2.1. Fish and Flow Parameters
The table below summarizes the data related to (i) the biomass for each pond (i.e., total biomass, number of fish, average fish weight and stocking densities) that were obtained from the fish farmers, and (ii) flow values that were measured for each monitored pond (
Table 1 and
Table 2).
Table 1 shows the range of fish sizes being reared at the time of the experiment (170–1600 g/fish). Different stocking densities were also observed in the different ponds (4–89 kg/m
3).
The flow measurements showed a flow dynamic slightly different than what was expected initially in terms of number of water passes. For example, a negative flow value was obtained between 2
1 and 2
2 ponds (
Table 2). This might be a consequence of the recirculation pump in operation in 2
1 that would have applied a suction of the water from 2
2 (average recirculation flow of 66 L/s, see
Table 2). This demonstrated that 2
2 was completely isolated from 2
1 and therefore isolated from any recirculated water. This is the reason why, contrary to initial expectations where it would have been a “3-pass pond”, 2
2 was considered as a “2-pass pond”, as it only received water from 1
8–12 ponds (i.e., “1-pass ponds”). Furthermore, we observed that the average flow from 2
2 to 2
3 pond was very limited (i.e., 6 L/s, see
Table 2). This is why 2
3 pond was considered a “mostly 2-pass pond”, as it received water from six “1-pass ponds” (i.e., 1
13–18 ponds at 7 L/s each, see
Table 2) and only one “2-pass pond” (i.e., 2
2 pond at 6 L/s, see
Table 2).
Temperature was monitored in different ponds during the experiment and water temperature was 8–9.5 °C in all ponds during the whole experiment.
2.2. Impact of the Whole Fish Farm on Water Quality
The objective was to assess the overall impact of the farm on water quality (i.e., farm influent vs. effluent water quality). Farm inlet (before oxygenation, feeding channel, i.e., 0
0) and farm outlet water quality were compared. Differential concentrations (Δ
C(
t), see Equation (1)) were calculated between inlet and mean outlet concentrations between the two outlets (considering the two farm outlets from 2
3 and 3
3 ponds had similar flows, see
Table 2) monitored during the two days of experiment for all the studied parameters (see
Section 3.2).
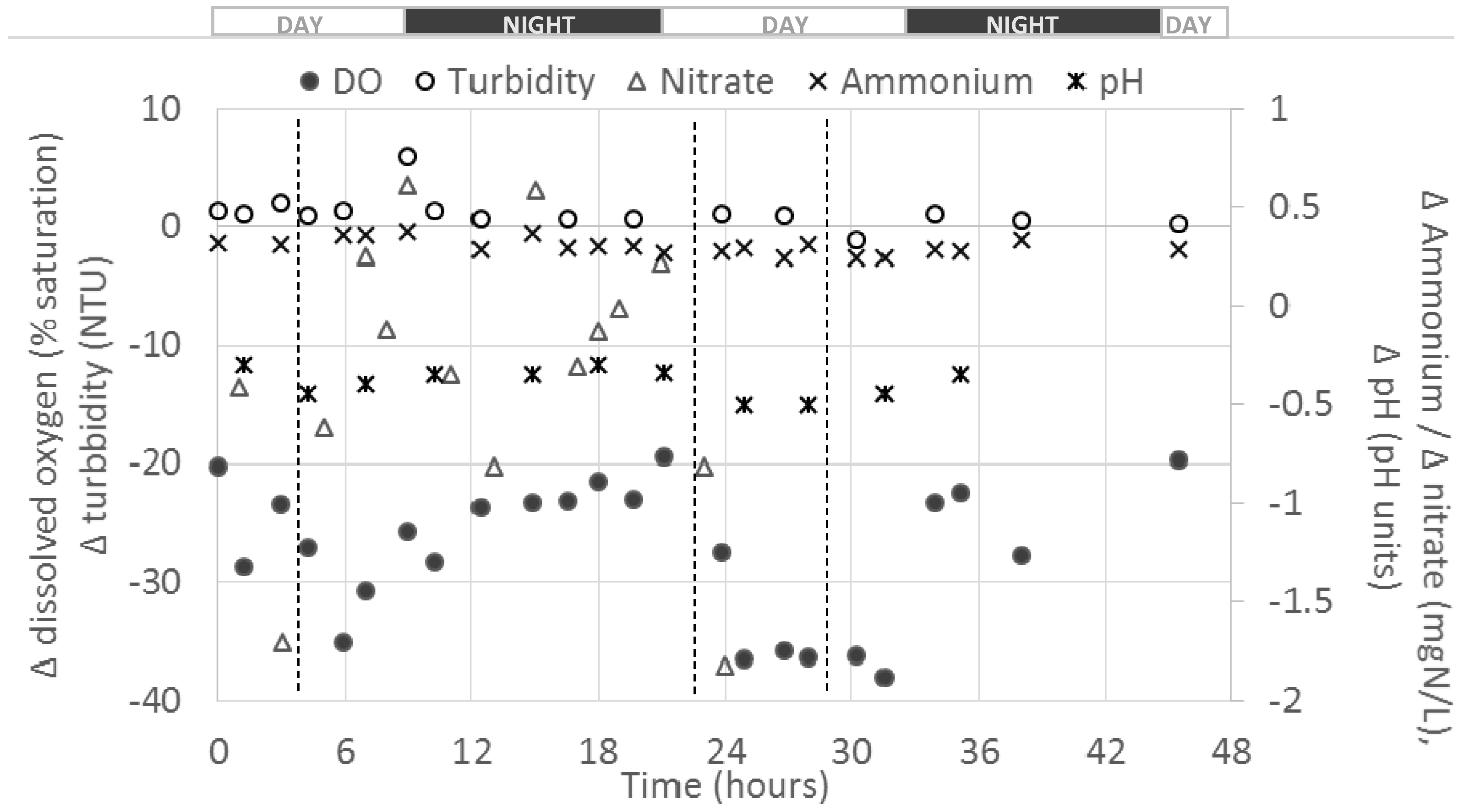
The figure below presents an outcome of the differential values obtained between the fish farm outlets and inlet for five parameters (i.e., DO, turbidity, nitrate (NO
3–N), ammonium (NH
4–N) and pH) during the two days of monitoring (
Figure 1).
Globally, negative Δ
C were observed for DO, pH and NO
3–N for the duration of the monitoring. Such results were expected for DO and pH due to O
2 consumption by fish and sediments and the CO
2 production leading to a pH drop, as observed on other studies [
16,
17]. However, positive Δ
C were observed for turbidity and NH
4–N, meaning that the farm activity “created” solids and NH
4–N. Except for NO
3–N and DO, Δ
C(
t) appeared relatively stable all along the experiment and no diurnal effect was observed at this overall farm scale. For DO, higher absolute Δ
C(
t) (i.e., down to almost −40%) were observed during the days compared to night times (i.e., about −20%); this might be due to the higher O
2 consumption by fish during the day due to the occurrences of feedings and higher fish activity [
18].
The table below summarizes these data by providing average and associated standard deviation of the overall farm Δ
C(
t) values (
Table 3). This presentation of the overall farm mean Δ
C shows a global picture of the influence of the fish farm on water quality. Associated fluxes calculated as presented in
Section 3.4 (Equations (2) and (3)) are also presented.
One of the parameters most affected by the farm was the DO saturation rate, with an average global “loss” of about 27% between farm inlet and outlet despite supplementary aeration/oxygenation steps on the farm (see Figure 7). Considering the total biomass and the outlet flows measured, mean DO F value was −18.4 mgO2/h/kgfish.
An increase in turbidity values from farm inlet to outlet was observed with an average positive Δ
C of about 1.2 Nephelometric Turbidity Unit (NTU). This value appears relatively low compared to values measured at the outlets of some ponds (up to 20 NTU), and this may be due to the sedimentation of suspended solids in ponds, which would have lowered the release of suspended solids at the farm outlet. A peak in turbidity increase (i.e., Δ
C(
t) of 6 NTU) was observed at the beginning of the first night of the monitoring (
Figure 1) which could be due to fish metabolism and the release of faeces a few hours after the afternoon feeding [
16,
17,
18]. In terms of flux, average F value was 7.1 NTU/h/kg
fish.
A global average drop in NO
3–N concentrations of about 0.36 mgN/L between farm inlet and outlet was observed (
Table 3). As shown on
Figure 1, and regarding the relatively high standard deviation associated to the average differential value for this parameter (i.e., 0.72 mgN/L), a high variability of NO
3–N Δ
C during the experiment (i.e., range −1.83–+0.61) was observed. A negative value of average flux of −2.2 mgN/h/kg
fish was obtained.
As expected, NH
4–N appeared to be “produced” by the farm, with a positive average Δ
C of 0.30 mgN/L (see
Table 3). This represents a significant increase regarding the average farm inlet concentration of about 0.30 mgN/L (see
Supplementary Materials). This Δ
C remained stable all along the experiment, with a relatively narrow range observed (0.24–0.38 mgN/L). Therefore, the release of NH
4–N by the farm seemed not be affected by light and feeding regimes. It is noticed that this apparent NH
4–N “production” might be an artefact due to the overall pH drop of 0.39 units (
Table 3) that would have changed the ammonia equilibrium through the ionized form, NH
4–N. An average F value of 1.8 mgN/h/kg
fish was obtained.
An average pH decrease of about 0.39 pH units between farm inlet and outlet was observed (
Table 3) that might a consequence of a CO
2 production by fish metabolism [
16,
17,
18]. This Δ
C remained stable all along the experiment with a relatively narrow range observed (−0.50–−0.30 pH units, see
Table 3).
2.3. Comparison of the Water Quality from Inlet/1-Pass/2-Pass/3 Pass Ponds
The objective of this part of the study was to identify trends between the water quality of the farm inlet, 1-pass, 2-pass and 3-pass waters in order (i) to check if fish farm inlet water impacted water quality flowing through the farm and (ii) to identify potential trends in water quality parameters throughout the farm.
According to the flow measurements performed, only 2
2 and 2
3 ponds were considered as “2-pass ponds” (see
Table 2 and
Section 2.1). Ponds 3
2 and 3
3 were considered as “at least 3-pass ponds” because, as explained before, they received some recirculated water from 2
1 (see
Table 2 and
Section 2.1). It is noticed that 2
1 pond was kept alone for this analysis because it had the specificity to receive directly recirculated water from 1
1–7. This made the number of water passes of this pond difficult to evaluate from the flow measurements performed.
Average values for inlets (after supplementary oxygenation, i.e., 1
13 and 1
15 inlets), 1-pass (1
13 and 1
15 outlets), 2-pass (or “mostly 2-pass ponds”, i.e., 2
2 and 2
3 outlets) and “at least 3-pass ponds” (i.e., 3
2 and 3
3 outlets), 2
1 outlet and associated standard deviation were calculated. The two “1-pass ponds” monitored (i.e., 1
13 and 1
15) were considered separately because of the 1
13 pond harvesting that occurred after 24 h into the monitoring period. For more simplicity, in the following paragraphs the terms “2-pass ponds” and “3-pass ponds” will be employed instead of “mostly 2-pass ponds” and “at least 3-pass ponds”, respectively. The overall results for all the different pond categories (number of water passes) and for all the monitored parameters are presented on the figure below (
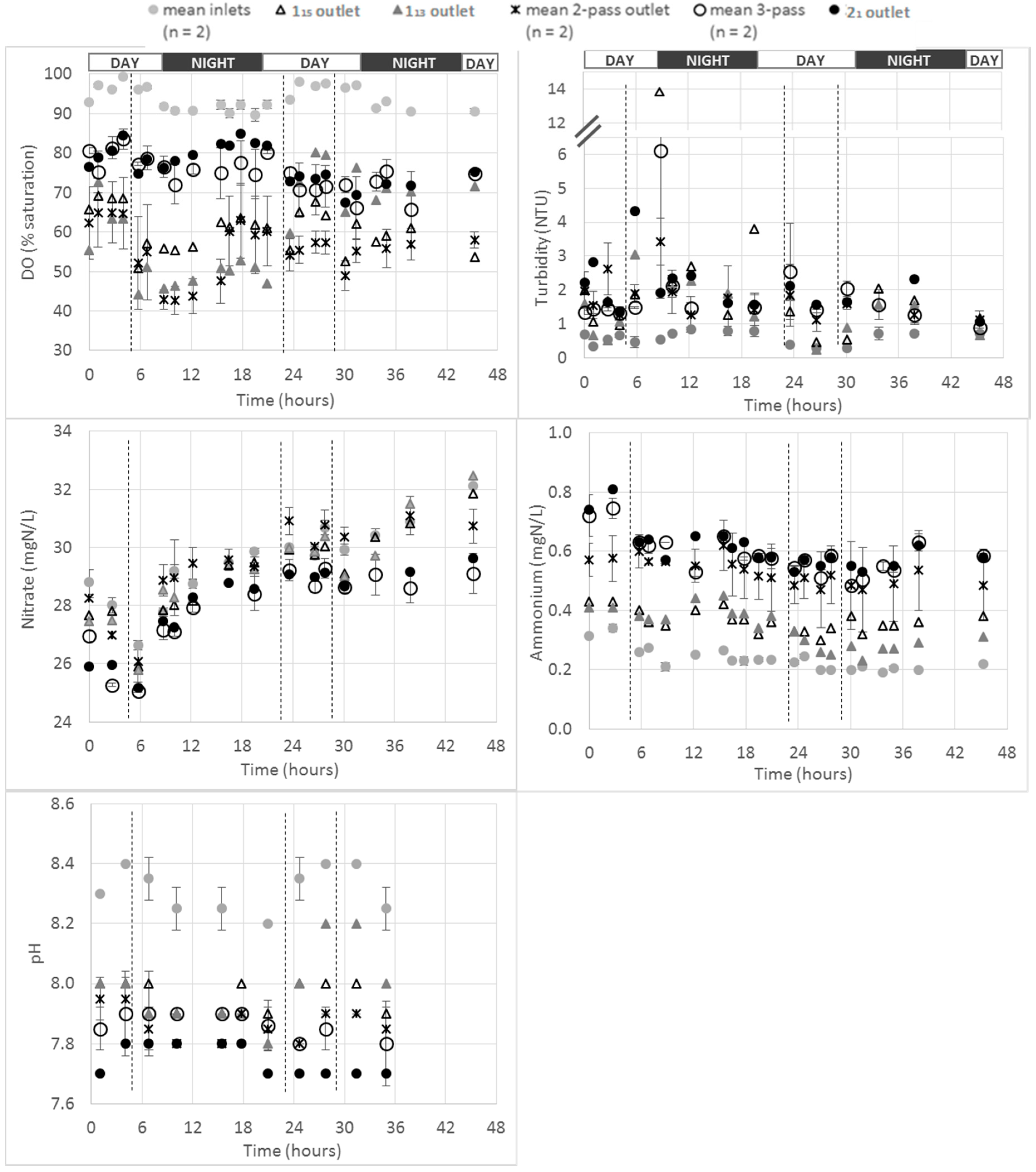
Figure 2).
2.3.1. Dissolved Oxygen
As expected, over the whole farm, the most oxygenated waters were the fish farm inlet waters, with saturation values in the range 85–100%. A higher DO saturation rate was measured during day time compared to night time (average DO values of 96% and 84%, respectively). These higher DO values during the day might be a consequence of the plants and phytoplankton photosynthesis occurring with sunlight and producing O
2 [
17]. The same trend was observed at the inlets of 1
13/1
15 ponds after supplementary oxygenation by the two oxygenation cones located in the inlet channels 0
2 and 0
3 (see Figure 7) and that were in operation continuously during the whole monitoring program. However, the impact of the oxygenation cones on the level of DO saturation of the water appeared to be negligible during day time (i.e., 2–3% higher in the oxygenated water (i.e., 1
13/1
15 ponds inlets) than in the inlet water from the inlet channel 0
0, see
Supplementary Materials). This impact was revealed to be higher at night time with a difference between oxygenated and non-oxygenated water up to 10% (i.e., 84% and 94% for non-oxygenated and oxygenated water respectively, see
Supplementary Materials).
The monitoring of the three “1-pass ponds” showed that a systematic drop in the DO level was observed after every feeding event. This drop appeared to occur immediately after feeding. This would coincide with an increase in fish activity and in the metabolic demands associated with digestion [
18].
Interestingly, the “3-pass ponds” did not show the least oxygenated water, with values in the range 65–85% of DO saturation at their outlets. According to the flow measurement performed (see
Table 1), it was demonstrated that those ponds were fed more or less directly by recirculated water (i.e., water from 2
1 pond). This demonstrated the efficiency of applying recirculation/supplementary oxygenation and the associated impact on DO saturation rate observed all along the farm from 2
1 pond to the 3
3 farm outlet (see Figure 7).
The least oxygenated ponds were the “2-pass ponds”, with values in the range 45–65%. These values were in the same range than the ones observed for “1-pass ponds” water from 1
15 (i.e., 50–70%), demonstrating the ability of paddle-wheel aerators to compensate for the loss of oxygen that occurred in those “2-pass ponds” due to fish/sediments consumption. Furthermore, the two “2-pass ponds” were not fed in any way by recirculated water (aeration of these ponds relied only on paddle-wheel aerators, see
Table 2 and
Section 3.1), and that could explain the relative low oxygen saturation levels observed in those ponds compared to the “3-pass ponds”. Among the two “2-pass ponds”, 2
3 pond had a higher DO saturation at its outlet than 2
2 pond during the whole monitoring period (see
Supplementary Materials); this might be due to the differences in aeration systems from one pond to another (i.e., 2
2 being aerated with two paddle-wheel aerators and 2
3 with only one paddle-wheel, see Figure 7). This observation would confirm the ability of paddle-wheel aerators in compensating for the DO loss due to fish and sediments. Furthermore, 2
1 and 3
2 ponds outlets had similar DO profiles during the experiment (see
Supplementary Materials). Thus, the aeration systems in operation in 3
2 (i.e., two paddle-wheels see Figure 7) were efficient enough to maintain the DO saturation levels higher than 70%. However, it was observed that DO levels were generally lower in 3
3 than in 2
1/3
2 ponds showing that the aeration system (i.e., one paddle-wheel, see Figure 7) was not efficient enough to maintain the same DO level in this pond.
2.3.2. Turbidity
As expected, the lowest turbidity levels and variability were observed for the farm inlet waters. However, the comparison of the different pond classes is not straightforward. The potential sedimentation of solids along the farm might be a potential explanation of the difficulty to analyze the results for this parameter [
16,
18]. The relatively low turbidity values measured (a few NTU) and the potentially high uncertainty associated with turbidity measurements at these low levels might be another potential explanation. However, the same peak of turbidity was observed for the two “1-pass ponds” (1
13 and 1
15) on the first day of monitoring (at about 8 h monitoring time) and the same general turbidity profiles were observed for those two ponds (
Figure 2). The fact that the turbidity measurements were repeatable from those two ponds with similar fish characteristics (i.e., same total biomass and average fish weight, see
Table 1) indicates that the turbidity measurements performed were accurate and reliable. The greater variability in turbidity was observed for the 1-pass ponds outlets (i.e., 1
13 and 1
15). This may be due to the sedimentation of suspended solids all along the farm that would have smoothed the associated turbidity profiles [
16,
18].
2.3.3. Nitrate
The same profile was observed for NO3–N concentrations at any farm level for the duration of the monitoring. The general increase of concentration observed at farm inlet was also observed at each level of water passes. However, the ponds associated to the highest levels of passes (i.e., “3-pass ponds” and 21) were associated to the lowest NO3–N concentrations (i.e., in the range 25–29 mgN/L) while the farm inlet concentrations were in the range 26–32 mg/L. It is noteworthy that the “3-pass ponds” and 21 pond (all fed with recirculated water) showed similar NO3–N profile. This demonstrated that, as for the DO profile, 21 pond could not be considered a “2-pass pond” for the NO3–N profile. Additionally, the “2-pass ponds” were not affected in terms of the NO3–N concentration profile by the fish farming activity. This was highlighted by the comparison with the farm inlet NO3–N concentrations that were observed to be in similar range than 2-pass ponds all along the experiment (range of 2-pass ponds 27–32 mgN/L).
2.3.4. Ammonium
A clear distinction between NH
4–N levels at the farm inlets, “1-pass”, “2-pass” and “3-pass ponds” outlets was observed. As expected, the higher the number of passes is, the higher the NH
4–N concentration. This demonstrates the progressive accumulation of NH
4–N across the whole farm due to fish excretion and/or to progressive dissolution of sediments [
16,
18].
Farm inlet NH4–N concentrations were in the range 0.20–0.34 mgN/L.
The “1-pass pond” outlets’ NH4–N concentrations were in the range 0.30–0.45 mgN/L (before 113 harvest). It is noteworthy that almost the exact same NH4–N profile was obtained at the outlet of both “1-pass ponds” (i.e., 113 before harvest and 115); then, lower values were observed after 113 harvest during the second day of monitoring. This highlighted both (i) the good quality and reproducibility of the data generated regarding the use of the multisensor probes in terms of NH4–N monitoring and (ii) the impact of fish in NH4–N production in a pond.
The “2-pass ponds” average NH
4–N outlet concentrations were in the range 0.47–0.62 mgN/L, which is a higher level than the ones observed for “1-pass ponds”. A great variability between the values measured for the two “2-pass ponds” considered (i.e., 2
2 and 2
3) was observed; the NH
4–N concentrations were indeed systematically higher for 2
2, by about 0.1 mgN/L. This could be due to the different receiving waters for those two ponds (2
2 received water from the five 1
8–12 “1-pass ponds” (not monitored during this study) and 2
3 pond received water from the six 1
13–18 “1-pass ponds”). This could also be due to the different biomass reared in both 2
2 and 2
3 ponds (see
Table 1).
The “3-pass” and 21 ponds NH4–N outlet concentrations were in the range 0.49–0.81 mgN/L, which was the highest range recorded over the whole farm. This observation is in agreement with both (i) the expected NH4–N accumulation across the whole farm and (ii) the water recirculation without any nitrification step to oxidize NH4–N (into NO2–N and NO3–N) leading to an NH4–N accumulation within the recirculation loop. This underpinned the impact of recirculation on the 21 pond water quality. It is noticed that the 22 pond outlet NH4–N concentrations were in the same range that those measured at “3-pass pond” outlets.
2.3.5. pH
As expected, an impact of the farm on pH values was observed with a general decrease of pH across the whole farm, likely due to the CO
2 production by fish metabolism [
17].
The inlet mean values were in the range 8.2–8.4 pH units with lower values generally observed at night. The “1-pass pond” outlets’ pH values were measured in the range 7.9–8.0 (before harvest for 113 pond). It is interesting to observe values up to 8.2 pH units after harvest at the 113 pond outlet (similar to the values observed at the inlet). This highlights the impact of fish on pH variation. The “2-pass ponds”, “3-pass ponds” and 21 pond outlets pH values were in the range 7.8–7.95 and 7.8–7.9 and 7.7–7.8 pH units, respectively. The lowest values were therefore observed for the recirculated water from the 21 pond. This again demonstrated the impact of recirculation on water quality. This could be due to the recirculation loop that could have brought back water with low pH into this pond. As mentioned before, this could explain the high NH4–N concentration measured in this pond.
Of note is that the ponds with the highest number of water passes had the lowest pH values and the highest NH4–N concentrations. At low pH (i.e., <8), and at the temperatures measured during this monitoring program, the ionized form of ammonia (NH4–N, relatively non-toxic to fish) is highly predominating compared to the toxic unionized form (NH3–N). Therefore, the highest NH4–N concentrations observed in the high number of passes ponds could be just an artefact due to pH variation and not due to an accumulation of fish wastes.
2.4. Impact of 1-Pass Ponds on Water Quality
Three different “1-pass ponds” were intensively monitored during the experiment (i.e., 1
5, 1
13 and 1
15, see Figure 7). As for the whole farm, Δ
C(
t), calculated as explained in
Section 3.4 and using Equation (1), are presented. Fluxes, calculated as explained in
Section 3.4 (Equations (2) and (3)), are also presented. The flux values will be specifically discussed in
Section 2.5 below.
2.4.1. 115 Pond
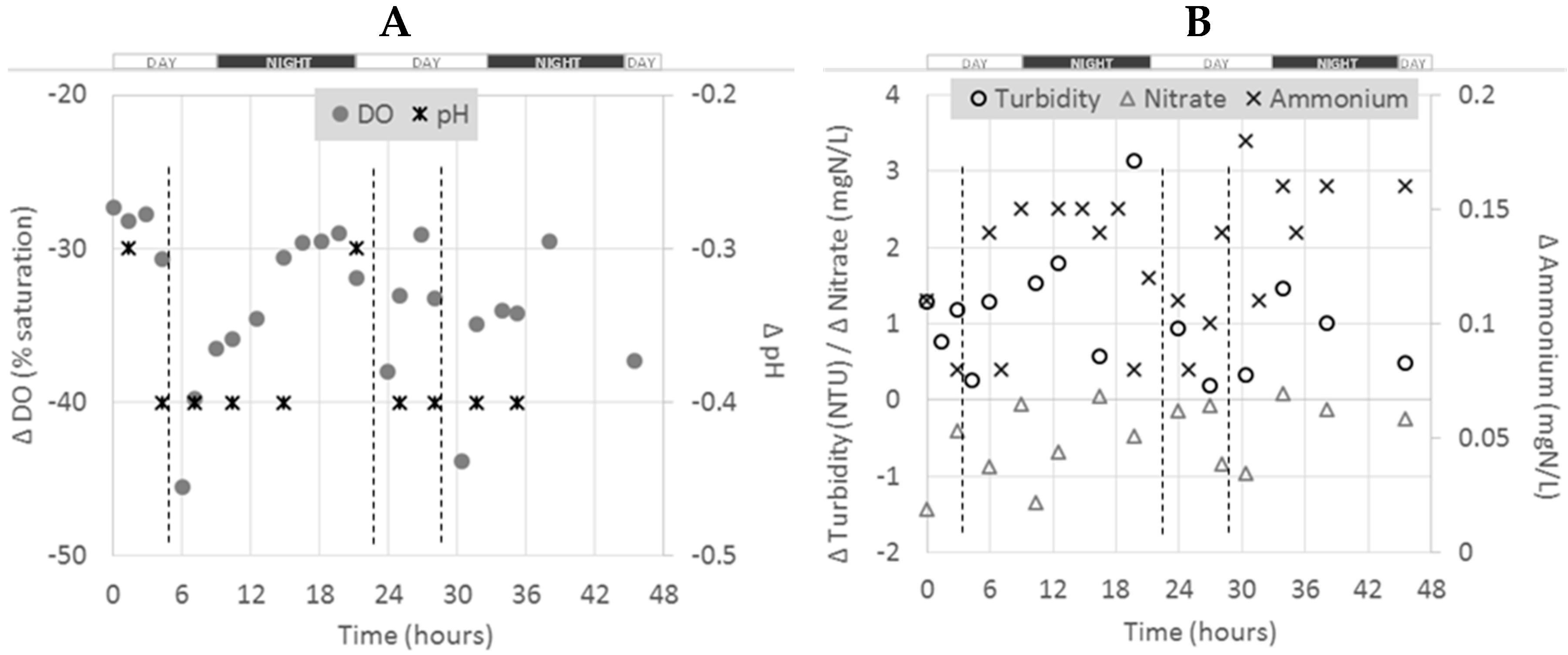
The figure below presents the pattern of Δ
C between the outlet and inlet of 1
15 pond during the monitoring program (
Figure 3).
As for the whole farm scale, different trends were observed, depending on the parameter considered at this pond scale. Negative Δ
C were obtained for pH, DO and NO
3–N. Positive values were obtained for turbidity and NH
4–N. For NO
3–N, a general increase in Δ
C from the beginning to the end of the experiment (from −1.5 to about 0 mgN/L) was observed. The Δ
C for NH
4–N was higher during night times. Dissolved oxygen showed diurnal variation also, with higher consumption (or lower Δ
C) during day time with drops likely to be due to feedings [
19]. No clear trends or diurnal variation were observed for the other parameters.
The Δ
C data are summarized in the table below, which provides average values, associated standard deviation and the calculated fluxes for each parameter (
Table 4). Fluxes data, as described in
Section 3.4 are also presented.
2.4.2. 113 Pond
As for 1
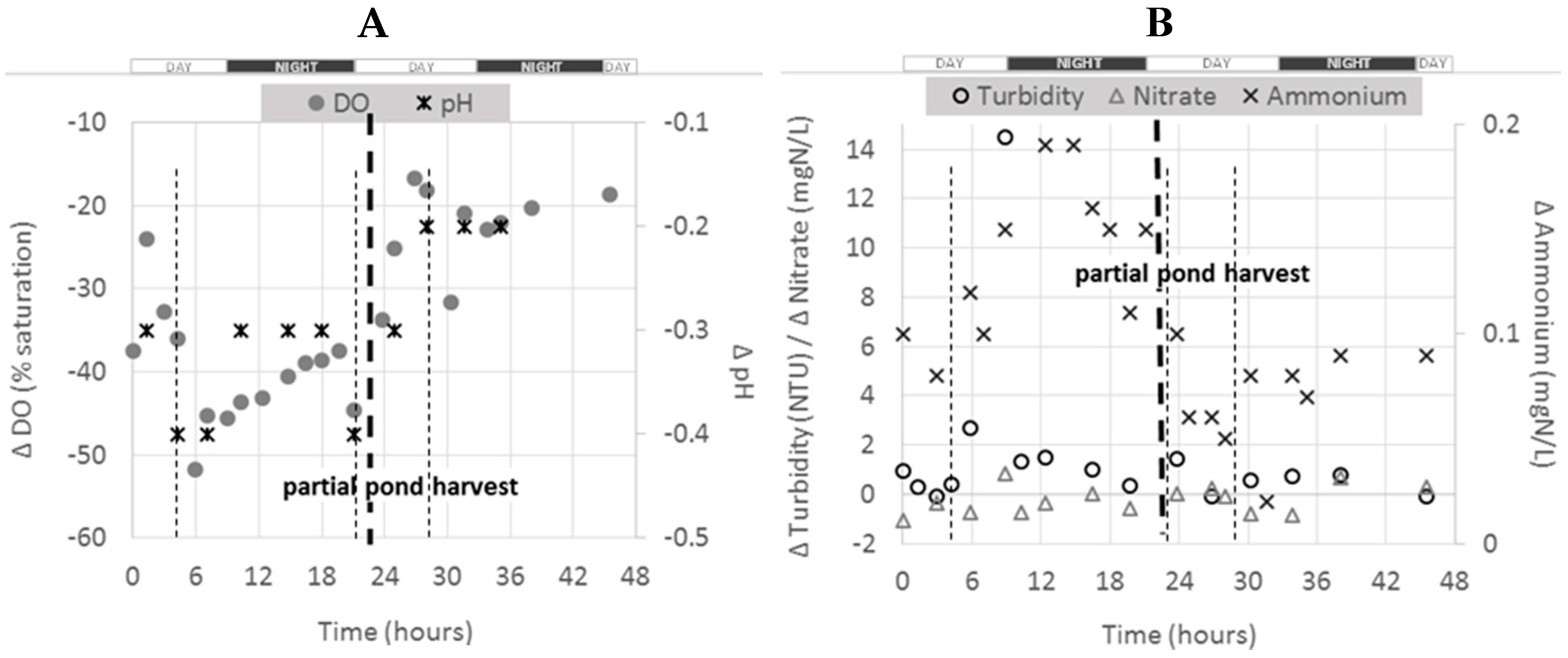
15 pond, the figure below presents the evolution of Δ
C between outlet and inlet of 1
13 pond during all the monitoring program (
Figure 4). It is noticed that this pond was partially harvested on day two of the experiment (after about 24 h of monitoring) (see
Table 1).
As for the whole farm and the 1
15 pond, negative Δ
C values were observed for NO
3–N, pH and DO and positive ones for turbidity and NH
4–N. Before harvest, Δ
C for NH
4–N were higher at night, confirming the observations made for 1
15 pond (see
Section 2.4.1). After harvest, all Δ
C profiles changed for all parameters and all the absolute values were lower. As expected, the effect of the fish activity in this pond was therefore reduced after harvesting, as the total biomass was reduced (see
Table 1).
The Δ
C data are summarized in the table below, which provides average values, associated standard deviation and the calculated fluxes for each parameter before and after harvest (
Table 5). Fluxes data, as described in
Section 2.4 and according to Equations (2) and (3) are also presented.
The comparison of 1
15 and 1
13 ponds before harvest (similar total and average fish weight in those two ponds as presented in
Table 1) showed very similar values, demonstrating the reproducibility of our approach. The effect of harvesting on the differential values appears very clear when results before and after harvest are compared. Mean DO consumption dropped from 40 to 23%, the mean pH difference dropped from 0.34 to 0.23 pH units, mean turbidity production dropped from 2.30 to 0.56 NTU and the mean NH
4–N production dropped from 0.14 to 0.07 mgN/L.
It is noteworthy that despite the fact that the 1
13 pond was harvested and that only 600 kg of fish remained after harvest, the pond fluxes in terms of DO and NH
4–N are not negligible. This underpins the potential impact of the sediments accumulated at the bottom of the tank might have on DO consumption or NH
4–N production. The role of sediments on the residual Δ
C values obtained after harvest will be discussed in
Section 2.5.
2.4.3. 15 Pond
The figure below presents the evolution of Δ
C between outlet and inlet of 1
13 pond during the entire monitoring program (
Figure 5).
This pond had three main particularities compared to 113/115 ponds regarding DO:
- (1)
It was supplied with super-saturated water from the recirculation loop (average 140% saturation rate, see
Supplementary Materials),
- (2)
It had a paddle-wheel aerator running during the experiment (see Figure 7),
- (3)
Bigger fish than in 1
13/1
15 ponds were reared (average weight of 800 g and 220–240 g respectively, see
Table 1)
The table below presents a summary of the Δ
C values observed for all parameters during the whole experiment (
Table 6). The amount of data is lower for this pond because the initial inlet monitoring location had to be changed for another during the experiment as the initial one was observed to be unsuitable for monitoring.
A relatively high DO consumption rate of 59 mgO
2/h/kg
fish compared to the ones found for 1
13/1
15 ponds (43 and 48 mgO
2/h/kg
fish, respectively; see
Table 3 and
Table 4) was observed. The NH
4–N production rate was found to be similar to what was found for the other “1-pass ponds” and for the whole farm (
Table 3).
Contrary to the other “1-pass ponds” monitored (i.e., 113 and 115), a paddle-wheel aerator was employed in this pond for supplementary aeration (Figure 7) and thus, had to be considered in the DO mass balance for this 15 pond. However, the impact of the paddle-wheel aerator in increasing DO levels in the pond could not be assessed.
These results regarding the higher DO consumption rate in 15 compared to 113/115 ponds was unexpected for at least two reasons:
- (1)
The presence of a paddle-wheel aerator should have increased the DO amount received by the pond, and therefore decreased the overall consumption rate (because the calculation did not into account the O2 input from the paddle-wheel aerator).
- (2)
From the literature [
16], bigger fish are supposed to consume less O
2 by mass unit than smaller ones, and the comparisons of results obtained for 1
5 and 1
13/1
15 ponds appeared not to confirm this statement.
The different hypotheses that might explain these results could be:
In relation to the very high initial DO saturation rate at the inlet of this 15 pond (i.e., 140% on average) that would have modified the O2 liquid/air equilibrium and therefore could have caused a faster decrease in DO concentration within the pond (which would have caused the O2 loss by liquid/air transfer higher and give a higher apparent consumption rate).
In relation to sediment amount at the bottom of this pond that could be higher per square meter than in 113/115 ponds that could have caused a higher sediment DO consumption rate for this 15 pond.
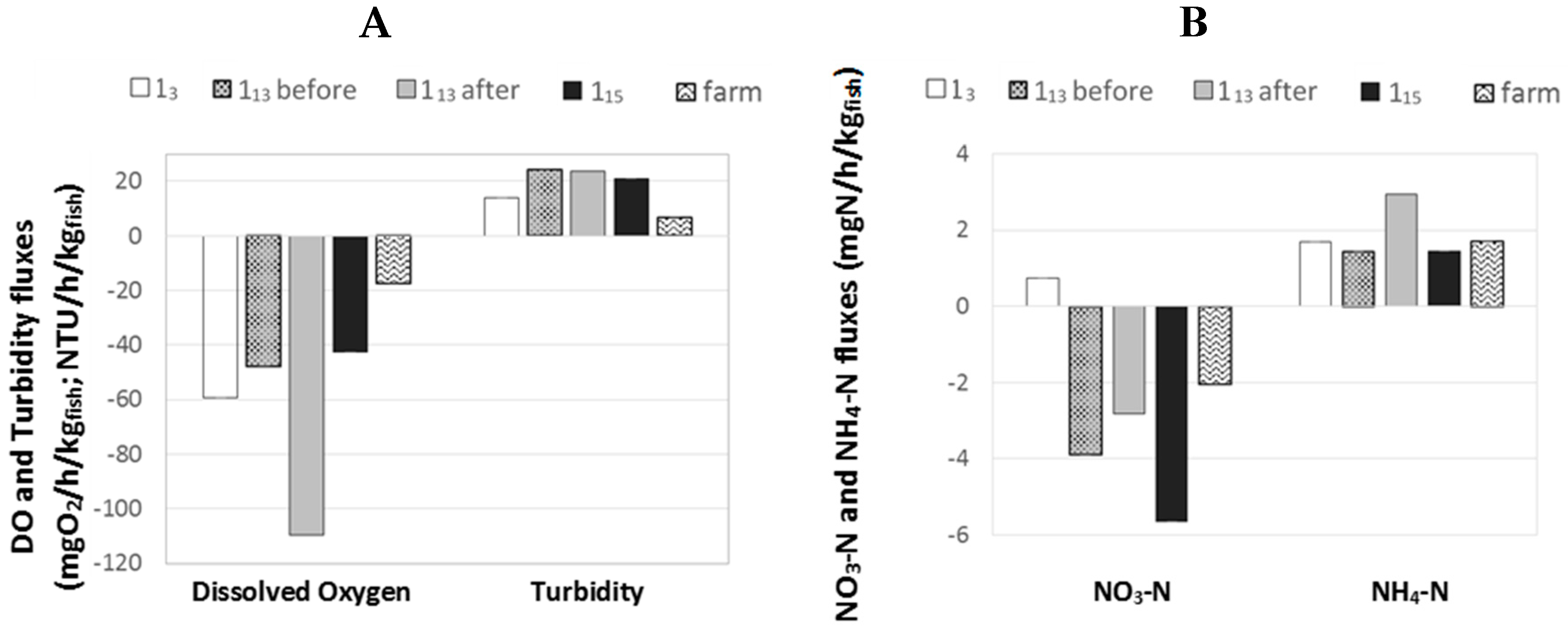
2.5. Outcome on Fluxes
The figure below presents the mean
F(
t) values calculated from Equation (3) for (i) the three “1-pass ponds” monitored (i.e., 1
5, 1
13 before and after harvest and 1
15 ponds) and (ii) the whole farm (
Figure 6).
The differences in DO fluxes between 1
3 and the other “1-pass ponds” were already discussed in a previous Section (
Section 2.4.3) and it was assumed that the higher fluxes observed in 1
3 pond were due to a higher initial DO saturation rate. It is confirmed here that 1
13 pond before harvest and 1
15 pond had similar DO fluxes, with F values being −48 and −43 mgO
2/h/kg
fish respectively. The lower F value observed for the whole farm (i.e., F = −18 mgO
2/h/kg
fish) can be explained by the supply of oxygen given by both aeration and oxygenation devices across the farm that would have lowered the apparent loss of O
2 at farm scale. The highest F value observed for 1
13 pond after harvest (i.e., −109 mgO
2/h/kg
fish) could be an artefact due the impact of sediments (fish faeces and uneaten feed) on the overall DO consumption. The same observation can be done for NH
4–N flux with a very high F value observed for 1
13 after harvest compared to other ponds. It is noteworthy that no data were generated in order to quantify the potential impact of other compartments (plants, algae, phytoplankton, soil etc.) on the DO/NH
4–N levels in ponds. However, no diurnal variation was observed in DO consumption after harvest in pond 1
13 (
Figure 5); this observation would confirm that the impact of phytoplankton and plants was negligible compared to the impact of sediments and fish.
For turbidity, F values for 1
13 before and after harvest and for 1
15 were similar (i.e., 24.1, 23.6 and 20.8 NTU/h/kg
fish respectively). The F value for 1
5 pond was slightly lower (i.e., 13.8 NTU/h/kg
fish), which could be caused by the higher size of this pond and lower water velocity, which would enhance the settling capacities of this pond, increasing the amount of solids removed from water and thus the F value for turbidity. At farm scale, F value was even lower (i.e., 7.1 NTU/h/kg
fish); this observation could be due to the settling capacity of the whole farm [
16,
18].
For NO3–N, a negative flux was observed for all ponds except 13 pond. This pond was the only one to receive some recirculated water which could have created this flux difference and this apparent NO3–N production by the pond by bringing back some low NO3–N content water into the loop.