Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC Voltage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Latex balloons as an elastomeric shell;
- Spiral weave material of 8 mm internal diameter made of 0.5 mm diameter polycaprolactam fiber as an external reinforcement material;
- Woven mesh braided material of 8 mm internal diameter made of polyethylene terephthalate which has the ability to stretch twice;
- Conductive copper wires used as electrodes;
- Heat-shrinkable tubes made of polyolefins, 10 mm long with a diameter of 4- and 2-mm. Heat-shrinkable tubes are required to properly connect and fix the external reinforcements, elastomeric shell, and electrodes in the prepared the actuators.
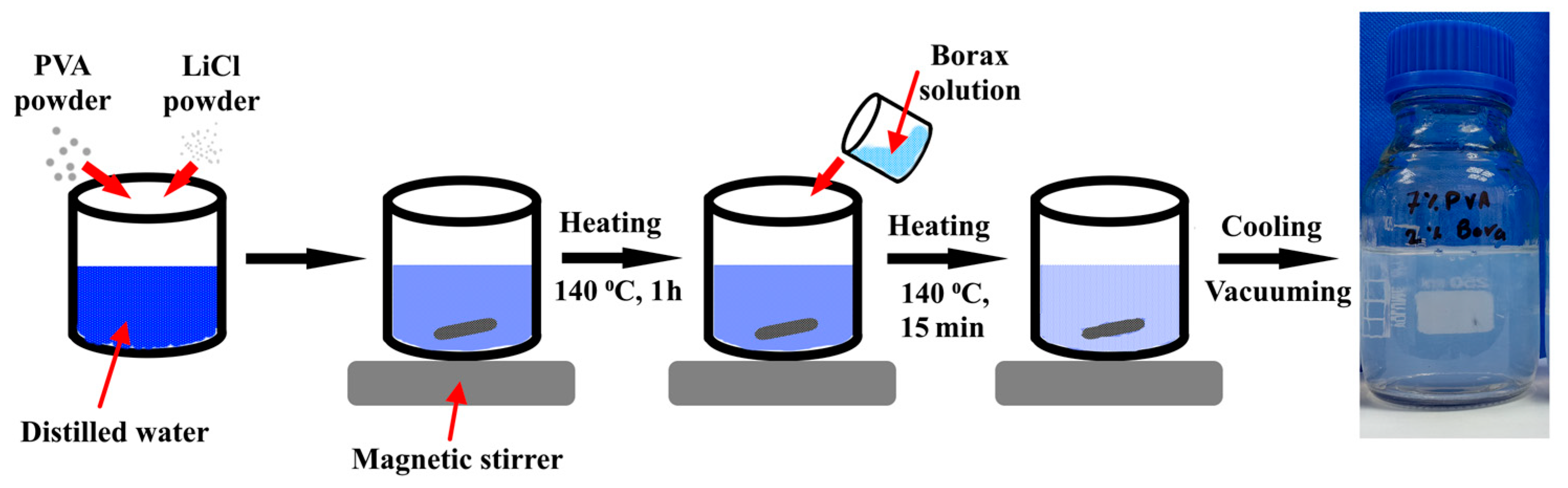
2.2. Preparation of PVA Hydrogels
2.3. Preparation of Hydrogel Actuators
2.4. Procedures
2.4.1. Hydrogel Actuators’ Electrical Resistance Measurements
2.4.2. Actuation Tests
2.4.3. Actuation Deformation Measurement
2.4.4. PVA/LiCl Hydrogels’ Generated Force Measurement
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozsecen, M.Y.; Mavroidis, C. Nonlinear force control of dielectric electroactive polymer actuators. Electroact. Polym. Actuators Devices 2010, 7642, 673–680. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Materials Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- Anderson, I.A.; Gisby, T.A.; McKay, T.G.; O’Brien, B.M.; Calius, E. Multi-functional dielectric elastomer artificial muscles for soft and smart machines. J. Appl. Phys. 2012, 112, 041101. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Electroactive Polymers as Artificial Muscles—Reality, Potential and Challenges, Vol. PM136, 2nd ed.; SPIE Press: Bellingham, WA, USA, 2004; pp. 1–765. ISBN 0-8194-5297-1. [Google Scholar]
- Cardoso, V.F.; Ribeiro, C.; Lanceros-Mendez, S. Metamorphic biomaterials. In Bioinspired Materials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–99. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Dayyoub, T.; Telyshev, D.V.; Gerasimenko, A.Y. Electroactive Polymer-Based Composites for Artificial Muscle-like Actuators: A Review. Nanomaterials 2022, 12, 2272. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Twigg, S.; Lin, J.-H.; Hatipoglu, G.; Liu, S.; Zhang, Q.M. Ion distribution in ionic electroactive polymer actuators. Electroact. Polym. Actuators Devices 2011, 79762, 776–783. [Google Scholar] [CrossRef]
- Biddiss, E.; Chau, T. Electroactive polymeric sensors in hand prostheses: Bending response of an ionic polymer metal composite. Med. Eng. Phys. 2006, 28, 568–578. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.V.; Filippova, O.V.; Tcherdyntsev, V.V.; Telyshev, D.V. Shape Memory Polymers as Smart Materials: A Review. Polymers 2022, 14, 3511. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Shehzahdi, S.M.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowan, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.; Larionov, D.I.; Filippova, O.V.; Telyshev, D.V.; Gerasimenko, A.Y. Preparation of Linear Actuators Based on Polyvinyl Alcohol Hydrogels Activated by AC Voltage. Polymers 2023, 15, 2739. [Google Scholar] [CrossRef]
- Morrow, R.; McKenzie, D.R. The Time-Dependent Development of Electric Double-Layers in Pure Water at Metal Electrodes: The Effect of an Applied Voltage on the Local pH. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 18–34. [Google Scholar] [CrossRef]
- Morrow, R.; McKenzie, D.R.; Bilek, M.M.M. The Time-Dependent Development of Electric Double-Layers in Saline Solutions. J. Phys. D Appl. Phys. 2006, 39, 937–943. [Google Scholar] [CrossRef]
- Han, Z.J.; Morrow, R.; Tay, B.K.; McKenzie, D. Time-Dependent Electrical Double Layer with Blocking Electrode. Appl. Phys. Lett. 2009, 94, 043118. [Google Scholar] [CrossRef]
- Hossain, R.; Adamiak, K. Dynamic Properties of the Electric Double Layer in Electrolytes. J. Electrost. 2013, 71, 829–838. [Google Scholar] [CrossRef]
- Hong, F.; Cao, J.; Cheng, P. A parametric study of AC electrothermal flow in microchannels with asymmetrical interdigitated electrodes. Int. Commun. Heat Mass Transf. 2011, 38, 275–279. [Google Scholar] [CrossRef]
- Lian, M.; Wu, J. Microfluidic flow reversal at low frequency by AC electrothermal effect. Microfluid. Nanofluid. 2009, 7, 757–765. [Google Scholar] [CrossRef]
- Zehavi, M.; Boymelgreen, A.; Yossifon, G. Competition between Induced-Charge Electro-Osmosis and Electrothermal Effects at Low Frequencies around a Weakly Polarizable Microchannel Corner. Phys. Rev. Applied 2016, 5, 044013. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Simon, G.P. Response to Osmotic Pressure versus Swelling Pressure: Comment on “Bifunctional Polymer Hydrogel Layers as Forward Osmosis Draw Agents for Continuous Production of Fresh Water Using Solar Energy”. Environ. Sci. Technol. 2014, 48, 4214–4215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Simon, G.P.; Wang, H. Forward osmosis desalination using polymer hydrogels as a draw agent: Influence of draw agent, feed solution and membrane on process performance. Water Res. 2013, 47, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Yao, J.; Zeng, Y.; Simon, G.P.; Wang, H. Composite polymer hydrogels as draw agents in forward osmosis and solar dewatering. Soft Matter 2011, 7, 10048–10056. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, B.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl. Phys. Lett. 2014, 105, 151903. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2016, 341, 984–987. [Google Scholar] [CrossRef]
- Chen, B.; Lu, J.J.; Yang, C.H.; Zhou, J.; Chen, Y.M.; Suo, Z. Highly Stretchable and Transparent Ionogels as Nonvolatile Conductors for Dielectric Elastomer Transducers. ACS Appl. Mater. Interfaces 2014, 6, 7840–7845. [Google Scholar] [CrossRef]
- De Ninno, A.; Nikollari, E.; Missori, M.; Frezza, F. Dielectric permittivity of aqueous solutions of electrolytes probed by THz time-domain and FTIR spectroscopy. Phys. Lett. A 2020, 384, 126865. [Google Scholar] [CrossRef]
- Valiskó, M.; Boda, D. The effect of concentration- and temperature-dependent dielectric constant on the activity coefficient of NaCl electrolyte solutions. J. Chem. Phys. 2014, 140, 234508. [Google Scholar] [CrossRef]
- Marcus, Y. Electrostriction in Electrolyte Solutions. Chem. Rev. 2011, 111, 2761–2783. [Google Scholar] [CrossRef]
- Anand, G.; Safaripour, S.; Snoeyink, C. Effects of Frequency and Joule Heating on Height Rise between Parallel Electrodes with AC Electric Fields. Langmuir 2022, 38, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Hlushak, S.; dos Ramos, M.C.; McCabe, C. Predicting the thermodynamic properties and dielectric behavior of electrolyte solutions using the SAFT-VR+DE equation of state. AIChE J. 2015, 61, 3053–3072. [Google Scholar] [CrossRef]
- de Sousa, F.F.; da Silva, L.D.P.; Freitas, K.H.G. Electrical and dielectric properties of water. Sci. Plena 2017, 13. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Sabri Kilic, M.; Storey, B.D.; Ajdari, A. Nonlinear electrokinetics at large voltages. New J. Phys. 2009, 11, 075016. [Google Scholar] [CrossRef]








| Material No. | Material Code | LiCl Concentration, wt. % of PVA Mass Content | LiCl Concentration, mol/L |
|---|---|---|---|
| 1 | PS1 | 10 | 0.1652 |
| 2 | PS3 | 30 | 0.4956 |
| 3 | PS5 | 50 | 0.8261 |
| 4 | PS10 | 100 | 1.6521 |
| Material | PVA | SP1 | SP3 | SP5 | SP10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency, Hz | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 |
| Electrical resistance, Ω | 1352 ± 11 | 1102 ± 8 | 890 ± 12 | 813 ± 6 | 414 ± 10 | 393 ± 2 | 151 ± 7 | 137 ± 6 | 111 ± 2 | 101 ± 1 |
| Hydrogel | PS1 | PS10 | ||||||
|---|---|---|---|---|---|---|---|---|
| Voltage, V | 90 | 110 | 90 | 110 | ||||
| Frequency, Hz | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 |
| Current, A | 0.042 ± 0.002 | 0.039 ± 0.006 | 0.008 ± 0.001 | 0.009 ± 0.001 | 0.007 ± 0.001 | 0.006 ± 0.001 | - | - |
| P1, Watt | 3.78 | 3.51 | 0.88 | 0.99 | 0.63 | 0.54 | - | - |
| P2 (extension), Watt | 0.0056 | 0.0054 | 0.0148 | 0.0042 | 0.0294 | 0.0176 | - | - |
| Efficiency (extension), % | 1.48 | 1.45 | 1.68 | 0.42 | 4.67 | 3.27 | - | - |
| P2 (contraction), Watt | 0.0024 | 0.0042 | 0.0031 | 0.0049 | 0.0122 | 0.0116 | - | - |
| Efficiency (contraction), % | 0.06 | 0.12 | 0.35 | 0.50 | 1.94 | 2.15 | - | - |
| Material | PS1 | PS10 | ||||||
|---|---|---|---|---|---|---|---|---|
| Voltage, V | 90 | 110 | 90 | 110 | ||||
| Frequency, Hz | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 |
| Activation force, kg | 2.11 ± 0.18 | 2.59 ± 0.24 | 3.25 ± 0.16 | 4.56 ± 0.22 | 3.14 ± 0.16 | 2.56 ± 0.13 | - | - |
| Activation force, kPa | 263.59 ± 22.49 | 323.56 ± 29.98 | 406.01 ± 19.99 | 569.66 ± 27.45 | 392.27 ± 19.91 | 319.80 ± 16.18 | - | - |
| Required time, sec | 1.56 ± 0.23 | 1.42 ± 0.32 | 1.88 ± 0.26 | 1.36 ± 0.27 | 1.15 ± 0.12 | 0.75 ± 0.03 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayyoub, T.; Zadorozhnyy, M.; Filippova, K.V.; Iudina, L.D.; Telyshev, D.V.; Zhemchugov, P.V.; Ladokhin, D.G.; Maksimkin, A. Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC Voltage. J. Compos. Sci. 2024, 8, 323. https://doi.org/10.3390/jcs8080323
Dayyoub T, Zadorozhnyy M, Filippova KV, Iudina LD, Telyshev DV, Zhemchugov PV, Ladokhin DG, Maksimkin A. Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC Voltage. Journal of Composites Science. 2024; 8(8):323. https://doi.org/10.3390/jcs8080323
Chicago/Turabian StyleDayyoub, Tarek, Mikhail Zadorozhnyy, Kseniia V. Filippova, Lidiia D. Iudina, Dmitry V. Telyshev, Pavel V. Zhemchugov, Dmitriy G. Ladokhin, and Aleksey Maksimkin. 2024. "Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC Voltage" Journal of Composites Science 8, no. 8: 323. https://doi.org/10.3390/jcs8080323
APA StyleDayyoub, T., Zadorozhnyy, M., Filippova, K. V., Iudina, L. D., Telyshev, D. V., Zhemchugov, P. V., Ladokhin, D. G., & Maksimkin, A. (2024). Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC Voltage. Journal of Composites Science, 8(8), 323. https://doi.org/10.3390/jcs8080323








