Morpholine’s Effects on the Repair Strength of a Saliva-Contaminated CAD/CAM Resin-Based Composite Mended with Resin Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures for Preparing RBC-CAD/CAM Specimens
2.2. Sandblast Process
2.3. Surface Treatment Specimens Grouping
2.3.1. Artificial Saliva Contamination
2.3.2. Phosphoric Acid Etching
2.3.3. Morpholine Treatment
2.3.4. Adhesive Agent Treatment
2.4. Resin Composite Application
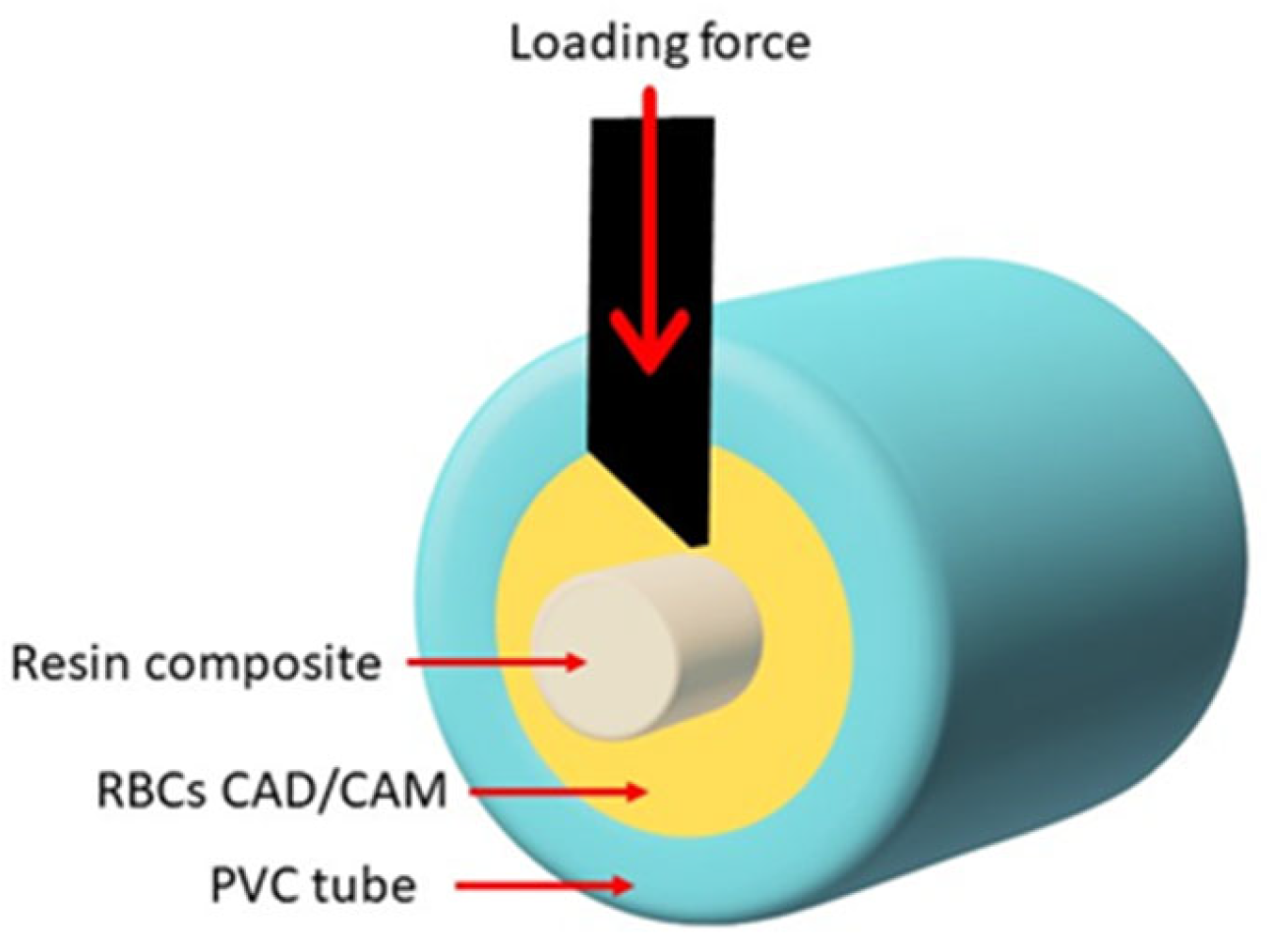
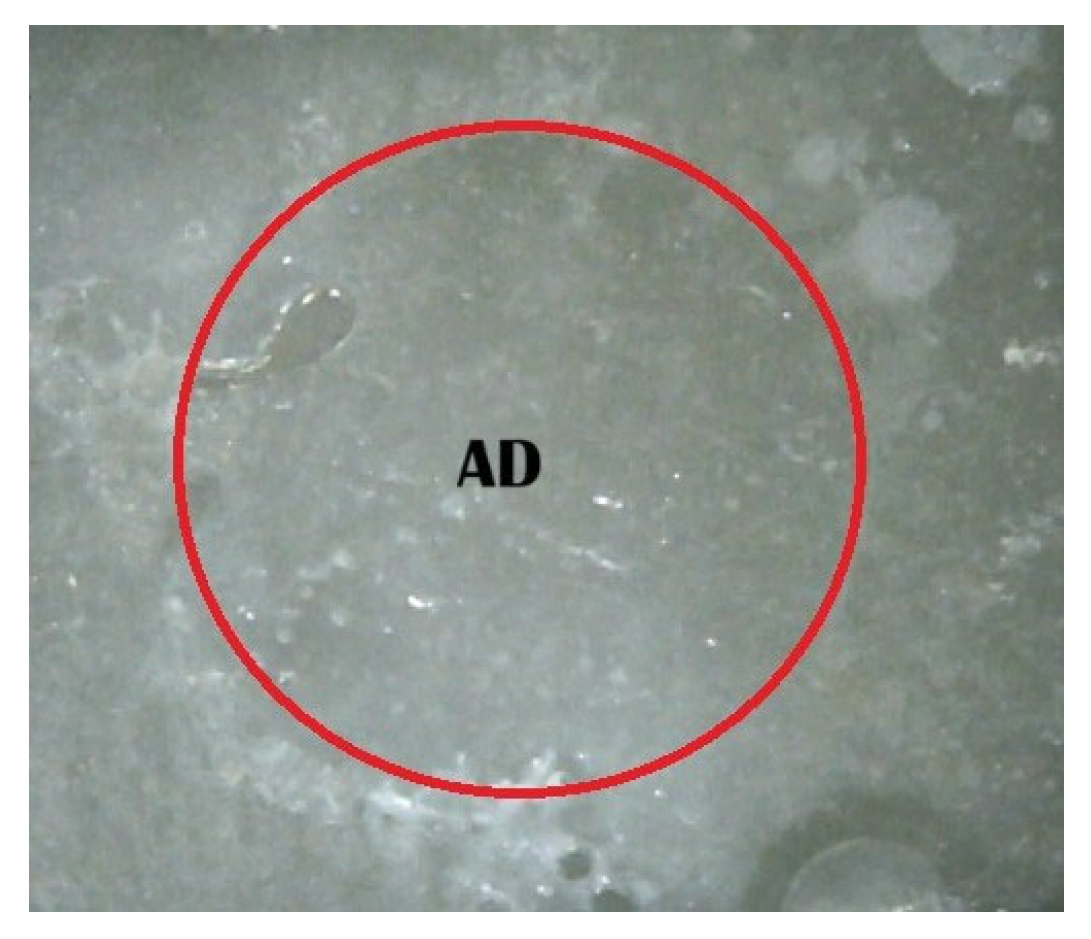
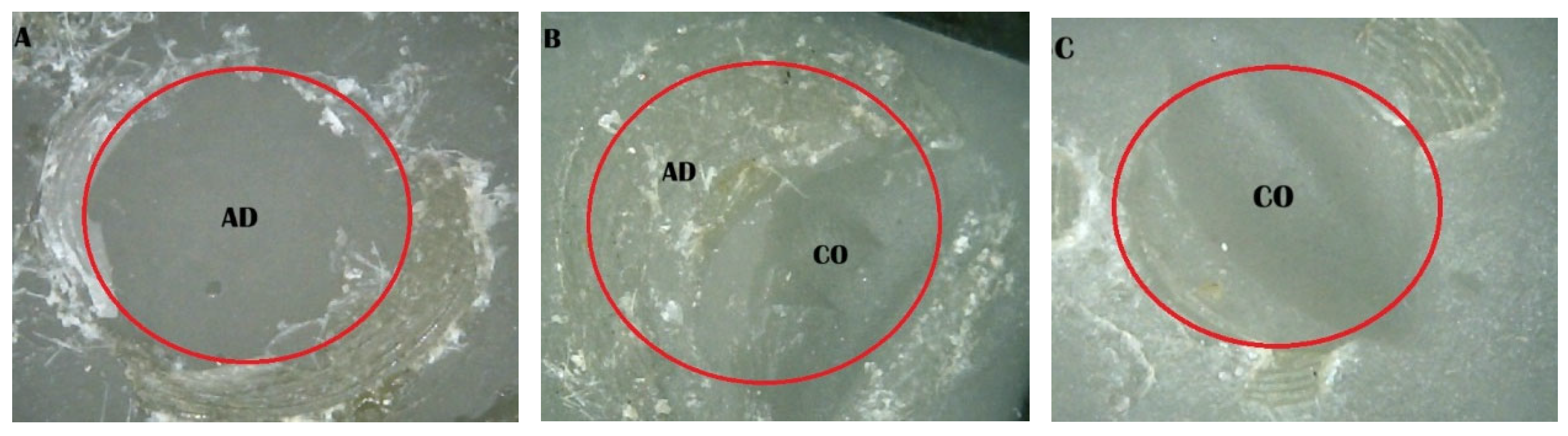
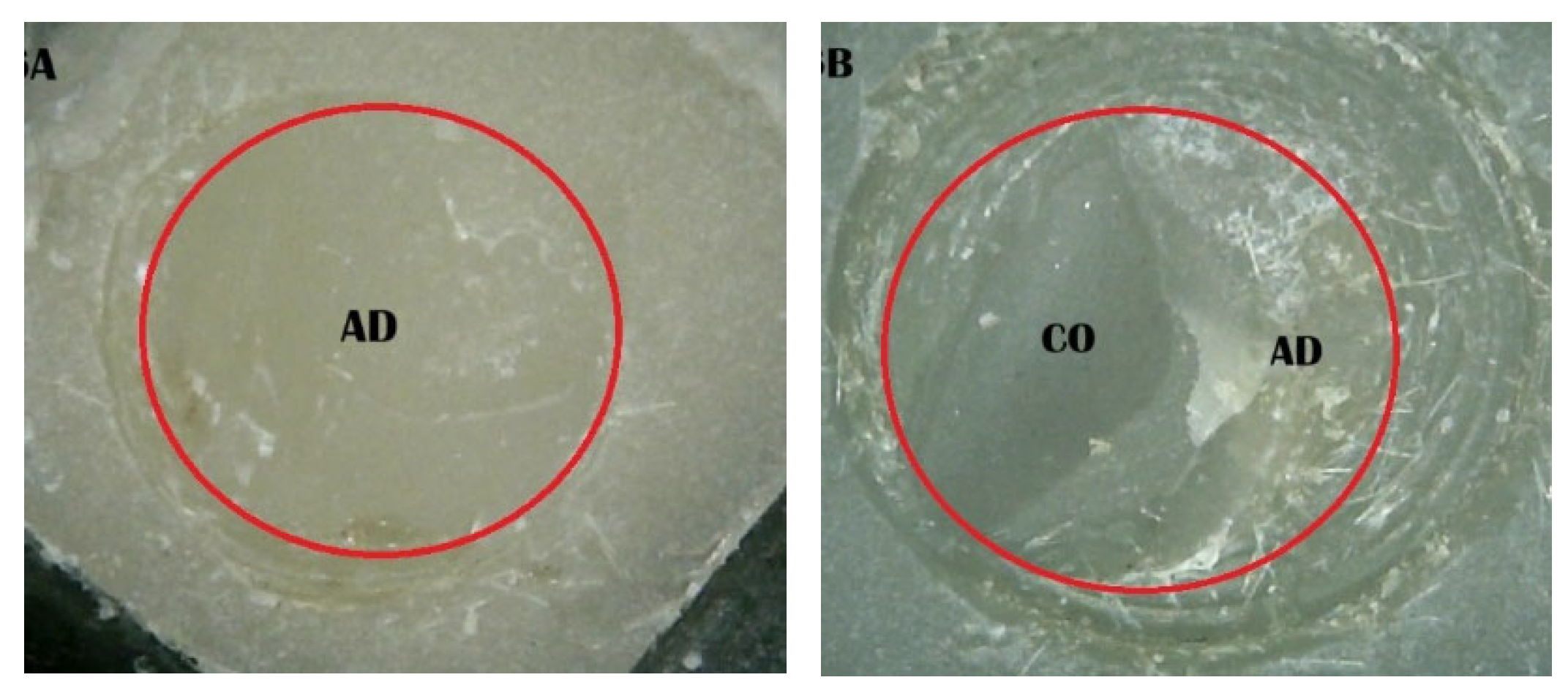
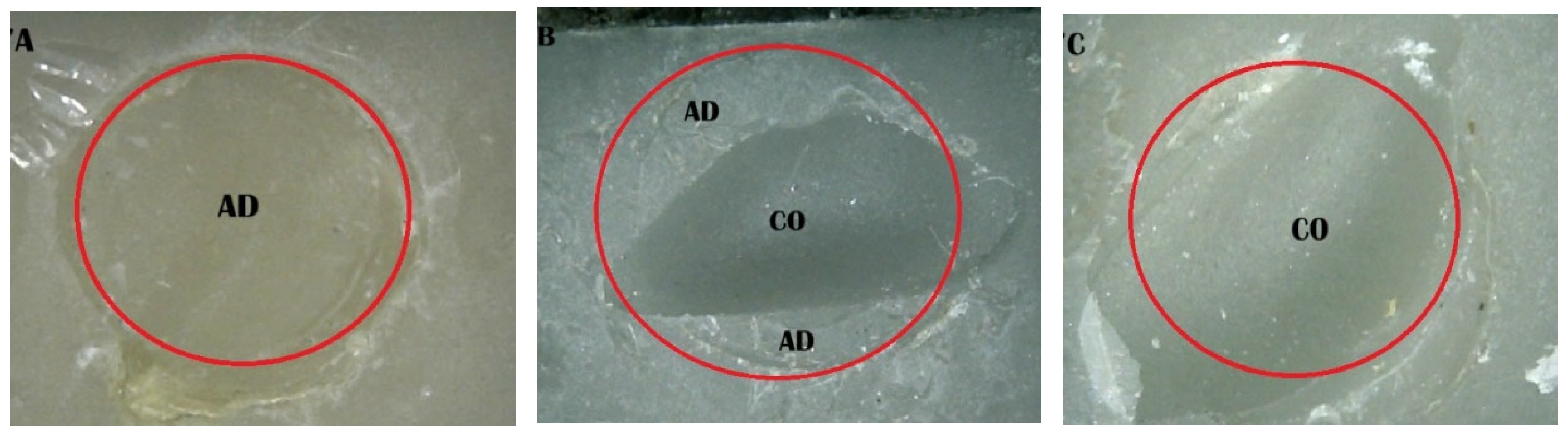
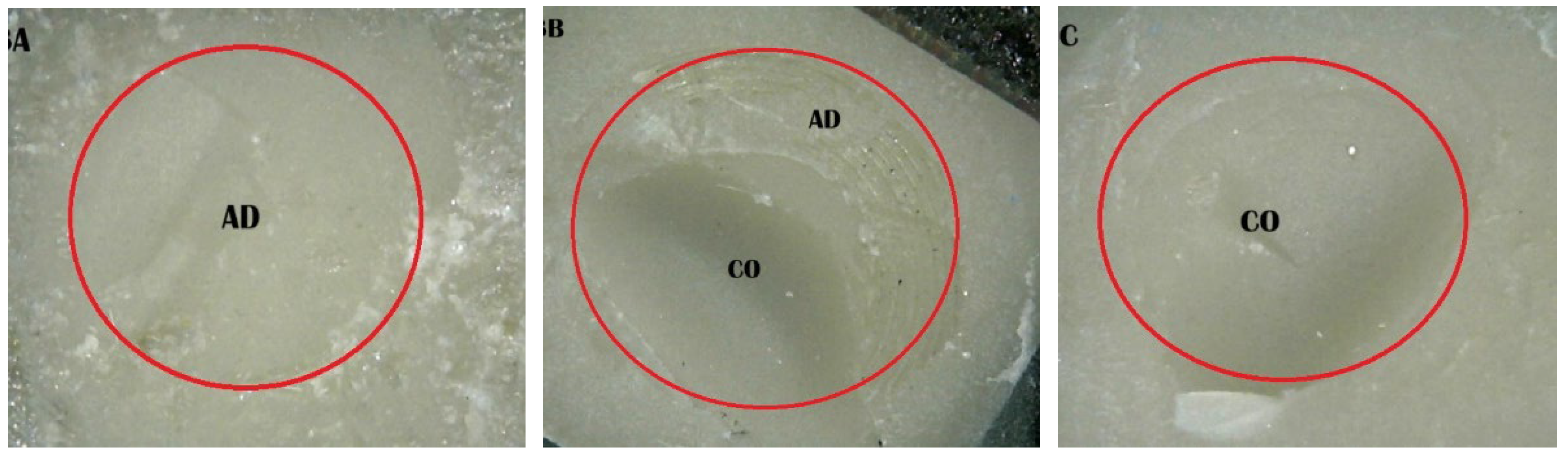
2.5. Shear Bond Strength (SBS) Test and Fracture Pattern Investigation
- (A)
- An adhesive design refers to the failure that occurs at the contact between RBC-CAD/CAM and resin composites.
- (B)
- A cohesive design is one which features fractures in RBC-CAD/CAM or resin composites.
- (C)
- A mixed design includes both adhesive and cohesive failure designs.
2.6. Data Analysis
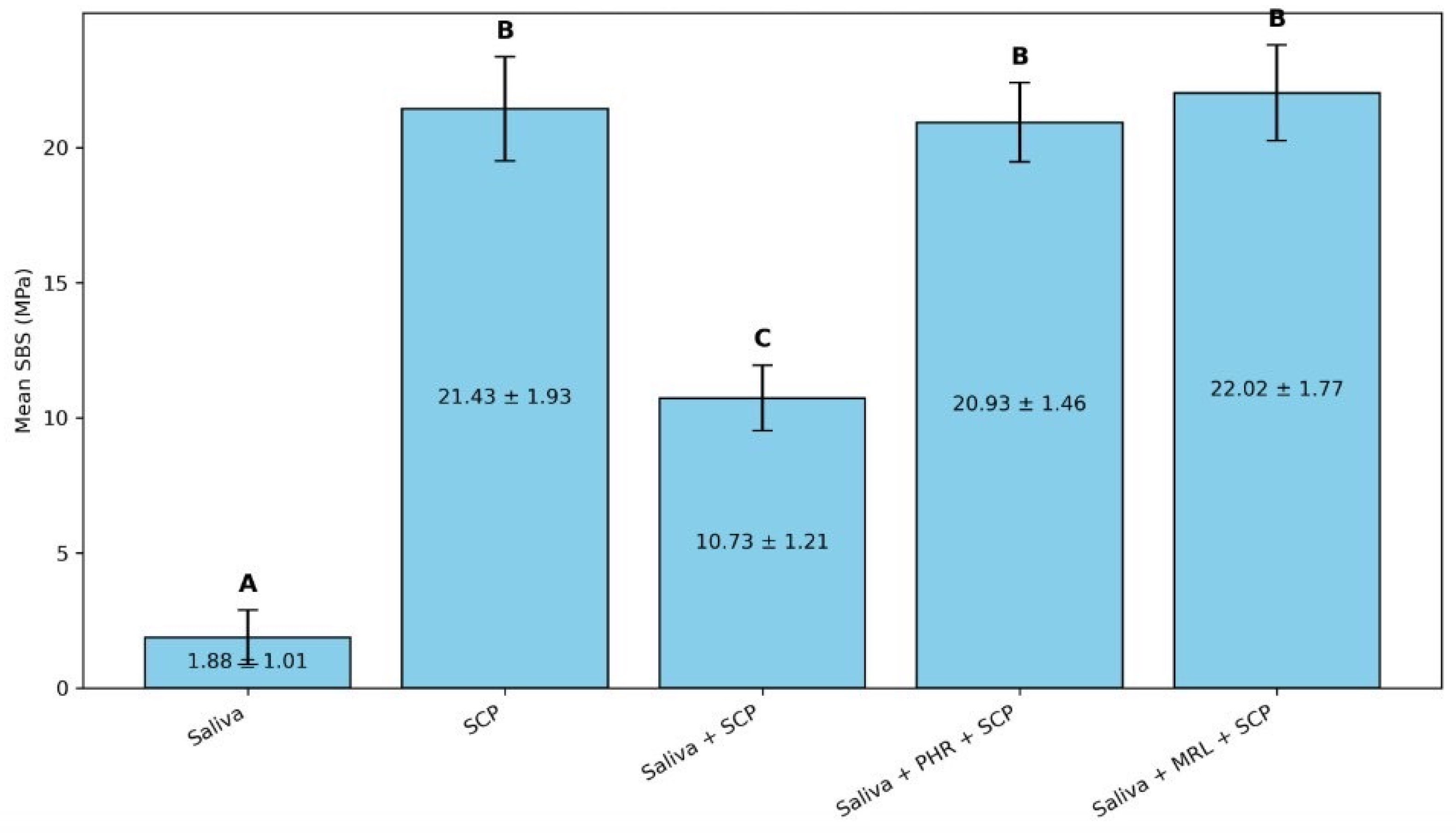
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dejak, B.; Młotkowski, A. A comparison of mvM stress of inlays, onlays and endocrowns made from various materials and their bonding with molars in a computer simulation of mastication—FEA. Dent. Mater. 2020, 36, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Boldt, J.; Gierthmuehlen, P.C. CAD/CAM Ceramic Restorative Materials for Natural Teeth. J. Dent. Res. 2018, 97, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Aslan, Y.U.; Coskun, E.; Ozkan, Y.; Dard, M. Clinical Evaluation of Three Types of CAD/CAM Inlay/ Onlay Materials After 1-Year Clinical Follow Up. Eur. J. Prosthodont. Restor. Dent. 2019, 27, 131–140. [Google Scholar]
- Coşkun, E.; Aslan, Y.U.; Özkan, Y.K. Evaluation of two different CAD-CAM inlay-onlays in a split-mouth study: 2-year clinical follow-up. J. Esthet. Restor. Dent. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Fathy, H.; Hamama, H.H.; El-Wassefy, N.; Mahmoud, S.H. Clinical performance of resin-matrix ceramic partial coverage restorations: A systematic review. Clin. Oral Investig. 2022, 26, 3807–3822. [Google Scholar] [CrossRef]
- Yu, H.; Özcan, M.; Yoshida, K.; Cheng, H.; Sawase, T. Bonding to industrial indirect composite blocks: A systematic review and meta-analysis. Dent. Mater. 2020, 36, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef]
- Spitznagel, F.A.; Scholz, K.J.; Strub, J.R.; Vach, K.; Gierthmuehlen, P.C. Polymer-infiltrated ceramic CAD/CAM inlays and partial coverage restorations: 3-year results of a prospective clinical study over 5 years. Clin. Oral Investig. 2018, 22, 1973–1983. [Google Scholar] [CrossRef]
- Lu, T.; Peng, L.; Xiong, F.; Lin, X.-Y.; Zhang, P.; Lin, Z.-T.; Wu, B.-L. A 3-year clinical evaluation of endodontically treated posterior teeth restored with two different materials using the CEREC AC chair-side system. J. Prosthet. Dent. 2018, 119, 363–368. [Google Scholar] [CrossRef]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical proper ties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- Tekçe, N.; Pala, K.; Demirci, M.; Tuncer, S. Influence of different composite materials and cavity preparation designs on the fracture resistance of mesio-occluso-distal inlay restoration. Dent. Mater. 2016, 35, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Limsiriwong, W.; Klaisiri, A.; Krajangta, N. Effect of anti-COVID-19 mouthwashes on shear bond strength of resin-matrix ceramics repaired with resin composite using universal adhesive: An in vitro study. J. Funct. Biomater. 2023, 14, 158. [Google Scholar] [CrossRef]
- Prabriputaloong, S.; Krajangta, N.; Klaisiri, A. The effect of different chemical surface treatments on the bond strength of resin-matrix ceramic repaired with resin composite. Eur. J. Dent. 2025, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Furuse, A.Y.; da Cunha, L.F.; Benetti, A.R.; Mondelli, J. Bond strength of resin-resin interfaces contaminated with saliva and submitted to different surface treatments. J. Appl. Oral Sci. 2007, 15, 501–505. [Google Scholar] [CrossRef]
- Bolme, J.; Gjerdet, N.R.; Laegreid, T. Effect of saliva contamination on the bond strength of single-step and three-step adhesive systems. Eur. J. Oral Sci. 2022, 130, e12838. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Suebnukarn, S.; Krajangta, N.; Rakmanee, T.; Sriamporn, T.; Thamrongananskul, N. The effect of morpholine on composite-to-composite repair strength contaminated with saliva. Polymers 2022, 14, 4718. [Google Scholar] [CrossRef]
- Klaisiri, A.; Krajangta, N.; Assawarattanaphan, K.; Sriperm, J.; Prawatvatchara, W.; Thamrongananskul, N.; Sriamporn, T. Does Applying Morpholine to Saliva-Contaminated Acrylic Resin Improve the Repair Bond Strength? J. Compos. Sci. 2024, 8, 349. [Google Scholar] [CrossRef]
- Komagata, Y.; Ikeda, H.; Fujio, Y.; Nagamatsu, Y.; Shimizu, H. Effect of phosphoric acid and sodium hydroxide on cleaning and bonding of saliva-contaminated feldspar porcelain. J. Prosthodont. Res. 2023, 67, 132–137. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef]
- Prawatvatchara, W.; Angkanawiriyarak, S.; Klaisiri, A.; Sriamporn, T.; Thamrongananskul, N. Effect of aprotic solvents on the microtensile bond strength of composite core and fiber-reinforced composite posts. Polymers 2023, 15, 3984. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lassila, L.V. Enhanced resin-composite bonding to zirconia framework after pretreatment with selected silane monomers. Dent. Mater. 2011, 27, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Krajangta, N.; Sriamporn, T.; Phumpatrakom, P.; Thamrongananskul, N. The use of silane coupling agents on lithium disilicate glass ceramic repaired with resin composite. J. Int. Dent. Med. Res. 2021, 14, 169–172. [Google Scholar]
- Arkoy, S.; Ulusoy, M. Effect of Different Surface Treatments on Repair Bond Strength of CAD/CAM Resin-Matrix Ceramics. Materials 2022, 15, 6314. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Maneenacarith, A.; Sriamporn, T. The effect of micro-mechanical surface preparation and adhesive surface modification strategies on resin-matrix ceramic repair bond strength using universal adhesive containing silane agent. Eur. J. Gen. Dent. 2025, 14, 61–66. [Google Scholar]
- Fouquet, V.; Lachard, F.; Abdel-Gawad, S.; Dursun, E.; Attal, J.P.; François, P. Shear bond strength of a direct resin composite to CAD-CAM composite blocks: Relative contribution of micromechanical and chemical block surface treatment. Materials 2022, 15, 5018. [Google Scholar] [CrossRef]
- Yang, B.; Wolfart, S.; Scharnberg, M.; Ludwig, K.; Adelung, R.; Kern, M. Influence of contamination on zirconia ceramic bonding. J. Dent. Res. 2007, 86, 749–753. [Google Scholar] [CrossRef]
- Neelagiri, K.; Kundabala, M.; Shashi, R.A.; Thomas, M.S.; Parolia, A. Effects of saliva contamination and decontamination procedures on shear bond strength of self-etch dentine bonding systems: An invitro study. J. Conserv. Dent. 2010, 13, 71–75. [Google Scholar]
- Ulker, E.; Bilgin, S.; Kahvecioglu, F.; Erkan, A.I. Effect of saliva decontamination procedures on shear bond strength of a one-step adhesive system. Niger. J. Clin. Pract. 2017, 20, 1201–1205. [Google Scholar]
- Pinzon, L.M.; Powers, J.M.; O’Keefe, K.L.; Dusevish, V.; Spencer, P.; Marshall, G.W. Effect of mucoprotein on the bond strength of resin composite to human dentin. Odontology 2011, 99, 119–128. [Google Scholar] [CrossRef]
- Yin, H.; Kwon, S.; Chung, S.H.; Kim, R.J.Y. Performance of universal adhesives in composite resin repair. Biomed. Res. Int. 2022, 2022, 7663490. [Google Scholar] [CrossRef]
- Ghosh, P.; Pal, G. Photopolymerization of methyl methacrylate using morpholine–bromine charge transfer complex as the photoinitiator. Eur. Polym. J. 1998, 35, 677–682. [Google Scholar] [CrossRef]
- Francois, P.; Vennat, E.; Le Goff, S.; Ruscassier, N.; Attal, J.P.; Dursun, E. Shear bond strength and interface analysis between a resin composite and a recent high-viscous glass ionomer cement bonded with various adhesive systems. Clin. Oral Investig. 2019, 23, 2599–2608. [Google Scholar] [CrossRef]
- Alnafaiy, S.; Labban, N.; Maawadh, A.; Alshehri, H.; Albaijan, R. Repair Bond Strength of Composite Resin to Aged Resin and Glass-Matrix CAD/CAM Ceramic Materials Using Two Different Repair Systems. Coatings 2021, 11, 1331. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Bukhary, D.M.; Al Qahtani, M.A.; Alenazi, N.O.; Alzahrani, A.H.; Alobaid, H.A. Repair of temporary fixed dental prostheses using a flowable resin composite: Effect of material, bonding, and aging. Saudi. Dent. J. 2021, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Albergardi, A.B.S.; Limírio, J.P.J.O.; Gomes, J.M.L.; Pesqueira, A.A.; Pellizzer, E.P. Effect of surface treatments on the bond strength of resin-repaired resin matrix CAD-CAM ceramic: A scoping review. J. Dent. 2025, 154, 105594. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Stucki, L.; Hoffmann, R.; Attin, T.; Stawarczyk, B. Repairability of CAD/CAM high-density PMMA- and composite-based polymers. Clin. Oral Investig. 2015, 19, 2007–2013. [Google Scholar] [CrossRef]
- Güngör, M.B.; Nemli, S.K.; Bal, B.T.; Ünver, S.; Doğan, A. Effect of surface treatments on shear bond strength of resin composite bonded to CAD/CAM resin-ceramic hybrid materials. J. Adv. Prosthodont. 2016, 8, 259–266. [Google Scholar] [CrossRef]
- Şişmanoğlu, S.; Gürcan, A.T.; Yıldırım-Bilmez, Z.; Turunç-Oğuzman, R.; Gümüştaş, B. Effect of surface treatments and universal adhesive application on the microshear bond strength of CAD/CAM materials. J. Adv. Prosthodont. 2020, 12, 22–32. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Hong, Q.; Yang, H.; Zhang, L.; Zhang, M.; Yu, L. Organometallic modification of silica with europium endowing the fluorescence properties: The key technique for numerical quality monitoring. Chin. Chem. Lett. 2025, 36, 110165. [Google Scholar] [CrossRef]
| Material | Chemical Composition |
|---|---|
| Resin-based composites CAD/CAM; Shofu Inc., Kyoto, Japan. | TEGDMA, UDMA, Filler; Silica powder, micro fumed silica, zirconium silicate, 61% by weight. |
| Scotchbond universal plus; 3M, Neuss, Germany. | HEMA, 2-propenoic acid, 2-methyl-, diesters with 4,6-dibromo-1,3-benzenediol 2-(2-hydroxyethoxy)ethyl 3-hydroxypropyl diethers, 2-propenoic acid, 2-methyl-, reaction products with 1,10-decanediol and phosphorus oxide, 2-propenoic acid, 2-methyl-, 3(triethoxysilyl)propyl ester, reaction products with silica and 3(triethoxysilyl)-1-propanamine, synthetic amorphous silica, fumed, crystalline-free, ethanol, water, (3-aminopropyl)triethoxysilane, camphorquinone, N,N-dimethylbenzocaine, methacrylic acid, Acetic acid, copper(2+) salt, monohydrate |
| Morpholine; Loba Chemie PVT Ltd., Mumbai, India. | 98% Extra-pure O(CH2CH2)2NH |
| Resin composite, Harmonize A4D; Kerr Corporation, CA, USA. | TEGDMA, Bis-GMA, EBPADMA, zirconia/silica cluster filler (2–3 m) comprising 20 nm spherical fumed silica and 5 nm zirconia particles, prepolymerized filler. |
| Groups | Surface Modification |
|---|---|
| 1 | Saliva-contaminated RBCs (saliva) |
| 2 | Treated with SCP (SCP) |
| 3 | Saliva-contaminated RBCs treated with SCP (saliva + SCP) |
| 4 | Saliva-contaminated RBCs treated with phosphoric acid prior to application of SCP (saliva + PHR + SCP) |
| 5 | Saliva-contaminated RBCs treated with morpholine prior to application of SCP (Saliva + MRL + SCP) |
| Groups | Failure Pattern Mode (%) | ||
|---|---|---|---|
| Adhesive | Mixed | Cohesive | |
| 1. Saliva | 100 | 0 | 0 |
| 2. SCP | 10 | 20 | 70 |
| 3. Saliva + SCP | 90 | 10 | 0 |
| 4. Saliva + PHR + SCP | 20 | 10 | 70 |
| 5. Saliva + MRL + SCP | 10 | 10 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaisiri, A.; Sriamporn, T.; Krajangta, N.; Thamrongananskul, N. Morpholine’s Effects on the Repair Strength of a Saliva-Contaminated CAD/CAM Resin-Based Composite Mended with Resin Composite. J. Compos. Sci. 2025, 9, 345. https://doi.org/10.3390/jcs9070345
Klaisiri A, Sriamporn T, Krajangta N, Thamrongananskul N. Morpholine’s Effects on the Repair Strength of a Saliva-Contaminated CAD/CAM Resin-Based Composite Mended with Resin Composite. Journal of Composites Science. 2025; 9(7):345. https://doi.org/10.3390/jcs9070345
Chicago/Turabian StyleKlaisiri, Awiruth, Tool Sriamporn, Nantawan Krajangta, and Niyom Thamrongananskul. 2025. "Morpholine’s Effects on the Repair Strength of a Saliva-Contaminated CAD/CAM Resin-Based Composite Mended with Resin Composite" Journal of Composites Science 9, no. 7: 345. https://doi.org/10.3390/jcs9070345
APA StyleKlaisiri, A., Sriamporn, T., Krajangta, N., & Thamrongananskul, N. (2025). Morpholine’s Effects on the Repair Strength of a Saliva-Contaminated CAD/CAM Resin-Based Composite Mended with Resin Composite. Journal of Composites Science, 9(7), 345. https://doi.org/10.3390/jcs9070345







