Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation and Presodiation for Batteries
Abstract
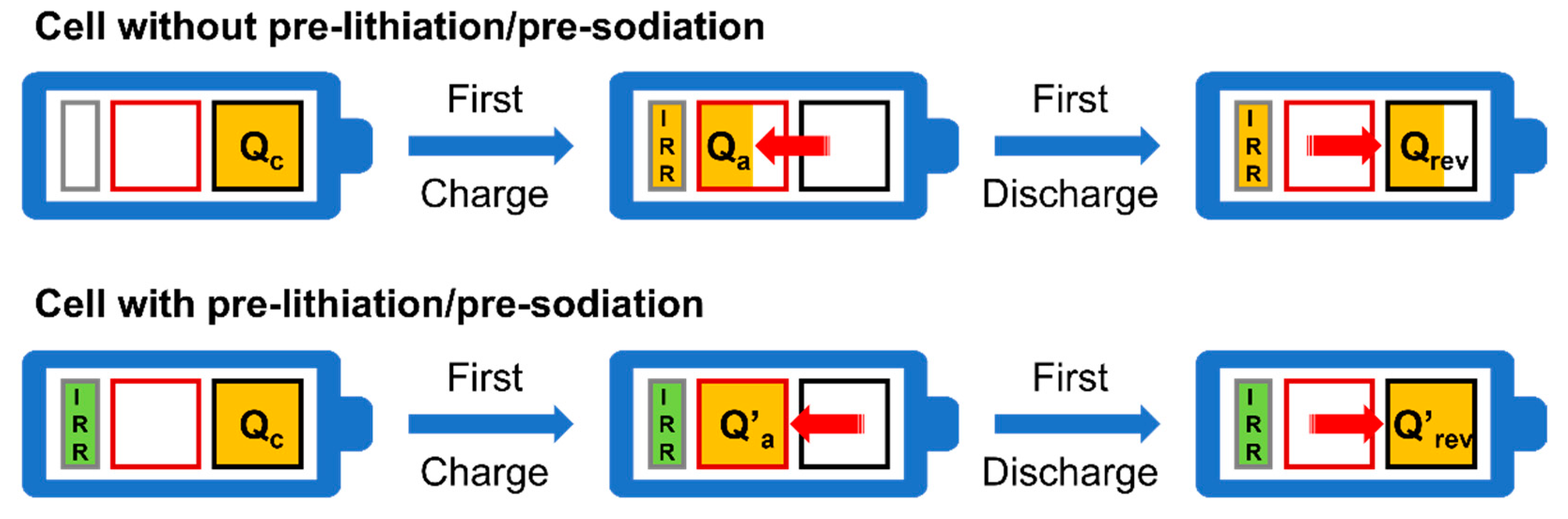
:1. Introduction
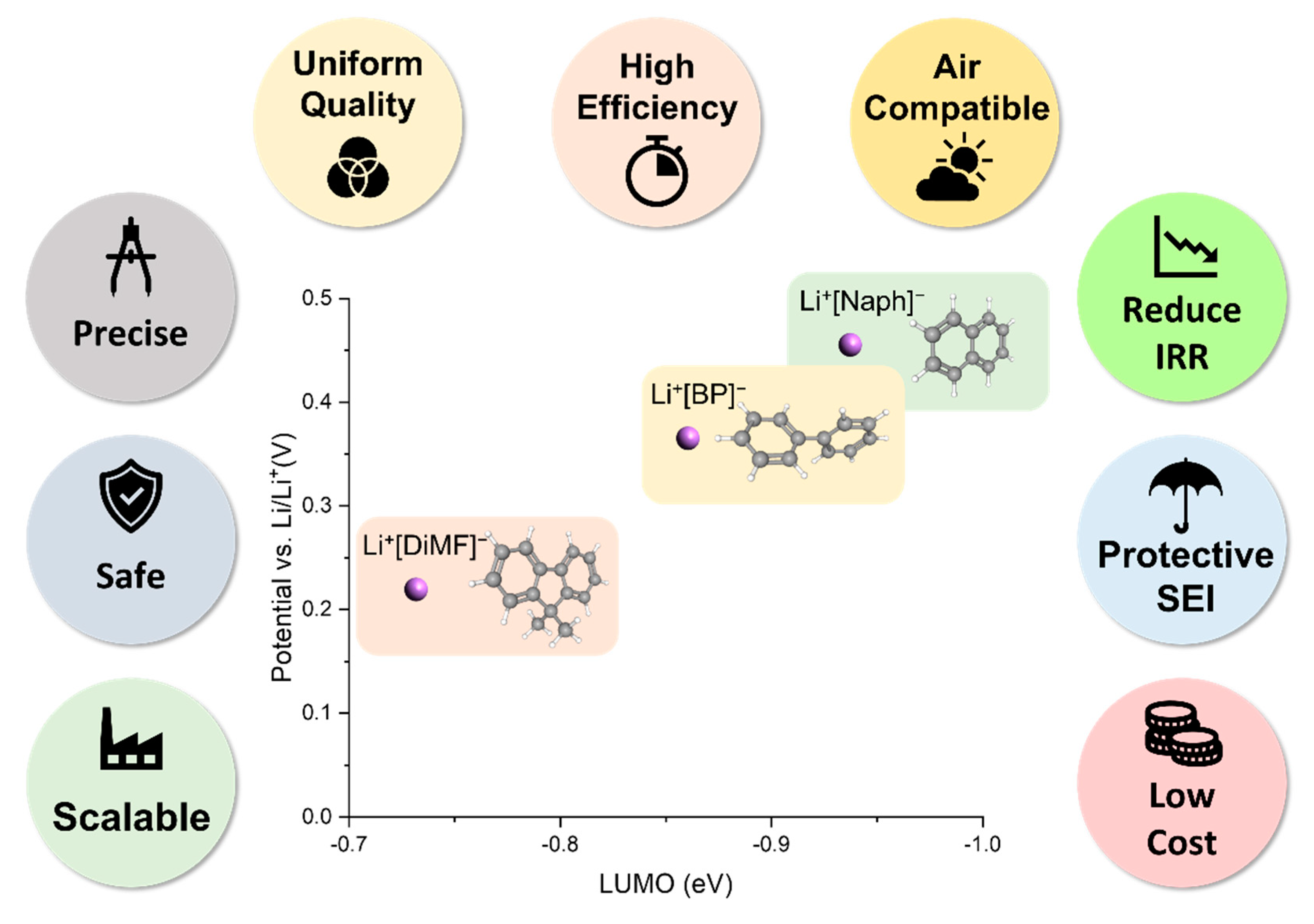
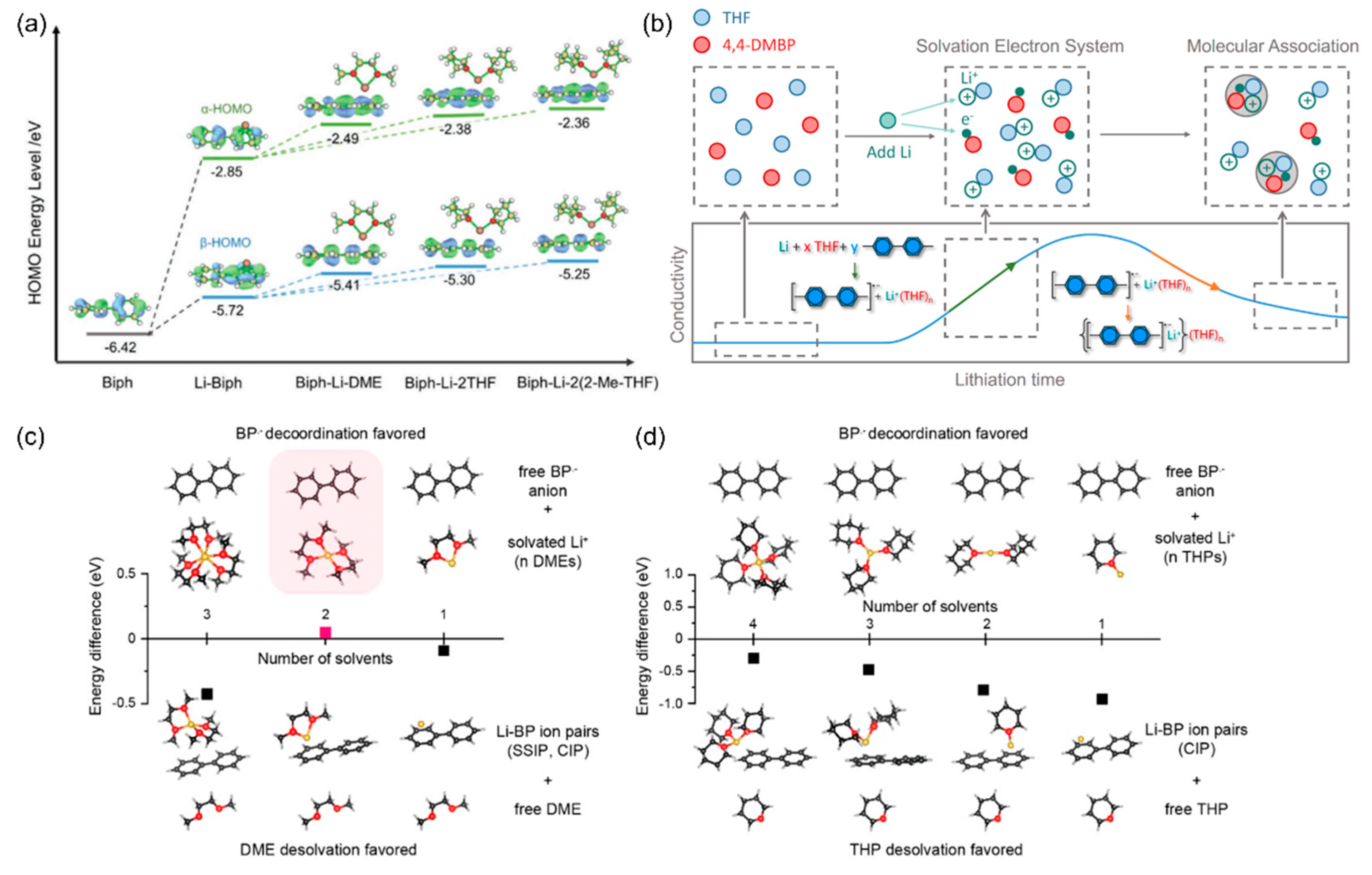
2. Features of Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation/Presodiation
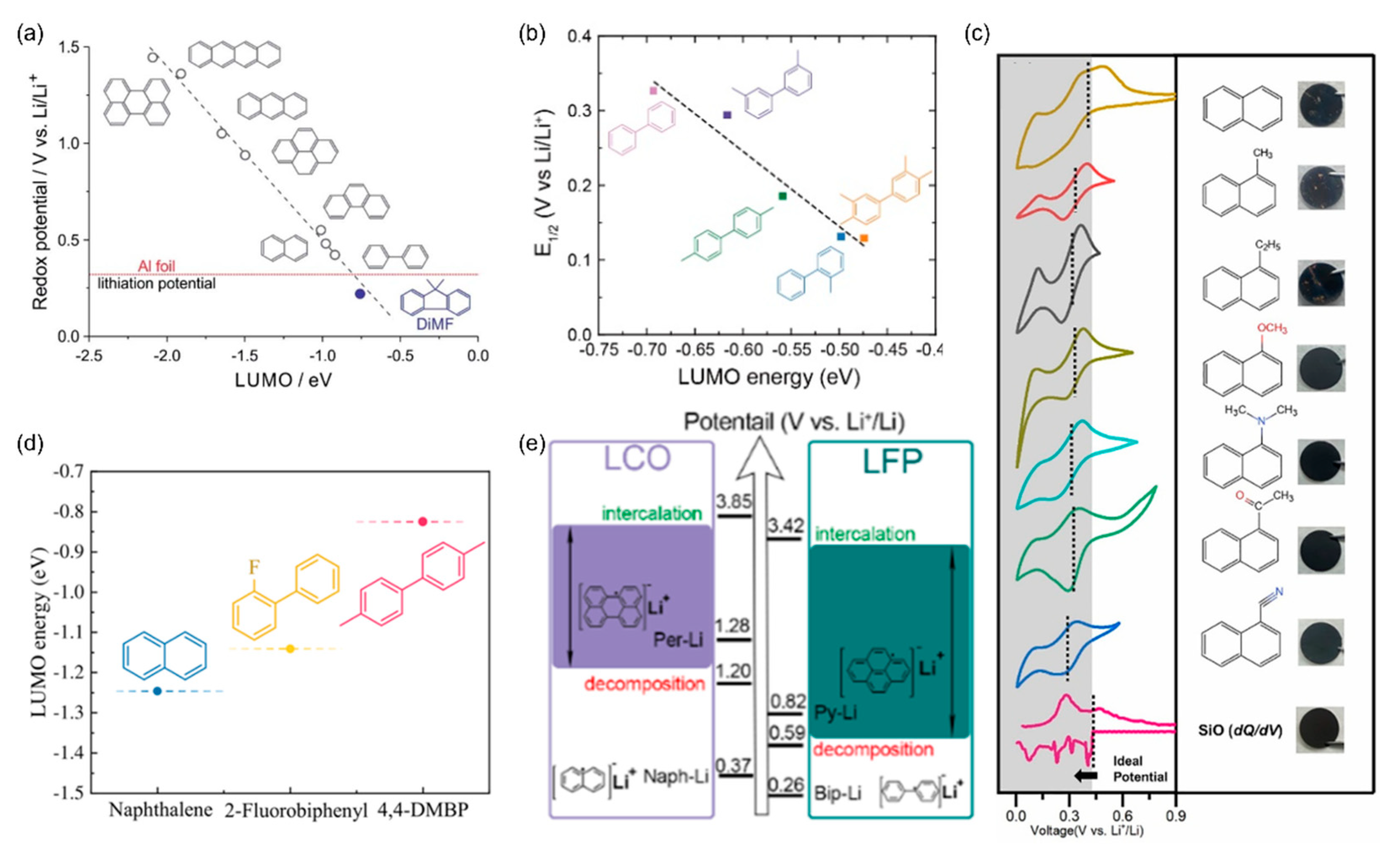
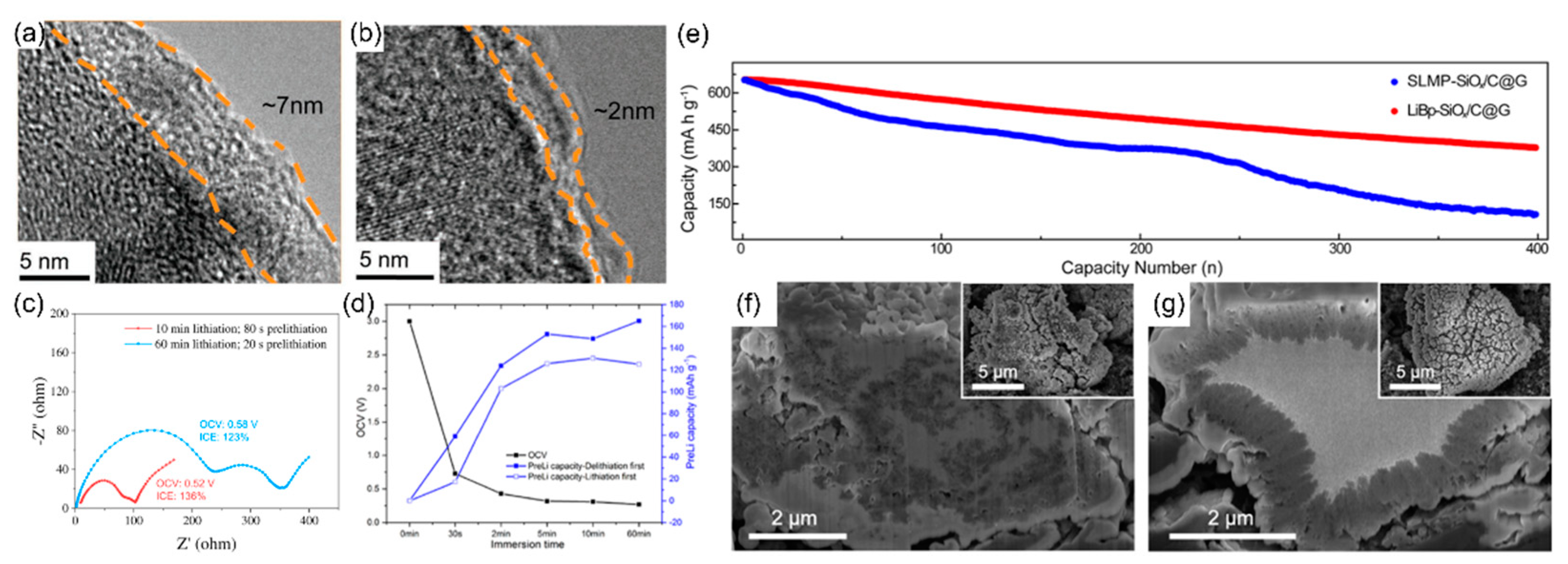
3. Adjusting Prelithiation/Presodiation Power and Rate of PAH Complex Solutions
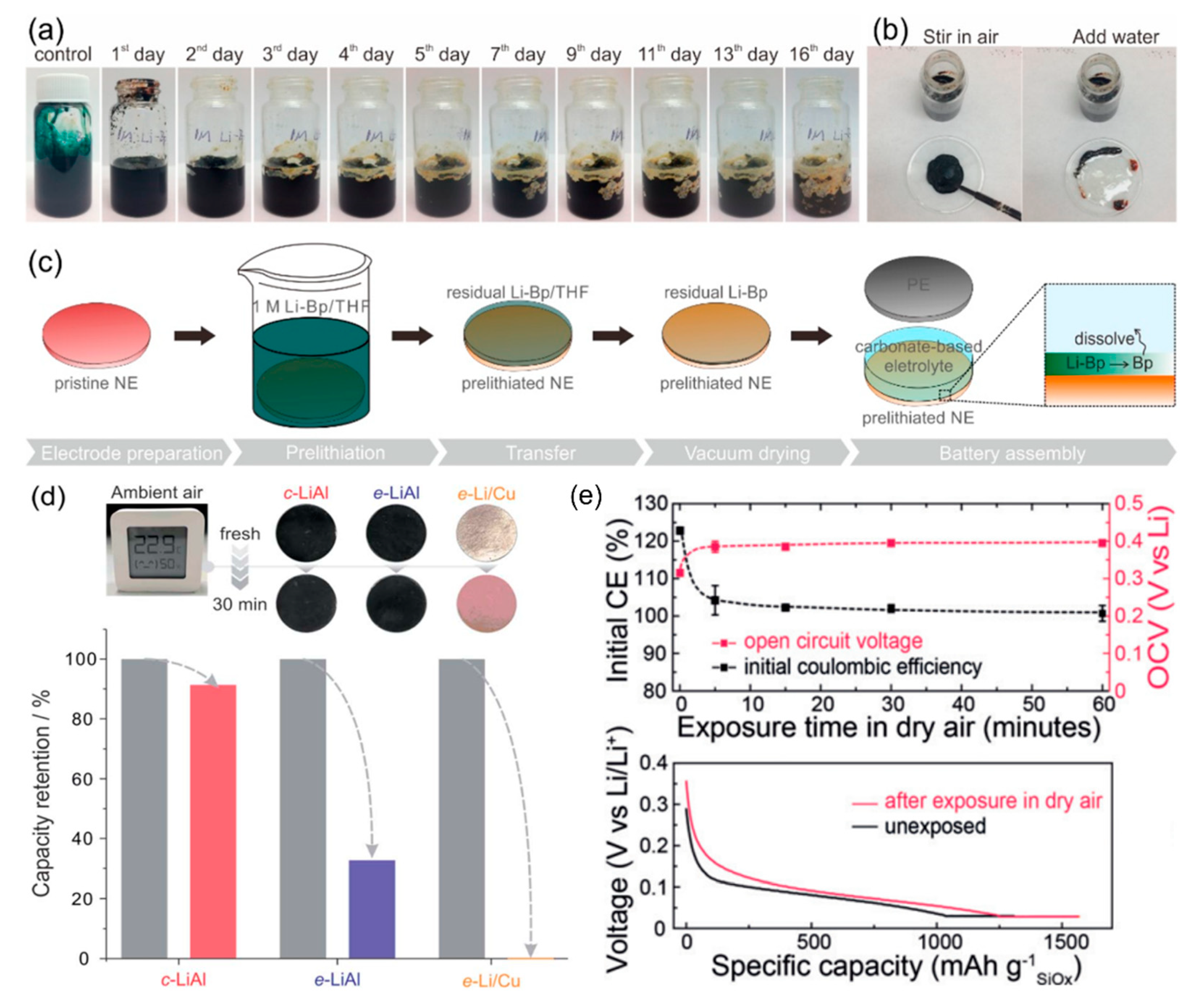
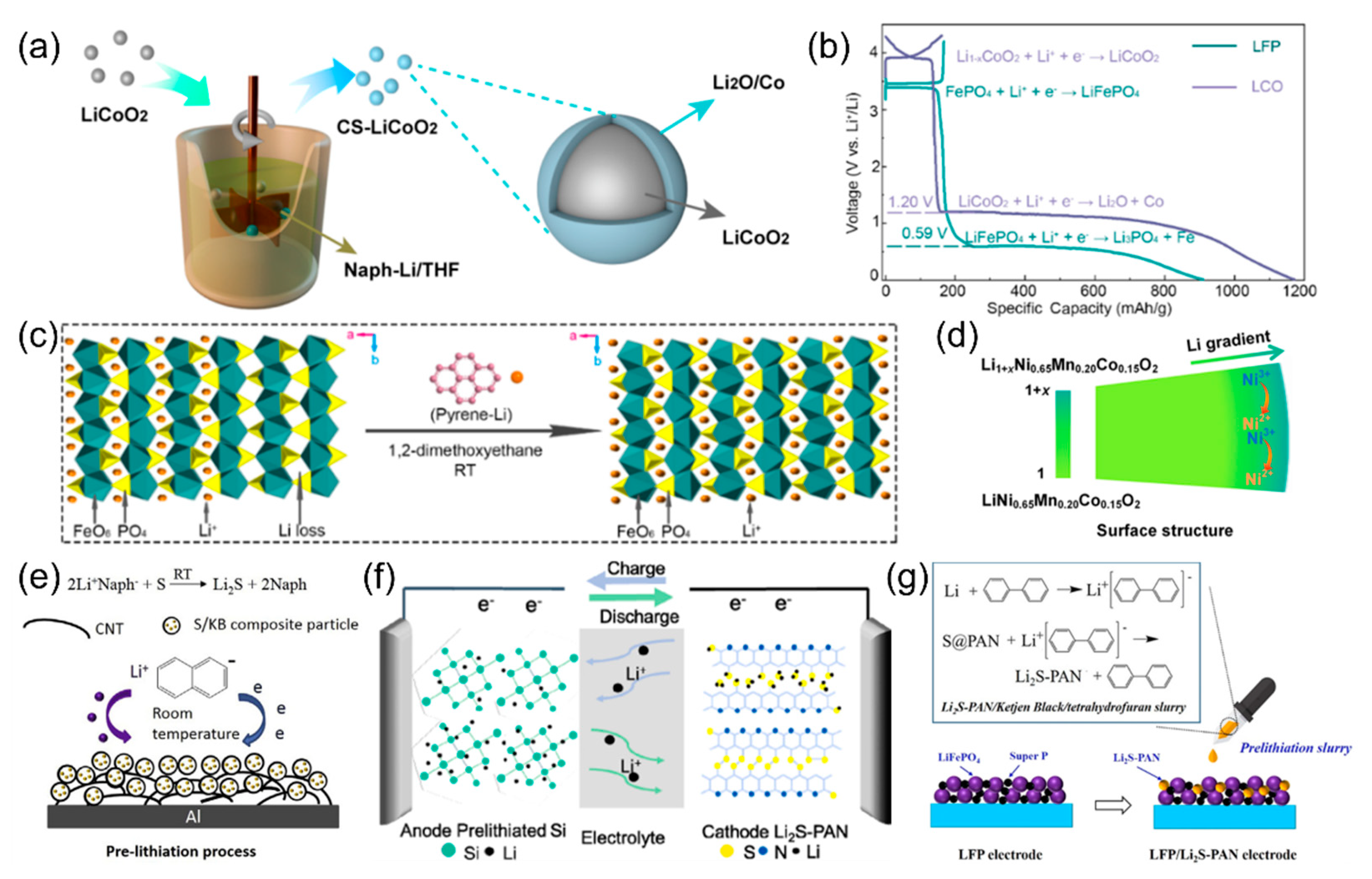
4. Anode Prelithiation Using PAH Complex Solutions
5. Cathode Prelithiation Using PAH Complex Solutions
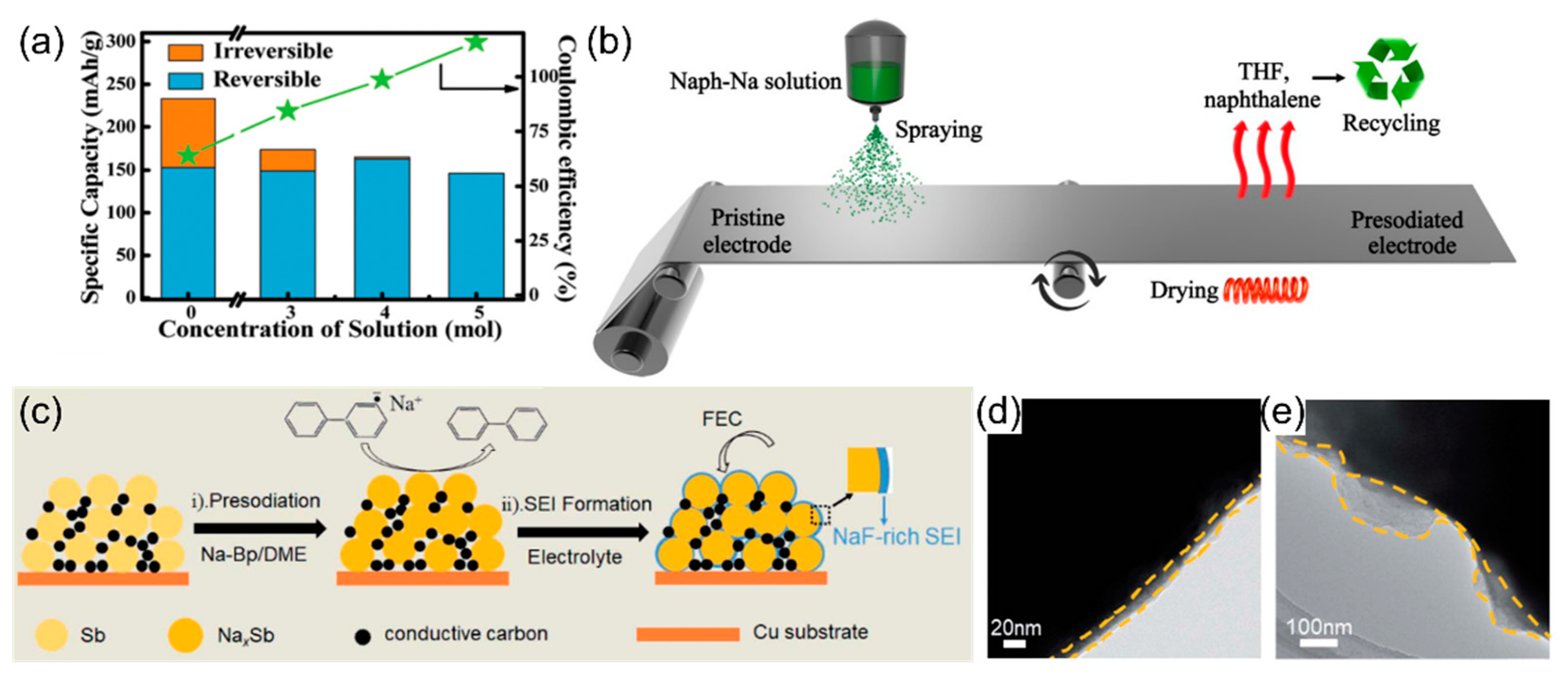
6. Presodiation Using PAH Complex Solutions
7. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Larramendi, I.R.; Guo, X.; Wang, G.; Rojo, T. Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhai, T. Reviving Lithium-Metal Anodes for Next-Generation High-Energy Batteries. Adv. Mater. 2017, 29, 1700007. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Cao, Y.; Li, M.; Lu, J.; Liu, J.; Amine, K. Bridging the Academic and Industrial Metrics for Next-Generation Practical Batteries. Nat. Nanotechnol. 2019, 14, 200–207. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-Lithiation Strategies for Rechargeable Energy Storage Technologies: Concepts, Promises and Challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
- Li, F.; Cao, Y.; Wu, W.; Wang, G.; Qu, D. Prelithiation Bridges the Gap for Developing Next-Generation Lithium-Ion Batteries/Capacitors. Small Methods 2022, 6, 2200411. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Wang, X.; Chen, Z.; Seh, Z.W.; Wang, L.; Sun, Y. Promises and Challenges of the Practical Implementation of Prelithiation in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2101565. [Google Scholar] [CrossRef]
- Zou, K.; Deng, W.; Cai, P.; Deng, X.; Wang, B.; Liu, C.; Li, J.; Hou, H.; Zou, G.; Ji, X. Prelithiation/Presodiation Techniques for Advanced Electrochemical Energy Storage Systems: Concepts, Applications, and Perspectives. Adv. Funct. Mater. 2021, 31, 2005581. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A Review of the Features and Analyses of the Solid Electrolyte Interphase in Li-Ion Batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.-C.; et al. The Truth about the 1st Cycle Coulombic Efficiency of LiNi1/3 Co1/3 Mn1/3 O2 (NCM) Cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef]
- Jarvis, C.R.; Lain, M.J.; Gao, Y.; Yakovleva, M. A Lithium Ion Cell Containing a Non-Lithiated Cathode. J. Power Sources 2005, 146, 331–334. [Google Scholar] [CrossRef]
- Jarvis, C.R.; Lain, M.J.; Yakovleva, M.V.; Gao, Y. A Prelithiated Carbon Anode for Lithium-Ion Battery Applications. J. Power Sources 2006, 162, 800–802. [Google Scholar] [CrossRef]
- Xiang, B.; Wang, L.; Liu, G.; Minor, A.M. Electromechanical Probing of Li/Li 2 CO 3 Core/Shell Particles in a TEM. J. Electrochem. Soc. 2013, 160, A415–A419. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Li, J.; Wang, B.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Prelithiation: A Crucial Strategy for Boosting the Practical Application of Next-Generation Lithium Ion Battery. ACS Nano 2021, 15, 2197–2218. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, X.; Li, C.; Wang, K.; Sun, X.; Ma, Y. Recent Advances in Prelithiation Materials and Approaches for Lithium-Ion Batteries and Capacitors. Energy Storage Mater. 2020, 32, 497–516. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.-G.; Zheng, J.P. Progress and Perspectives on Pre-Lithiation Technologies for Lithium Ion Capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.; Lee, S.J.; Seo, M.W.; Lee, J.G.; Deniz, E.; Lee, Y.J.; Kim, E.K.; Choi, J.W. Controlled Prelithiation of Silicon Monoxide for High Performance Lithium-Ion Rechargeable Full Cells. Nano Lett. 2016, 16, 282–288. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Zhang, C.; Xu, H.; Sun, X.; Zheng, Y.; Yu, Y.; Li, S.; Huang, Y.; Li, J. Gassing in Sn-Anode Sodium-Ion Batteries and Its Remedy by Metallurgically Prealloying Na. ACS Appl. Mater. Interfaces 2019, 11, 23207–23212. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Zhang, C.; Chen, X.; Liu, W.; Zheng, Y.; Xie, Y.; Huang, Y.; Li, J. Roll-to-Roll Prelithiation of Sn Foil Anode Suppresses Gassing and Enables Stable Full-Cell Cycling of Lithium Ion Batteries. Energy Environ. Sci. 2019, 12, 2991–3000. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Chen, X.; Zhang, C.; Liu, W.; Fan, H.; Yu, Y.; Huang, Y.; Li, J. Sn-Alloy Foil Electrode with Mechanical Prelithiation: Full-Cell Performance up to 200 Cycles. Adv. Energy Mater. 2019, 9, 1902150. [Google Scholar] [CrossRef]
- Fan, H.; Chen, B.; Li, S.; Yu, Y.; Xu, H.; Jiang, M.; Huang, Y.; Li, J. Nanocrystalline Li–Al–Mn–Si Foil as Reversible Li Host: Electronic Percolation and Electrochemical Cycling Stability. Nano Lett. 2020, 20, 896–904. [Google Scholar] [CrossRef]
- Yu, Y.; Li, S.; Fan, H.; Xu, H.; Jiang, M.; Huang, Y.; Li, J. Optimal Annealing of Al Foil Anode for Prelithiation and Full-Cell Cycling in Li-Ion Battery: The Role of Grain Boundaries in Lithiation/Delithiation Ductility. Nano Energy 2020, 67, 104274. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Chen, X.; Zhang, C.; Tang, Z.; Fan, H.; Yu, Y.; Liu, W.; Liang, N.; Huang, Y.; et al. Surpassing Lithium Metal Rechargeable Batteries with Self-Supporting Li–Sn–Sb Foil Anode. Nano Energy 2020, 74, 104815. [Google Scholar] [CrossRef]
- Jang, J.; Kang, I.; Choi, J.; Jeong, H.; Yi, K.; Hong, J.; Lee, M. Molecularly Tailored Lithium–Arene Complex Enables Chemical Prelithiation of High-Capacity Lithium-Ion Battery Anodes. Angew. Chem. Int. Ed. 2020, 59, 14473–14480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, H.; Ji, W.; Zheng, D.; Ding, T.; Qiu, D.; Qu, D. An Electrode-Level Prelithiation of SiO Anodes with Organolithium Compounds for Lithium-Ion Batteries. J. Power Sources 2020, 478, 229067. [Google Scholar] [CrossRef]
- Scott, M.G.; Whitehead, A.H.; Owen, J.R. Chemical Formation of a Solid Electrolyte Interface on the Carbon Electrode of a Li-Ion Cell. J. Electrochem. Soc. 1998, 145, 1506–1510. [Google Scholar] [CrossRef]
- Peramunage, D.; Abraham, K.M. Preparation and Electrochemical Characterization of Overlithiated Spinel LiMn2 O4. J. Electrochem. Soc. 1998, 145, 1131–1136. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yasuda, H.; Yamachi, M. Li-Doping Process for LixSiO-Negative Active Material Synthesized by Chemical Method for Lithium-Ion Cells. J. Power Sources 2005, 146, 507–509. [Google Scholar] [CrossRef]
- Tabuchi, T.; Yasuda, H.; Yamachi, M. Mechanism of Li-Doping into Li4Ti5O12 Negative Active Material for Li-Ion Cells by New Chemical Method. J. Power Sources 2006, 162, 813–817. [Google Scholar] [CrossRef]
- Liu, N.; Li, H.; Jiang, J.; Huang, X.; Chen, L. Li−Biphenyl−1,2-Dimethoxyethane Solution: Calculation and Its Application. J. Phys. Chem. B 2006, 110, 10341–10347. [Google Scholar] [CrossRef]
- Holy, N.L. Reactions of the Radical Anions and Dianions of Aromatic Hydrocarbons. Chem. Rev. 1974, 74, 243–277. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Wei, F.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Chemical Prelithiation of Al for Use as an Ambient Air Compatible and Polysulfide Resistant Anode for Li-Ion/S Batteries. J. Mater. Chem. A 2020, 8, 18715–18720. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Yang, H.; Zhong, F.; Ai, X. Chemically Prelithiated Hard-Carbon Anode for High Power and High Capacity Li-Ion Batteries. Small 2020, 16, 1907602. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, G.; Zheng, D.; Zhang, X.; Abegglen, C.J.; Qu, H.; Qu, D. Controlled Prelithiation of SnO2/C Nanocomposite Anodes for Building Full Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 19423–19430. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-Y.; Li, G.; Zhang, J.; Tian, Y.-F.; Yin, Y.-X.; Zhang, C.-J.; Jiang, K.-C.; Xu, Q.; Li, H.-L.; Guo, Y.-G. Enabling SiOx/C Anode with High Initial Coulombic Efficiency through a Chemical Pre-Lithiation Strategy for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27202–27209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, H.; Ji, W.; Zheng, D.; Ding, T.; Abegglen, C.; Qiu, D.; Qu, D. Fast and Controllable Prelithiation of Hard Carbon Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 11589–11599. [Google Scholar] [CrossRef]
- Wang, G.; Li, F.; Liu, D.; Zheng, D.; Abeggien, C.J.; Luo, Y.; Yang, X.-Q.; Ding, T.; Qu, D. High Performance Lithium-Ion and Lithium–Sulfur Batteries Using Prelithiated Phosphorus/Carbon Composite Anode. Energy Storage Mater. 2020, 24, 147–152. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Mu, X.; Li, R.; Yin, G.; Zuo, P. A Scalable Cathode Chemical Prelithiation Strategy for Advanced Silicon-Based Lithium Ion Full Batteries. ACS Appl. Mater. Interfaces 2021, 13, 11985–11994. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Y.; Wang, W.; Li, C.; Seh, Z.W.; Wang, L.; Sun, Y. Conformal Prelithiation Nanoshell on LiCoO2 Enabling High-Energy Lithium-Ion Batteries. Nano Lett. 2020, 20, 4558–4565. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Pu, Y.; Wang, H.; Wang, B.; Qian, J.; Cao, Y.; Zhong, F.; Ai, X.; Yang, H. Effective Chemical Prelithiation Strategy for Building a Silicon/Sulfur Li-Ion Battery. ACS Energy Lett. 2019, 4, 1717–1724. [Google Scholar] [CrossRef]
- Ren, Y.X.; Wei, L.; Jiang, H.R.; Zhao, C.; Zhao, T.S. On-Site Fluorination for Enhancing Utilization of Lithium in a Lithium–Sulfur Full Battery. ACS Appl. Mater. Interfaces 2020, 12, 53860–53868. [Google Scholar] [CrossRef]
- Liu, X.; Liu, T.; Wang, R.; Cai, Z.; Wang, W.; Yuan, Y.; Shahbazian-Yassar, R.; Li, X.; Wang, S.; Hu, E.; et al. Prelithiated Li-Enriched Gradient Interphase toward Practical High-Energy NMC–Silicon Full Cell. ACS Energy Lett. 2021, 6, 320–328. [Google Scholar] [CrossRef]
- Lin, L.; Qin, K.; Li, M.; Hu, Y.; Li, H.; Huang, X.; Chen, L.; Suo, L. Spinel-Related Li2Ni0.5Mn1.5O4 Cathode for 5-V Anode-Free Lithium Metal Batteries. Energy Storage Mater. 2022, 45, 821–827. [Google Scholar] [CrossRef]
- Wu, C.; Hu, J.; Ye, L.; Su, Z.; Fang, X.; Zhu, X.; Zhuang, L.; Ai, X.; Yang, H.; Qian, J. Direct Regeneration of Spent Li-Ion Battery Cathodes via Chemical Relithiation Reaction. ACS Sustain. Chem. Eng. 2021, 9, 16384–16393. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Y.; Zhao, Y.; Lin, N.; Qian, Y. Revealing the Interface-Rectifying Functions of a Li-Cyanonaphthalene Prelithiation System for SiO Electrode. Sci. Bull. 2022, 67, 636–645. [Google Scholar] [CrossRef]
- Yue, H.; Zhang, S.; Feng, T.; Chen, C.; Zhou, H.; Xu, Z.; Wu, M. Understanding of the Mechanism Enables Controllable Chemical Prelithiation of Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 53996–54004. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, X.; Yang, M.; Qian, J.; Cao, Y.; Yang, H.; Luo, Y.; Ai, X. Achieving Desirable Initial Coulombic Efficiencies and Full Capacity Utilization of Li-Ion Batteries by Chemical Prelithiation of Graphite Anode. Adv. Funct. Mater. 2021, 31, 2101181. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, H.; Jang, J.; Jeon, A.-R.; Kang, I.; Kwon, M.; Hong, J.; Lee, M. Weakly Solvating Solution Enables Chemical Prelithiation of Graphite–SiOx Anodes for High-Energy Li-Ion Batteries. J. Am. Chem. Soc. 2021, 143, 9169–9176. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, T.; Zhong, X.; Zhai, T.; Li, H. A Safe, Convenient Liquid Phase Pre-Sodiation Method for Titanium-Based SIB Materials. Chem. Commun. 2019, 55, 14761–14764. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Y.; Liu, T.; Wang, W.; Li, C.; Lu, J.; Sun, Y. A Simple Electrode-Level Chemical Presodiation Route by Solution Spraying to Improve the Energy Density of Sodium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1903795. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Guo, S.; Wang, B.; Shen, Y.; Ai, X.; Yang, H.; Qian, J. Chemically Presodiated Hard Carbon Anodes with Enhanced Initial Coulombic Efficiencies for High-Energy Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17620–17627. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Shen, Y.; Guo, S.; Zhang, J.; Ai, X.; Yang, H.; Qian, J. Chemically Presodiated Sb with a Fluoride-Rich Interphase as a Cycle-Stable Anode for High-Energy Sodium Ion Batteries. J. Mater. Chem. A 2021, 9, 5639–5647. [Google Scholar] [CrossRef]
- Zheng, G.; Lin, Q.; Ma, J.; Zhang, J.; He, Y.; Tang, X.; Kang, F.; Lv, W.; Yang, Q. Ultrafast Presodiation of Graphene Anodes for High-efficiency and High-rate Sodium-Ion Storage. InfoMat 2021, 3, 1445–1454. [Google Scholar] [CrossRef]
- Wang, G.; Li, F.; Liu, D.; Zheng, D.; Luo, Y.; Qu, D.; Ding, T.; Qu, D. Chemical Prelithiation of Negative Electrodes in Ambient Air for Advanced Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 8699–8703. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Hirota, N. Alkali Metal NMR Studies of Radical Anion Solutions. J. Chem. Phys. 1973, 58, 3745–3756. [Google Scholar] [CrossRef]
- Canters, G.W.; de Boer, E.; Alkali, N.M.R. Experiments on the Radical Ion Pairs of Biphenyl and Fluorenone: Part I. Analysis of N.M.R. Shifts. Mol. Phys. 1973, 26, 1185–1198. [Google Scholar] [CrossRef]
- Zheng, J.; Lochala, J.A.; Kwok, A.; Deng, Z.D.; Xiao, J. Research Progress towards Understanding the Unique Interfaces between Concentrated Electrolytes and Electrodes for Energy Storage Applications. Adv. Sci. 2017, 4, 1700032. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wahyudi, W.; Li, M.; Kumar, P.; Wu, Y.; Hwang, J.-Y.; Hedhili, M.N.; Cavallo, L.; Sun, Y.-K.; et al. New Insights on Graphite Anode Stability in Rechargeable Batteries: Li Ion Coordination Structures Prevail over Solid Electrolyte Interphases. ACS Energy Lett. 2018, 3, 335–340. [Google Scholar] [CrossRef]
- Su, Y.-S.; Hsiao, K.-C.; Sireesha, P.; Huang, J.-Y. Lithium Silicates in Anode Materials for Li-Ion and Li Metal Batteries. Batteries 2022, 8, 2. [Google Scholar] [CrossRef]
- Yu, S.-H.; Feng, X.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding Conversion-Type Electrodes for Lithium Rechargeable Batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion Cathodes for Rechargeable Lithium and Lithium-Ion Batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes. Angew. Chem. Int. Ed. 2013, 52, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Li2S-Carbon Sandwiched Electrodes with Superior Performance for Lithium-Sulfur Batteries. Adv. Energy Mater. 2014, 4, 1300655. [Google Scholar] [CrossRef]
- Wu, Y.; Momma, T.; Ahn, S.; Yokoshima, T.; Nara, H.; Osaka, T. On-Site Chemical Pre-Lithiation of S Cathode at Room Temperature on a 3D Nano-Structured Current Collector. J. Power Sources 2017, 366, 65–71. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, Ä.; Buchmeiser, M.R. Structure-Related Electrochemistry of Sulfur-Poly(Acrylonitrile) Composite Cathode Materials for Rechargeable Lithium Batteries. Chem. Mater. 2011, 23, 5024–5028. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Z.; Elia, G.A.; Wu, Y.; Wahyudi, W.; Abou-Hamad, E.; Emwas, A.-H.; Cavallo, L.; Li, L.-J.; Ming, J. Recognizing the Mechanism of Sulfurized Polyacrylonitrile Cathode Materials for Li–S Batteries and beyond in Al–S Batteries. ACS Energy Lett. 2018, 3, 2899–2907. [Google Scholar] [CrossRef]
- Warneke, S.; Hintennach, A.; Buchmeiser, M.R. Communication—Influence of Carbonate-Based Electrolyte Composition on Cell Performance of SPAN-Based Lithium-Sulfur-Batteries. J. Electrochem. Soc. 2018, 165, A2093–A2095. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. Boost Sodium-Ion Batteries to Commercialization: Strategies to Enhance Initial Coulombic Efficiency of Hard Carbon Anode. Nano Energy 2021, 82, 105738. [Google Scholar] [CrossRef]
- Chojnacka, A.; Pan, X.; Bachetzky, C.; Brunner, E.; Béguin, F. A Strategy for Optimizing the Output Energy and Durability of Metal-Ion Capacitors Fabricated with Alloy-Based Anodes. Energy Storage Mater. 2022, 51, 719–732. [Google Scholar] [CrossRef]
- Rodriguez, R.; Loeffler, K.E.; Nathan, S.S.; Sheavly, J.K.; Dolocan, A.; Heller, A.; Mullins, C.B. In Situ Optical Imaging of Sodium Electrodeposition: Effects of Fluoroethylene Carbonate. ACS Energy Lett. 2017, 2, 2051–2057. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Tai Nai, C.; Suk Shin, H.; Young Jeong, H.; Liu, B.; Ping Loh, K. High Yield Exfoliation of Two-Dimensional Chalcogenides Using Sodium Naphthalenide. Nat. Commun. 2014, 5, 2995. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofibers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef]
- Yildirim, H.; Kinaci, A.; Chan, M.K.Y.; Greeley, J.P. First-Principles Analysis of Defect Thermodynamics and Ion Transport in Inorganic SEI Compounds: LiF and NaF. ACS Appl. Mater. Interfaces 2015, 7, 18985–18996. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Jiang, H.; Zheng, Y.; Zheng, H.; Liang, X.; Sun, Y.; Chen, C.; Xiang, H. A Bilayer Interface Formed in High Concentration Electrolyte with SbF3 Additive for Long-Cycle and High-Rate Sodium Metal Battery. J. Power Sources 2020, 455, 227956. [Google Scholar] [CrossRef]
| Methods | Alkali Ion Sources | Material or Electrode Level | Processing | External | Pressure | Washing | Roll-to-Roll |
|---|---|---|---|---|---|---|---|
| Temperature | Circuit | Activation | Step | Capability | |||
| Wet Chemical | Li/Na Containing Compounds | Both | RT | NR | NR | Maybe | Yes |
| Molten Alkali Metal | Li/Na Metal | Material | Above MP | NR | NR | NR | NA |
| Electrochemical | Li/Na Metal Foil | Electrode | RT | Required | NR | Maybe | Yes |
| Short-circuiting | Li/Na Metal Foil | Electrode | RT | NR | Required | Maybe | Yes |
| Alkali Metal Addition | Li/Na Metal Foil/Powder | Both | RT | NR | Required | NR | Yes |
| Anode | Li-PAH Complex | Prelithiation | Reversible Capacity | ICE before | ICE after | Reference |
|---|---|---|---|---|---|---|
| Time (min) | (mAh g−1) | Prelithiation | Prelithiation | |||
| Graphite | 1 M Li-BP/2-Me-THF | 5 | 365 | 84.4% | 98.8% | [55] |
| Graphite | 0.2 M Li4-BP/2-Me-THF | 2 | 354 | 90.4% | 110% | [56] |
| Graphite/Si | 1 | 583 | 83.2% | 98.6% | ||
| Graphite/SiOx | 1 | 911 | 74.1% | 103.2% | ||
| Hard Carbon | 1 M Li4-4,4-DMBP/THF | 0.5 | ≈290 | 78.7% | 100% | [54] |
| Hard Carbon | 0.5 M Li-Naph/DME | 4 | ≈405 | 75.5% | 99.5% | [41] |
| Hard Carbon | 1 M Li-BP/THF | 0.5 | 295 (lithiation first) | 79.2% | 104.4% | [44] |
| 2 | 321 (delithiation first) | 91.2% | ||||
| Li4Ti5O12 | 0.25 M Li-Naph/BME | 180 | 161 | NA | NA | [37] |
| SiO | 0.25 M Li-Naph/BME | 4320 | 670 | NA | NA | [36] |
| SiO | 0.2 M Li8-CNaph/THF | 30 | ≈1700 | 77% | 107.6% | [53] |
| SiOx | 0.5 M Li4-4,4-DMBP/DME | 30 | 1587 | 57% | 107% | [32] |
| Graphene/SiO | 1 M Li-DiMF/THF | 10 | ≈1050 | 71.2% | 87.1% | [33] |
| Carbon/SiOx | 1 M Li-BP/THF | NA | 1349 | 75.6% | 87.3% | [43] |
| Carbon/SnO2 | 1 M Li-BP/THF | 5 | 870 | 45% | 90% | [42] |
| Carbon/P | 1 M Li-BP/THF | 10 | ≈1200 | 74% | 94% | [62] |
| Anode | Na-PAH Complex | Presodiation | Reversible Capacity | ICE before | ICE after | Reference |
|---|---|---|---|---|---|---|
| Time (min) | (mAh g−1) | Presodiation | Presodiation | |||
| Hard Carbon | 0.5 M Na-BP/DME | 1 | 300 | 71.6% | 103% | [59] |
| Hard Carbon | 0.1 M Na-Naph/THF | NA | 145 | 67% | 87% | [58] |
| Graphene | 0.5 M Na-Naph/DME | 10 | ≈315 | ≈80% | 96.8% | [61] |
| Na2Ti6O13 | 4 M Na-Naph/DME | 10 | 164 | 65.7% | 99% | [57] |
| Sb | 0.5 M Na-BP/DME | 15 | 540 | 75% | 100% | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-S.; Chang, J.-K. Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation and Presodiation for Batteries. Batteries 2022, 8, 99. https://doi.org/10.3390/batteries8080099
Su Y-S, Chang J-K. Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation and Presodiation for Batteries. Batteries. 2022; 8(8):99. https://doi.org/10.3390/batteries8080099
Chicago/Turabian StyleSu, Yu-Sheng, and Jeng-Kuei Chang. 2022. "Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation and Presodiation for Batteries" Batteries 8, no. 8: 99. https://doi.org/10.3390/batteries8080099
APA StyleSu, Y.-S., & Chang, J.-K. (2022). Polycyclic Aromatic Hydrocarbon-Enabled Wet Chemical Prelithiation and Presodiation for Batteries. Batteries, 8(8), 99. https://doi.org/10.3390/batteries8080099







