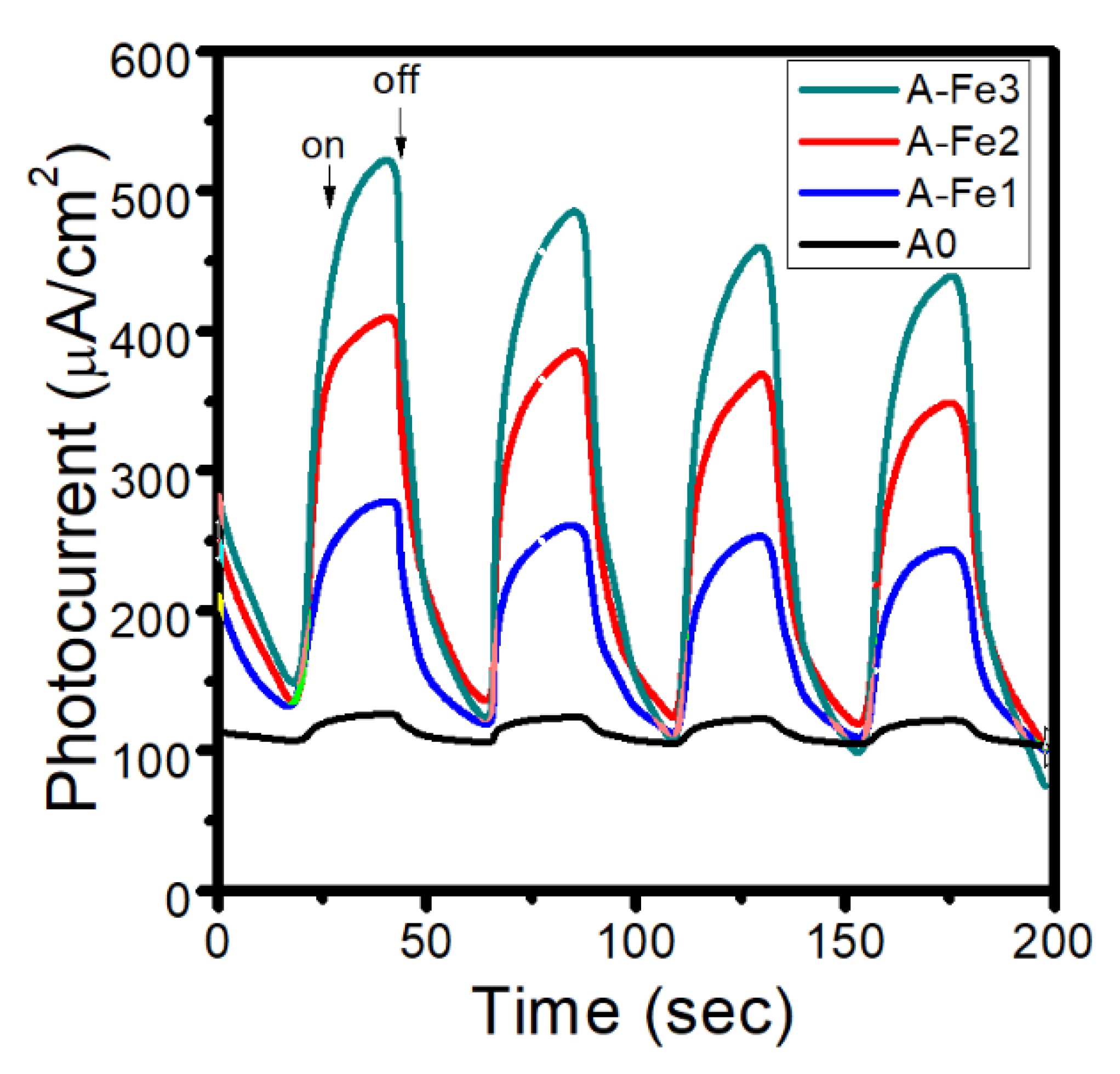



| Sample | Polyaniline Solution (µL) | Mn2Fe2O4 (mg) |
|---|---|---|
| A0 | 0.25 | 0 |
| A-Fe1 | 0.25 | 13 |
| A-Fe2 | 0.25 | 26 |
| A-Fe3 | 0.25 | 52 |
| Photoelectrode/FTO | Electrolyte | Max. Photocurrent | Ref. |
|---|---|---|---|
| PANI | 0.1 M Na2SO4/N2 | 80 μA/cm2 at 0.7 V | [52] |
| PANI@ZnO | 0.1 M Na2SO4 | 25 μA/cm2 at 0.8 V | [50] |
| PANi/TiO2 | 0.1 M NaCl | 50 μA/cm2 at 0.8 V | [45] |
| Camphor sulfonic acid doped PANi-WO3 | 0.1 M Na2SO4 | 200 μA/cm2 at 0.8 V | [51] |
| TiO2/polyaniline | 0.1 M Na2SO4 | 80 μA/cm2 at 0.8 V | [53] |
| PANI@ MnFe2O4 | H2SO4 and Na2SO4 | 400 μA/cm2 at 0.8 V | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsultan, M.; Al-Rubaye, S.; Al-Keisy, A.; Swiegers, G.F.; Taha, I.G. Stable and Efficient Photoinduced Charge Transfer of MnFe2O4/Polyaniline Photoelectrode in Highly Acidic Solution. Colloids Interfaces 2022, 6, 1. https://doi.org/10.3390/colloids6010001
Alsultan M, Al-Rubaye S, Al-Keisy A, Swiegers GF, Taha IG. Stable and Efficient Photoinduced Charge Transfer of MnFe2O4/Polyaniline Photoelectrode in Highly Acidic Solution. Colloids and Interfaces. 2022; 6(1):1. https://doi.org/10.3390/colloids6010001
Chicago/Turabian StyleAlsultan, Mohammed, Shaymaa Al-Rubaye, Amar Al-Keisy, Gerhard F. Swiegers, and Intisar Ghanim Taha. 2022. "Stable and Efficient Photoinduced Charge Transfer of MnFe2O4/Polyaniline Photoelectrode in Highly Acidic Solution" Colloids and Interfaces 6, no. 1: 1. https://doi.org/10.3390/colloids6010001






