Correlation of Rh Particle Size with CO Chemisorption: Effect on the Catalytic Oxidation of MTBE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2
2.2. Synthesis of Rh/TiO2 Catalysts
- RhTi1 was reduced at 500 °C for 3 h with a heating rate of 1 °C min−1;
- RhTi2 was reduced at 500 °C for 3 h with a heating rate of 2 °C min−1;
- RhTi3 was reduced at 400 °C for 3 h with a heating rate of 2 °C min−1;
- RhTi4 was reduced at 300 °C for 3 h with a heating rate of 2 °C min−1.
2.3. Characterization
2.4. Catalytic Activity
3. Results and Discussion
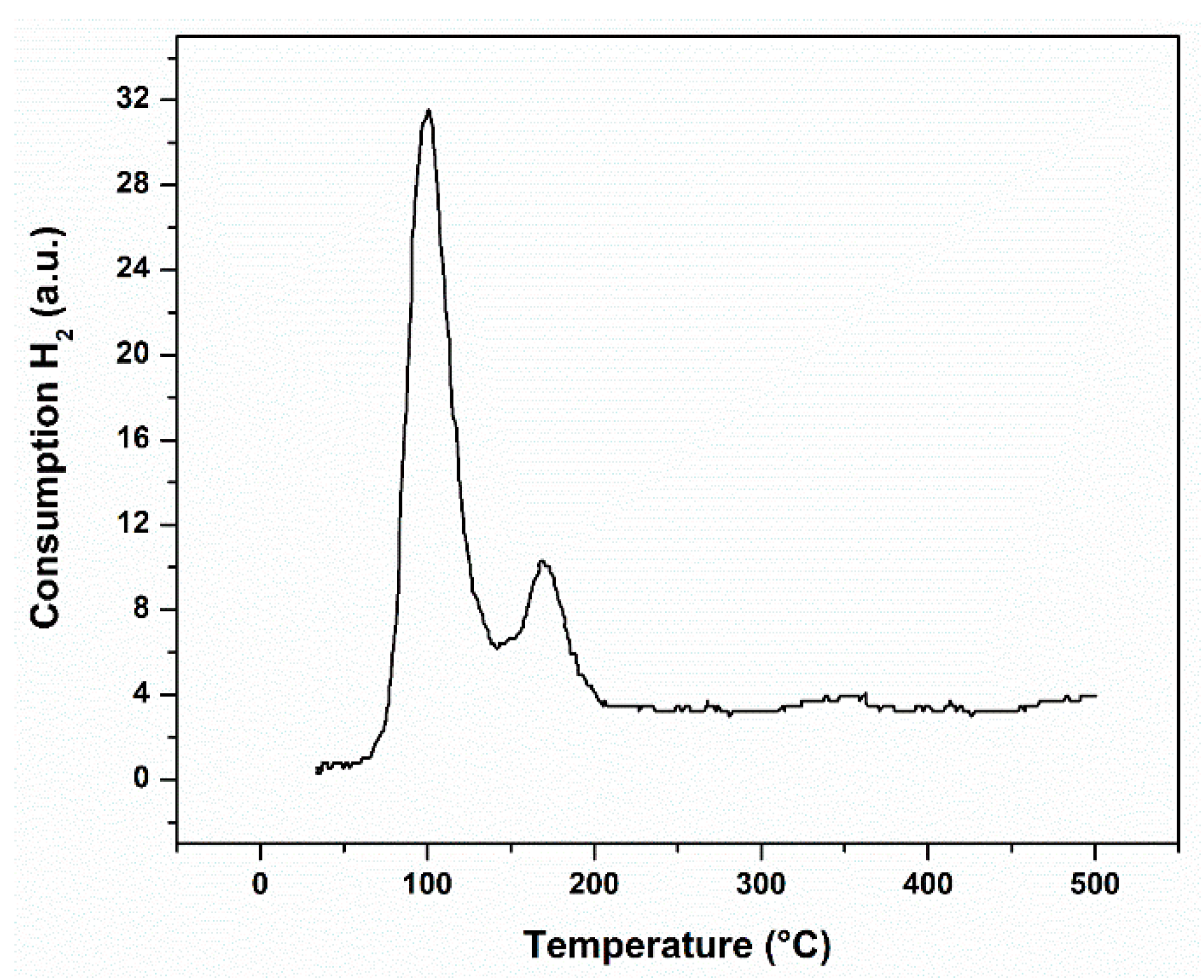
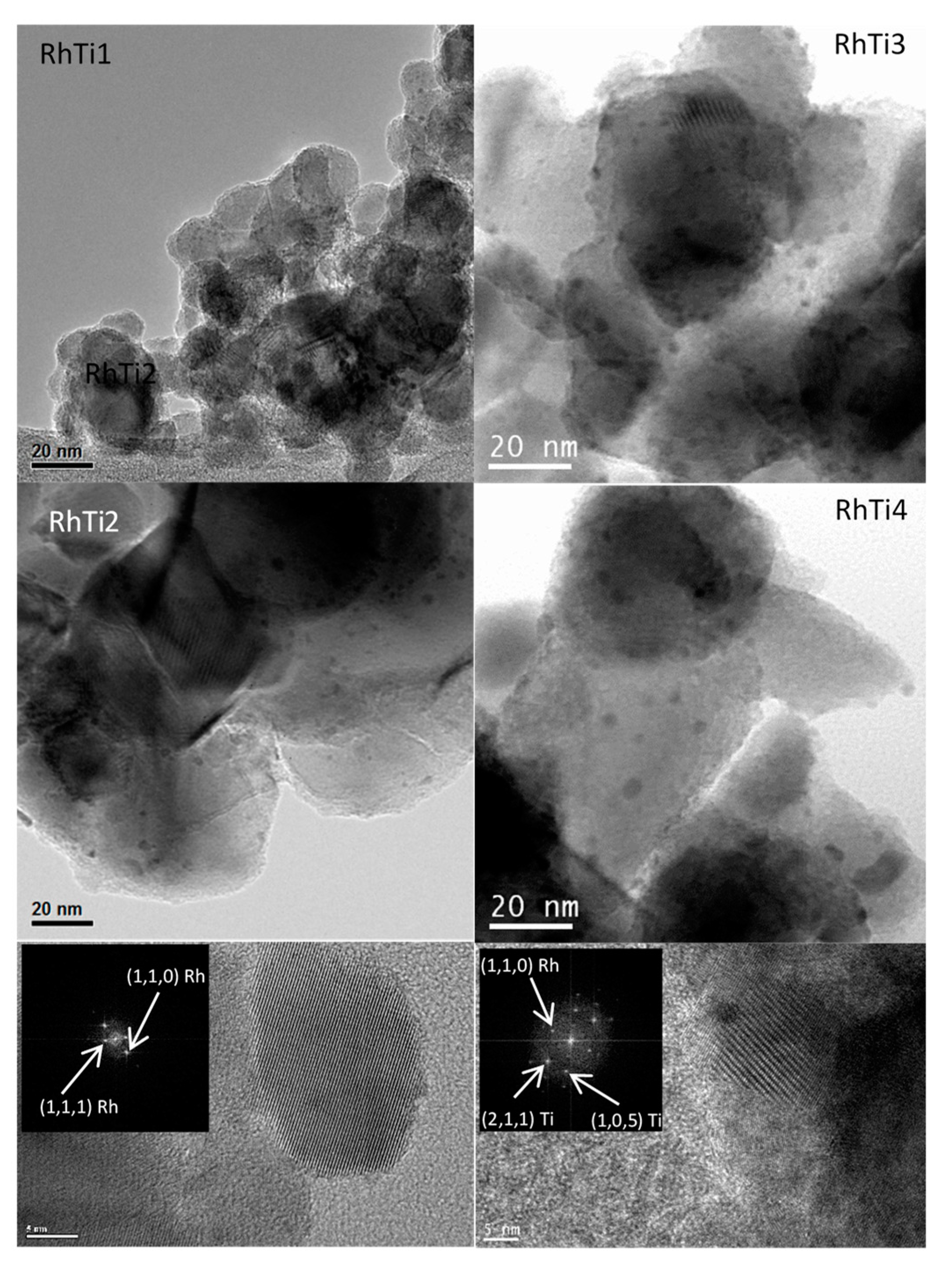
3.1. Catalysts Characterization
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Wu, X.; Huang, C.; Fan, W.; Qiu, X. Visible Light Photocatalytic Activity Induced by Rh(III) Modification on the Surface of BiOCl. Appl. Surf. Sci. 2016, 387, 45–50. [Google Scholar] [CrossRef]
- Tóth, M.; Varga, E.; Oszkó, A.; Baán, K.; Kiss, J.; Erdohelyi, A. Partial Oxidation of Ethanol on Supported Rh Catalysts: Effect of the Oxide Support. J. Mol. Catal. A Chem. 2016, 411, 377–387. [Google Scholar] [CrossRef]
- Nam, J.S.; Rong Kim, A.; Kim, D.M.; Chang, T.S.; Kim, B.S.; Bae, J.W. Novel Heterogeneous Rh-Incorporated Graphitic-Carbon Nitride for Liquid-Phase Carbonylation of Methanol to Acetic Acid. Catal. Commun. 2017, 99, 141–145. [Google Scholar] [CrossRef]
- Faroldi, B.; Múnera, J.; Falivene, J.M.; Ramos, I.R.; García, Á.G.; Fernández, L.T.; Carrazán, S.G.; Cornaglia, L. Well-Dispersed Rh Nanoparticles with High Activity for the Dry Reforming of Methane. Int. J. Hydrog. Energy 2017, 42, 16127–16138. [Google Scholar] [CrossRef]
- Oliviero, L.; Barbier, J.; Duprez, D.; Wahyu, H.; Ponton, J.W.; Metcalfe, I.S.; Mantzavinos, D. Wet Air Oxidation of Aqueous Solutions of Maleic Acid over Rh/CeO2 Catalysts. Appl. Catal. B Environ. 2001, 35, 1–12. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, T.; Lu, J.; Wu, X.; Hochstadt, H.; Zhao, Y. Three-Way Catalytic Reactions on Rh-Based Catalyst: Effect of Rh/ceria Interfaces. Appl. Catal. A Gen. 2017, 544, 30–39. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Cauqui, M.A.; Rodriguez-Izquierdo, J.M.; Vidal, H. Influence of the Preparation Procedure on the Chemical and Microstructural Properties of Lanthana Promoted Rh/SiO2 Catalysts. A FTIR Spectroscopic Study of Chemisorbed CO. J. Alloys Compd. 1997, 250, 461–466. [Google Scholar] [CrossRef]
- Back, S.; Lim, J.; Kim, N.-Y.; Kim, Y.-H.; Jung, Y. Single-Atom Catalysts for CO Electroreduction with Significant Activity and Selectivity Improvements. Chem. Sci. 2017, 594, 1–19. [Google Scholar]
- Karelovic, A.; Ruiz, P. Improving the Hydrogenation Function of Pd/γ-Al2O3 Catalyst by Rh/γ-Al2O3 Addition in CO2 Methanation at Low Temperature. ACS Catal. 2013, 3, 2799–2812. [Google Scholar] [CrossRef]
- Bódisa, J.; Németh, C.; Mink, J.; Keresztury, G.; Tétényi, P. Emission FT-IR Spectroscopic Study of Adsorbed Carbon Monoxide on Metal Powders and Supported Catalysts. J. Mol. Struct. 1997, 411, 179–182. [Google Scholar] [CrossRef]
- Worley, S.D.; Mattson, A.; Caudlll, R. An Infrared Study of the Hydrogenation of CO on Supported Rh Catalysts. J. Phys. Chem. 1983, 87, 1671–1673. [Google Scholar] [CrossRef]
- Diaz, A.L.; Quigley, W.W.C.; Yamamoto, H.D.; Bussell, M.E. Infrared Spectroscope and Temperature Programmed Desorption Study of CO on Rh/Al2O3 Catalysts: Probing Overlayer and Support Sites. Langmuir 1994, 10, 1461–1471. [Google Scholar] [CrossRef]
- Mink, J.; Gal, M.; Goggin, P.L.; Spencer, J.L. And Computerized Raman Studies of the Vibrational Spectra and Structure. J. Mol. Struct. 1986, 142, 467–472. [Google Scholar] [CrossRef]
- Sant, R.; Wolf, E. Elementary-Step Modeling and Transient Studies of Co Oxidation ON Rh/SiO2. Chem. Eng. Sci. 1990, 45, 3137–3147. [Google Scholar] [CrossRef]
- Yu, J.; Mao, D.; Ding, D.; Guo, X.; Lu, G. New Insights into the Effects of Mn and Li on the Mechanistic Pathway for CO Hydrogenation on Rh-Mn-Li/SiO2 Catalysts. J. Mol. Catal. A Chem. 2016, 423, 151–159. [Google Scholar] [CrossRef]
- Caballero, M.; Del Angel, G.; Bonilla-Sánchez, A.; Rangel-Vázquez, I.; Arrieta, A.; Vázquez-Zavala, A.; Huerta, L.; Salgado, M. High Selectivity to Hydrogen on the Methane Decomposition over Rh/γ-Al2O3–Nd2O3 Catalysts. Int. J. Hydrog. Energy 2016, 41, 23247–23259. [Google Scholar] [CrossRef]
- Cuauhtémoc, I.; Del Angel, G.; Torres, G.; Angeles-Chavez, C.; Navarrete, J.; Padilla, J.M. Enhancement of Catalytic Wet Air Oxidation of Tert-Amyl Methyl Ether by the Addition of Sn and CeO2 to Rh/Al2O3 Catalysts. Catal. Today 2011, 166, 180–187. [Google Scholar] [CrossRef]
- Samoila, P.; Boutzeloit, M.; Especel, C.; Epron, F.; Marécot, P. Relationship between the Structural Properties of Supported Bimetallic Pt-Rh Catalysts and Their Performances for Methylcyclopentane Ring Opening. J. Catal. 2010, 276, 237–248. [Google Scholar] [CrossRef]
- Liu, H.; Lin, Y.; Ma, Z. Rh2O3/mesoporous MOx-Al2O3 (M = Mn, Fe, Co, Ni, Cu, Ba) Catalysts: Synthesis, Characterization, and Catalytic Applications. Chin. J. Catal. 2016, 37, 73–82. [Google Scholar] [CrossRef]
- State, R.; Scurtu, M.; Miyazaki, A.; Papa, F.; Atkinson, I.; Munteanu, C.; Balint, I. Influence of metal-support interaction on nitrate hydrogenation over Rh and Rh-Cu nanoparticles dispersed on Al2O3 and TiO2 supports. Arab. J. Chem. 2017, 10, 975–984. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Chironi, I.; Karatchevtseva, I.; Triani, G.; Sorrell, C.C. Single and Mixed Phase TiO2 Powders Prepared by Excess Hydrolysis of Titanium Alkoxide. Adv. Appl. Ceram. 2012, 111, 149–158. [Google Scholar] [CrossRef]
- Ye, J.Y.; Jiang, Y.X.; Sheng, T.; Sun, S.G. In-situ FTIR Spectroscopic Studies of Electrocatalytic Reactions and Processes. Nano Energy 2016, 29, 414–427. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, With Special Reference to The Evaluation of Surface Area and Pore Size Distribution (Iupac Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Varga, E.; Oszko, A. Stability and Temperature-Induced Agglomeration of Rh Nanoparticles Supported by CeO2. Langmuir 2016, 32, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, R.R.; Yates, J.T.J. Site Distribution Studies of Rh Supported on Al2O3—An Infrared Study of Chemisorbed CO. J. Chem. Phys. 1981, 74, 4150–4155. [Google Scholar] [CrossRef]
- Miessner, H.; Gutschick, D.; Ewald, H.; Miller, H. The Influence of Support on the Geminal Dicabonyl Species Rh1(CO)2 on Supported Rhodium Catalysts: An Ir Spectroscopic. J. Mol. Catal. 1986, 36, 369–373. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Ivanova, E.; Dimitrov, L.; Kno, H. FTIR Spectroscopic Study of CO Adsorption on Rh–ZSM-5: Detection of Rh—CO Species. J. Mol. Struct. 2003, 662, 459–463. [Google Scholar] [CrossRef]
- Rice, C.A.; Worley, S.D.; Curtis, C.W.; Guin, J.A.; Tarrer, A.R. The Oxidation State of Dispersed Rh on Al2O3. J. Chem. Phys. 1981, 74, 6487–6497. [Google Scholar] [CrossRef]




| Catalyst | As(BET) (m2g−1) | Rh (%) | S (m2g−1) a | D (%) | d (nm) |
|---|---|---|---|---|---|
| TiO2 | 54 | ||||
| RhTi1 | 53 | 0.985 | 435 | 98 | 1.1 |
| RhTi2 | 51 | 0.986 | 370 | 83 | 1.3 |
| RhTi3 | 50 | 0.987 | 120 | 27 | 4.1 |
| RhTi4 | 49 | 0.983 | 60 | 13 | 8.1 |
| Catalyst | Frequency at 35 °C | |||
|---|---|---|---|---|
| Type I | Type II | Type III | Type IV | |
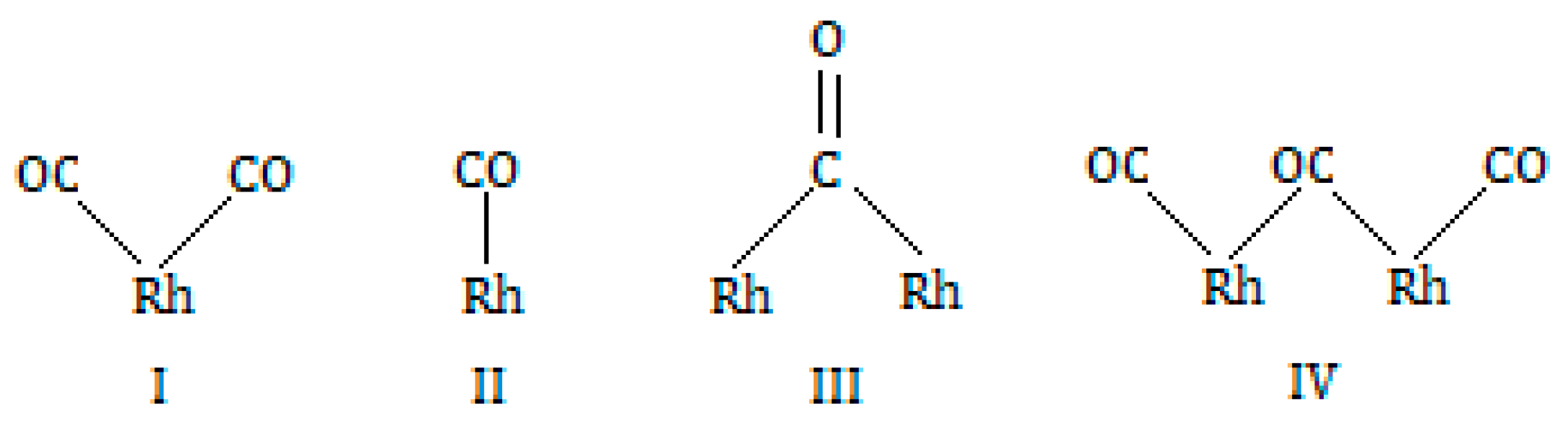
| RhTi1 | 2099, 2024 | - | - | - |
| RhTi2 | 2096, 2022 | 2070 | 1863 | - |
| RhTi3 | 2094, 2026 | 2072 | 2025, 1855 | - |
| RhTi4 | 2096, 2022 | 2070 | 1863 | 1925 |
| Catalyst | XMTBE (%) a | XTOC (%) a | −ri (mmol m−2h−1) |
|---|---|---|---|
| RhTi1 | 58 | 34 | 6.8 |
| RhTi2 | 31 | 29 | 4.3 |
| RhTi3 | 30 | 28 | 4.0 |
| RhTi4 | 25 | 23 | 3.6 |
| TiO2 | 19 | 16 | - |
| Catalyst | −ri (mmol m−2 h−1) | |||
|---|---|---|---|---|
| Methanol | Acetone | Isopropyl Alcohol | Tert-Butanol | |
| RhTi1 | 6.6 | 6.5 | 6.3 | 6.1 |
| RhTi4 | 2.8 | 2.7 | 2.6 | 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes Uribe, A.; Del Angel Montes, G.A.; Torres-Torres, G.; Vázquez-Zavala, A.; González-García, F.; Cordero-García, A.; Ojeda-López, R. Correlation of Rh Particle Size with CO Chemisorption: Effect on the Catalytic Oxidation of MTBE. J. Compos. Sci. 2019, 3, 81. https://doi.org/10.3390/jcs3030081
Cervantes Uribe A, Del Angel Montes GA, Torres-Torres G, Vázquez-Zavala A, González-García F, Cordero-García A, Ojeda-López R. Correlation of Rh Particle Size with CO Chemisorption: Effect on the Catalytic Oxidation of MTBE. Journal of Composites Science. 2019; 3(3):81. https://doi.org/10.3390/jcs3030081
Chicago/Turabian StyleCervantes Uribe, Adrián, Gloria Alicia Del Angel Montes, Gilberto Torres-Torres, Armando Vázquez-Zavala, Federico González-García, Adrián Cordero-García, and Reyna Ojeda-López. 2019. "Correlation of Rh Particle Size with CO Chemisorption: Effect on the Catalytic Oxidation of MTBE" Journal of Composites Science 3, no. 3: 81. https://doi.org/10.3390/jcs3030081





