Cardiovascular Outcomes in Renal Transplant Recipients: Feasibility and Clinical Role of 2D Speckle Tracking to Assess Myocardial Function
Abstract
:1. Introduction
2. Materials and Method
2.1. Population Studied
2.2. Cardiopulmonary Test
2.3. Echocardiographic Examination
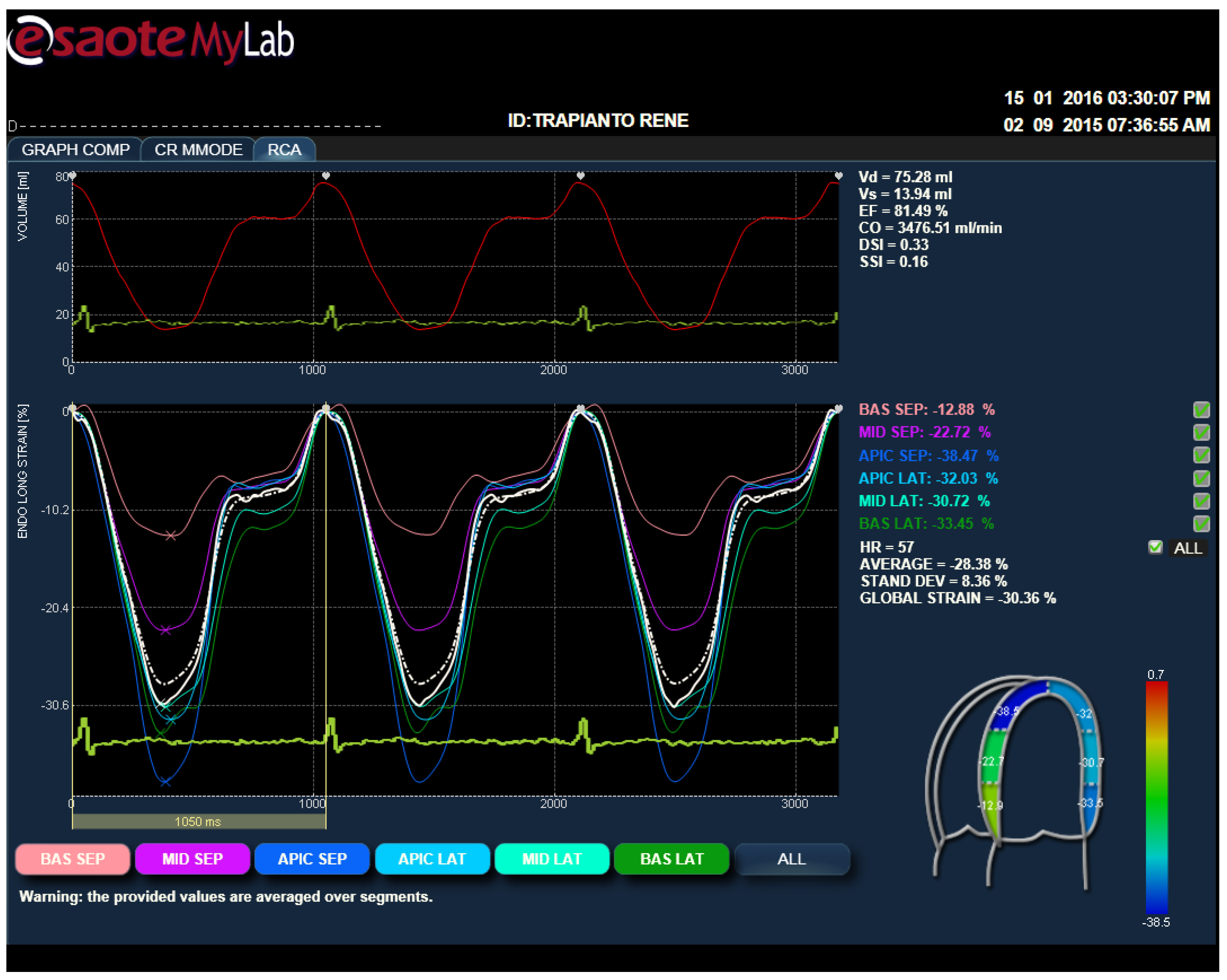
2.4. Strain Analysis by Speckle Tracking Model
2.5. Muscle Strength and Functional Abilities
3. Results
3.1. Cardiopulmonary Test Parameters of RTR
3.2. Echocardiographic Parameters
3.3. Strain Parameters
4. Discussion
Author Contributions
Conflicts of Interest
References
- Glicklich, D.; Vohra, P. Cardiovascular risk assessment before and after kidney transplantation. Cardiol. Rev. 2014, 22, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.P.; Fernandes, N.M.; Mata, G.F.; Chaoubah, A.; Paula, R.B.; Bastos, M.G. Prevalence of metabolic syndrome and its associated factors in renal transplant recipients. J. Bras. Nefrol. 2012, 34, 16–21. [Google Scholar] [PubMed]
- Mikolasevic, I.; Racki, S.; Lukenda, V.; Pavletic-Persic, M.; Milic, S.; Orlic, L. Non-alcoholic fatty liver disease; a part of the metabolic syndrome in the renal transplant recipient and possible cause of an allograft dysfunction. Med. Hypotheses 2014, 82, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Fellstrom, B.; Holdaas, H.; Jardine, A. Functional cardiopulmonary exercise testing in potential renal transplant recipients. J. Am. Soc. Nephrol. 2014, 25, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Galanti, G.; Stefani, L.; Mascherini, G.; Petri, C.; Corsani, I.; Francini, L.; Cattozzo, A.; Gianassi, M.; Minetti, E.; Pacini, A.; et al. Short-term prospective study of prescribed physical activityin kidney transplant recipients. Int. Emerg. Med. 2016, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Roi, G.S.; Stefoni, S.; Mosconi, G.; Brugin, E.; Burra, P.; Ermolao, A.; Granito, M.; Mancini, P.; Mastrosimone, S.; Nacchia, F.; et al. Physical activity in solid organ transplant recipients: Organizational aspects and preliminary results of the Italian project. Transpl. Proc. 2014, 46, 2345–2349. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Stefani, L.; Pedrizzetti, G.; Pedri, S.; Galanti, G. The effect of exercise training on left ventricular function in young elite athletes. Cardiovasc. Ultrasound 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.; de Luca, A.; Maffulli, N.; Mercuri, R.; Innocenti, G.; Suliman, I.; Toncelli, L.; Vono, M.C.R.; Cappelli, B.; Pedri, S.; et al. Speckle tracking for left ventricle performance in young athletes with bicuspid aortic valve and mild aortic regurgitation. Eur. J. Echocardiogr. 2009, 10, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Claus, P.; Omar, A.M.; Pedrizzetti, G.; Sengupta, P.P.; Nagel, E. Tissue tracking technology for assessing cardiac mechanics. Principles, normal values and clinical application. JACC Cardiovasc. Imaging 2015, 8, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, J.; Barbosa, D.; Gao, H.; Claus, P.; Prater, D.; Hamilton, J.; Lysyansky, P.; Abe, Y.; Ito, Y.; Houle, H.; et al. Two-dimensional speckle tracking echocardiography: Standardization efforts based on synthetic ultrasound data. Eur. Heart J. Cardiovasc. Imaging 2015. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The EACVI/ASE inter-vendor comparison study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Brzycki, M. A Practical Approach to Strength Training; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F.; Mark, D.B.; McCallister, B.D.; O’Reilly, M.G.; Winters, W.L., Jr.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002, 106, 1883–1892. [Google Scholar] [PubMed]
- Ghosh, A.K. Anaerobic threshold: Its concept and role in endurance sport. Malays. J. Med. Sci. 2004, 11, 24–36. [Google Scholar] [PubMed]
- Robertson, R.J.; Goss, F.L.; Dubé, J.; Rutkowski, J.; Dupain, M.; Brennan, C.; Andreacci, J. Validation of the adult OMNI scale of perceived exertion for cycle ergometer exercise. Med. Sci. Sports Exerc. 2004, 36, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Garcia, M.J.; Haines, D.E.; Lai, W.W.; Manning, W.J.; Patel, A.R.; Picard, M.H.; Polk, D.M.; Ragosta, M.; Ward, R.P.; et al. ACCF/ ASE/ AHA/ ASNC/HFSA/ HRS/ SCAI/ SCCM/ SCCT/ SCMR 2011 Appropriate Use Criteria for Echocardiography. J. Am. Coll. Cardiol. 2011, 57, 1126–1166. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension 1987, 9, II19–II26. [Google Scholar] [CrossRef] [PubMed]
- Riess, K.J.; Haykowsky, M.; Lawrance, R.; Tomczak, C.R.; Welsh, R.; Lewanczuk, R.; Tymchak, W.; Haennel, R.G.; Gourishankar, S. Exercise training improves aerobic capacity, muscle strength, and quality of life in renal transplant recipients. Appl. Physiol. Nutr. MeTable 2014, 39, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Laditka, J.N.; Hardin, J.W.; Blair, S.N. Estimated functional capacity predicts mortality in older adults. PED. J. Am. Geriatr. Soc. 2007, 55, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Bozbas, H.; Altin, C.; Karacaglar, E.; Kanyilmaz, S.; Yildirir, A.; Muderrisoglu, H.; Haberal, M. The prevalence and types of cardiovascular disease in patients with end-stage renal disease undergoing renal transplantation. Transpl. Proc. 2013, 45, 3478–3480. [Google Scholar] [CrossRef] [PubMed]
| Number 10 Transplant Recipients | Parameters | T0 | T6 | p |
|---|---|---|---|---|
| Aerobic Threshold | VO2 (mLO2/kg/min) | 11.5 ± 7.8 | 11.4 ± 5.4 | NS |
| HR (bpm) | 105 ± 23.2 | 101.7 ± 14.1 | NS | |
| Lactate (mmol) | 3.5 ± 1.4 | 3.6 ± 0.9 | NS | |
| Anaerobic Threshold | VO2 max (mLO2/kg/min) | 15.3 ± 6.8 | 20.5 ± 10.1 | <0.05 |
| HR (bpm) | 119.4 ± 19.7 | 118.6 ± 19.8 | NS | |
| Load (watt) | 67.5 ± 45.9 | 77.2 ± 67.0 | NS | |
| VO2 Peak | VO2 (mLO2/kg/min) | 22.6 ± 8.2 | 25.4 ± 13.0 | NS |
| HR max (bpm) | 140.1 ± 24.1 | 135.4 ± 20.2 | NS | |
| Load max (watt) | 112.5 ± 61.6 | 111.3 ± 62.4 | NS |
| N10 Transplant Recipients | T0 | T6 | p |
|---|---|---|---|
| Ao (mm) | 33.4 ± 4.0 | 33.1 ± 3.7 | NS |
| Left Atria (mm) | 36.7 ± 4.6 | 36.1 ± 3.9 | NS |
| IVS (mm) | 9.5 ± 0.9 | 9.4 ± 0.7 | NS |
| PW (mm) | 9.0 ± 0.6 | 9.3 ± 1 | NS |
| LVdD (mm) | 50.5 ± 3.8 | 49.2 ± 3.2 | NS |
| LVsD (mm) | 30.2 ± 2.6 | 30.1 ± 2.9 | NS |
| EF% | 62.7 ± 3 | 67 ± 2.3 | 0.02 |
| RV (mm) | 23.2 ± 0.9 | 23.6 ± 1 | NS |
| CMI (g/m2) | 107 ± 20.8 | 104.7 ± 1.4 | NS |
| T/R | 0.38 | 0.38 | NS |
| E | 72.5 ± 16 | 69.9 ± 11.7 | NS |
| A | 72.9 ± 19 | 68.4 ± 11.4 | NS |
| E/A | 2.05 ± 2.4 | 1.02 ± 0.2 | NS |
| IVRT | 76.8 ± 7.6 | 74.5 ± 10.4 | NS |
| DTc | 201.6 ± 48.1 | 194 ± 40 | NS |
| E 1 | 8.5 ± 1.9 | 7.8 ± 2.4 | NS |
| A 1 | 9.8 ± 3.4 | 9.2 ± 2.9 | NS |
| E/E 1 | 9 ± 2.7 | 9.4 ± 2 | NS |
| LV-GLS | LoS Medium | Bas Sept | Bas Lat | Ap Lat | Ap Sept | |
|---|---|---|---|---|---|---|
| RTR | −19.2 ± 5.1 | −19.7 ± 5.3 | −13.2 ± 4.1 | −16.6 ± 5.5 | −21 ± 2.3 | −25.7 ± −7.0 |
| Controls | −22.7 ± −2.6 | −21.4 ± 2.3 | −20.2 ± 3 | −17.9 ± 2.7 | −23.2 ± 5.7 | −25.4 ± 6 |
| p | NS | NS | 0.02 | NS | NS | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefani, L.; Pedrizzetti, G.; Pedri, S.; Minetti, E.; Mandoli, M.; Tosi, B.; Galanti, G. Cardiovascular Outcomes in Renal Transplant Recipients: Feasibility and Clinical Role of 2D Speckle Tracking to Assess Myocardial Function. J. Funct. Morphol. Kinesiol. 2016, 1, 109-117. https://doi.org/10.3390/jfmk1010109
Stefani L, Pedrizzetti G, Pedri S, Minetti E, Mandoli M, Tosi B, Galanti G. Cardiovascular Outcomes in Renal Transplant Recipients: Feasibility and Clinical Role of 2D Speckle Tracking to Assess Myocardial Function. Journal of Functional Morphology and Kinesiology. 2016; 1(1):109-117. https://doi.org/10.3390/jfmk1010109
Chicago/Turabian StyleStefani, Laura, Gianni Pedrizzetti, Stefano Pedri, Enrico Minetti, Marco Mandoli, Benedetta Tosi, and Giorgio Galanti. 2016. "Cardiovascular Outcomes in Renal Transplant Recipients: Feasibility and Clinical Role of 2D Speckle Tracking to Assess Myocardial Function" Journal of Functional Morphology and Kinesiology 1, no. 1: 109-117. https://doi.org/10.3390/jfmk1010109






