Effects of Cell Design Parameters on Zinc-Air Battery Performance
Abstract
:1. Introduction
1.1. Overview of Zn-Air Batteries
1.2. Electrolyte Additives
1.3. Bifunctional Catalyst for Air Electrode
1.4. Surface Morphology of Air Electrode
1.5. Effects of Air Electrode Catalyst on Cell Power Density
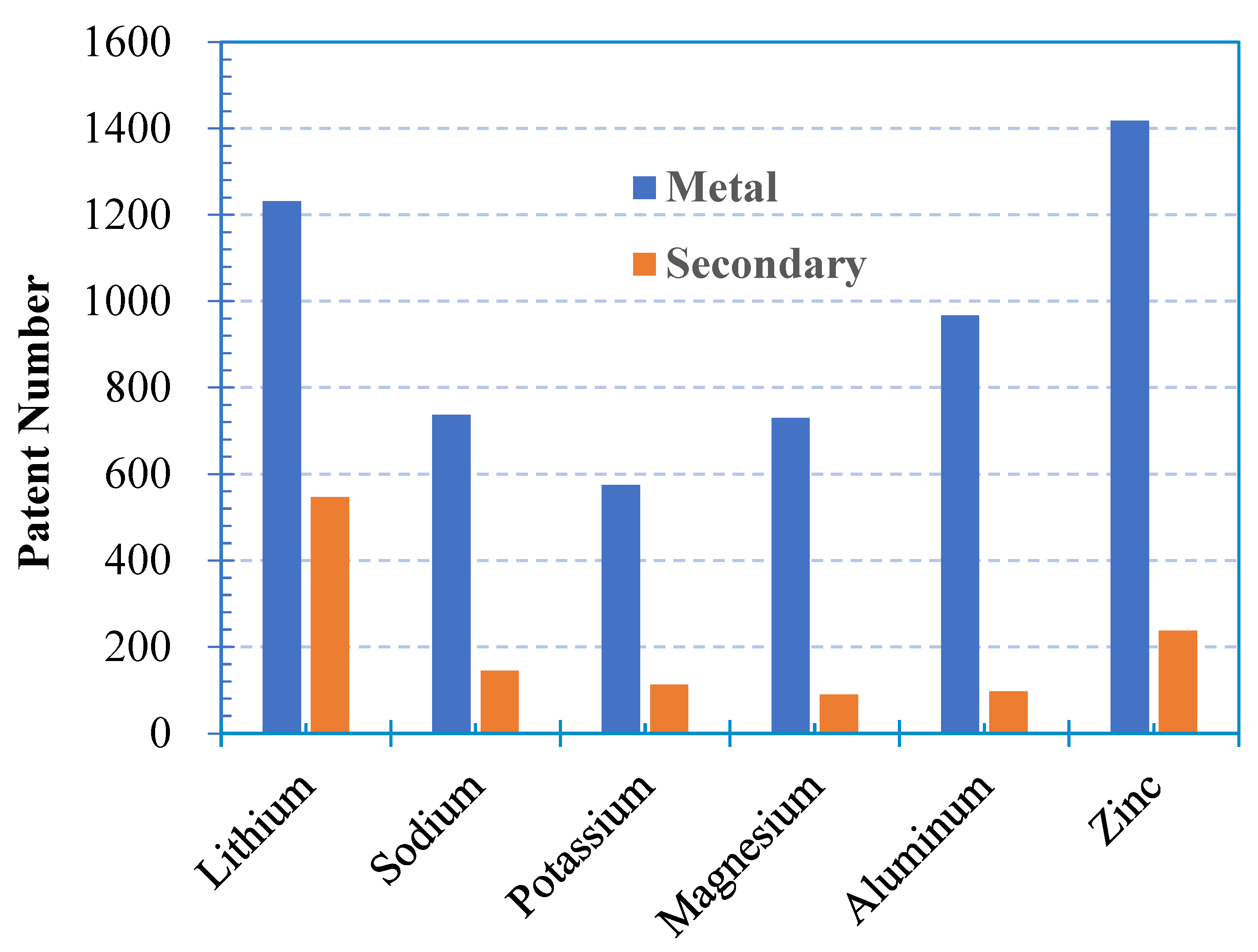
1.6. Effects of Zinc Type on Cell Power Density
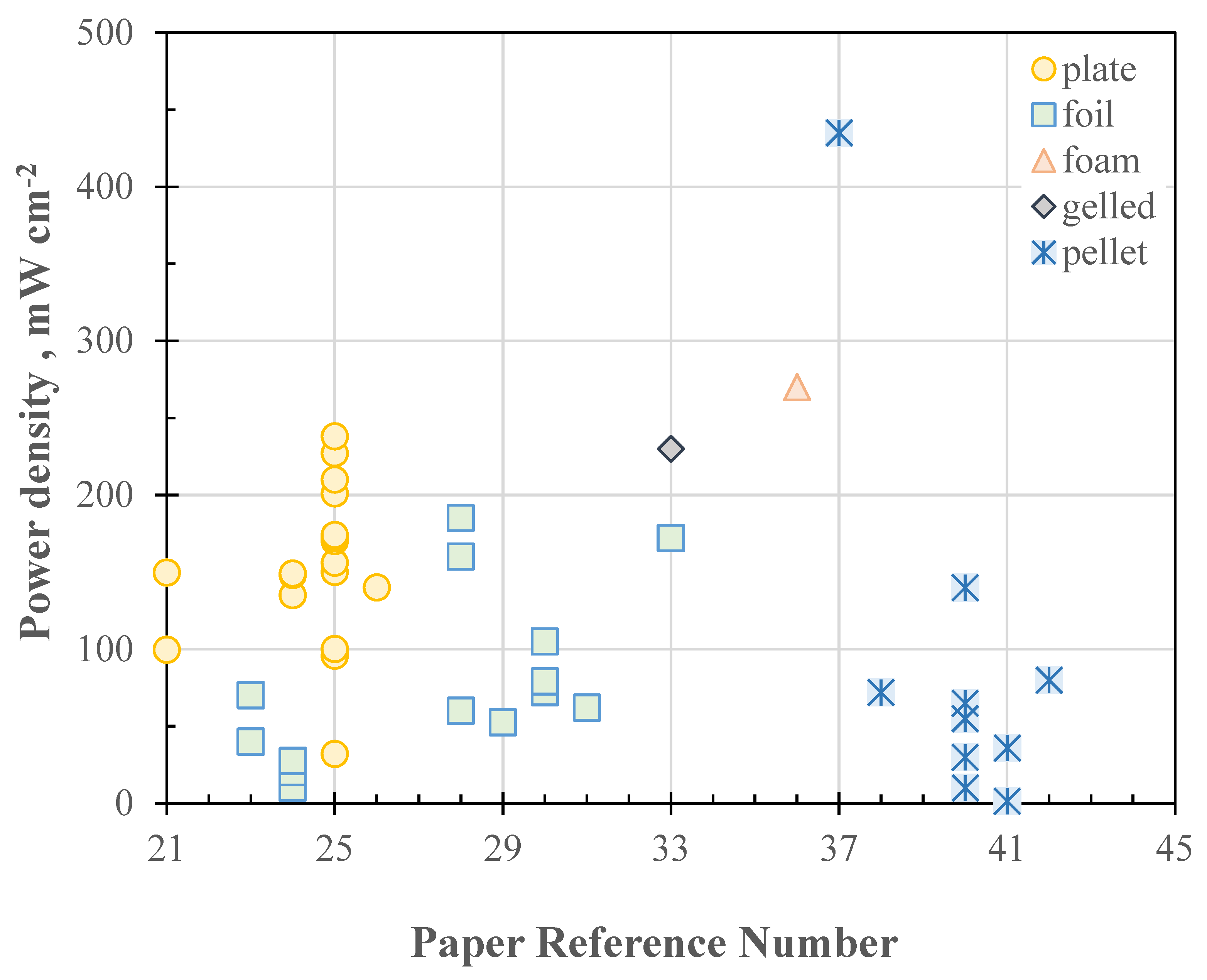
1.7. Effect of Cell Configuration on Power Density
1.8. Motivation and Scope of Present Study
- Cell 1 (proximity cell): electrodes are in close proximity (less than 1 mm),
- Cell 2 (aqueous electrolyte cell): equal-area electrodes separated with aqueous electrolyte,
- Cell 3 (cathode limited cell): large zinc electrode area and small air electrode area,
- Cell 4 (air flow channel cell): channel flow on the air side.
2. Experimental and Computer Modeling
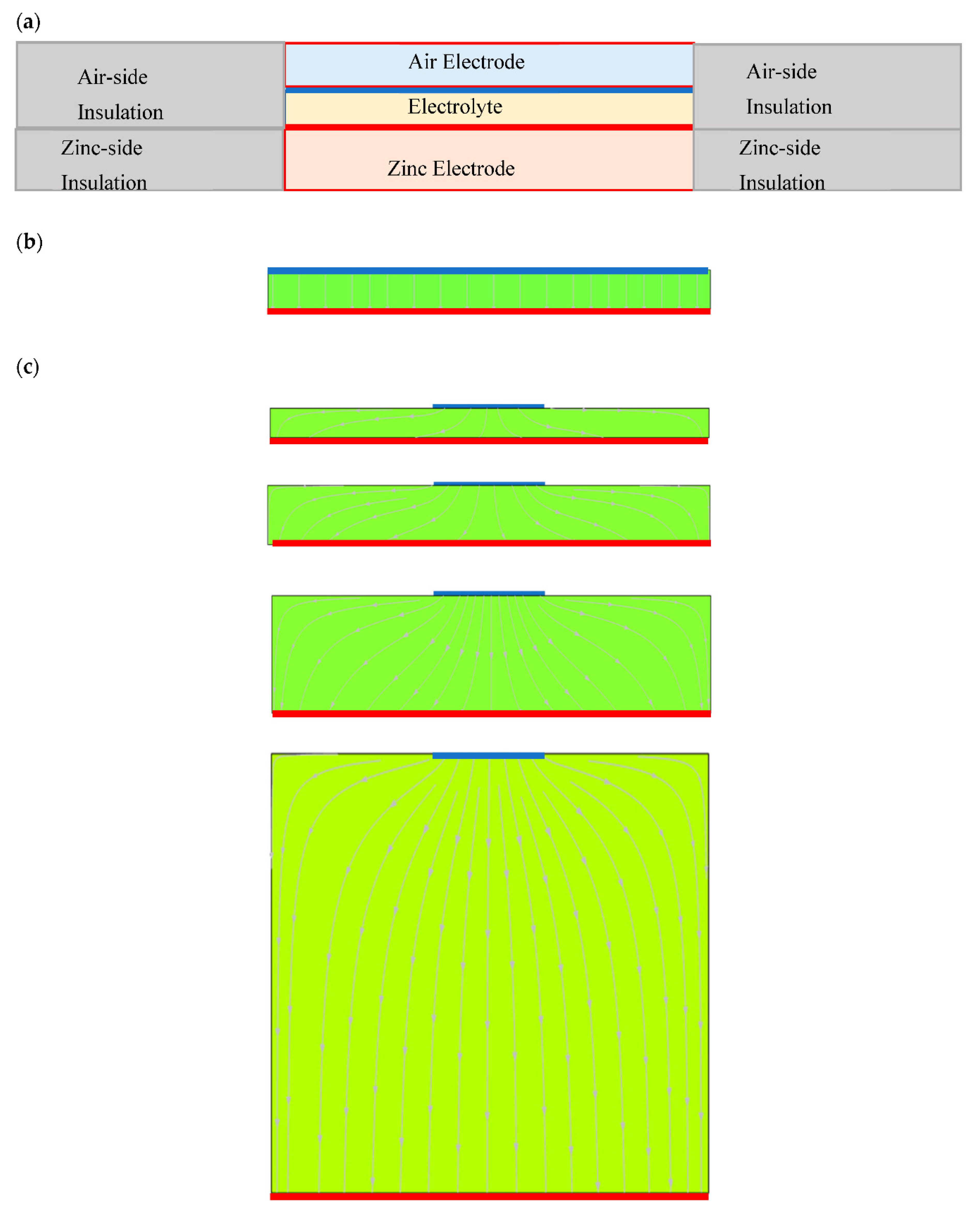
2.1. Configuration of Cells under Investigation
2.2. Configuration of Cells under Investigation—Cell 1
2.3. Configuration of Cells under Investigation—Cell 2
2.4. Configuration of Cells under Investigation—Cell 3
2.5. Configuration of Cells under Investigation—Cell 4
2.6. Modified 25BC Carbon Paper as Cathode
2.7. Computer Modeling of Testing Cells—Current Distribution
3. Results and Discussion
3.1. Cell 1
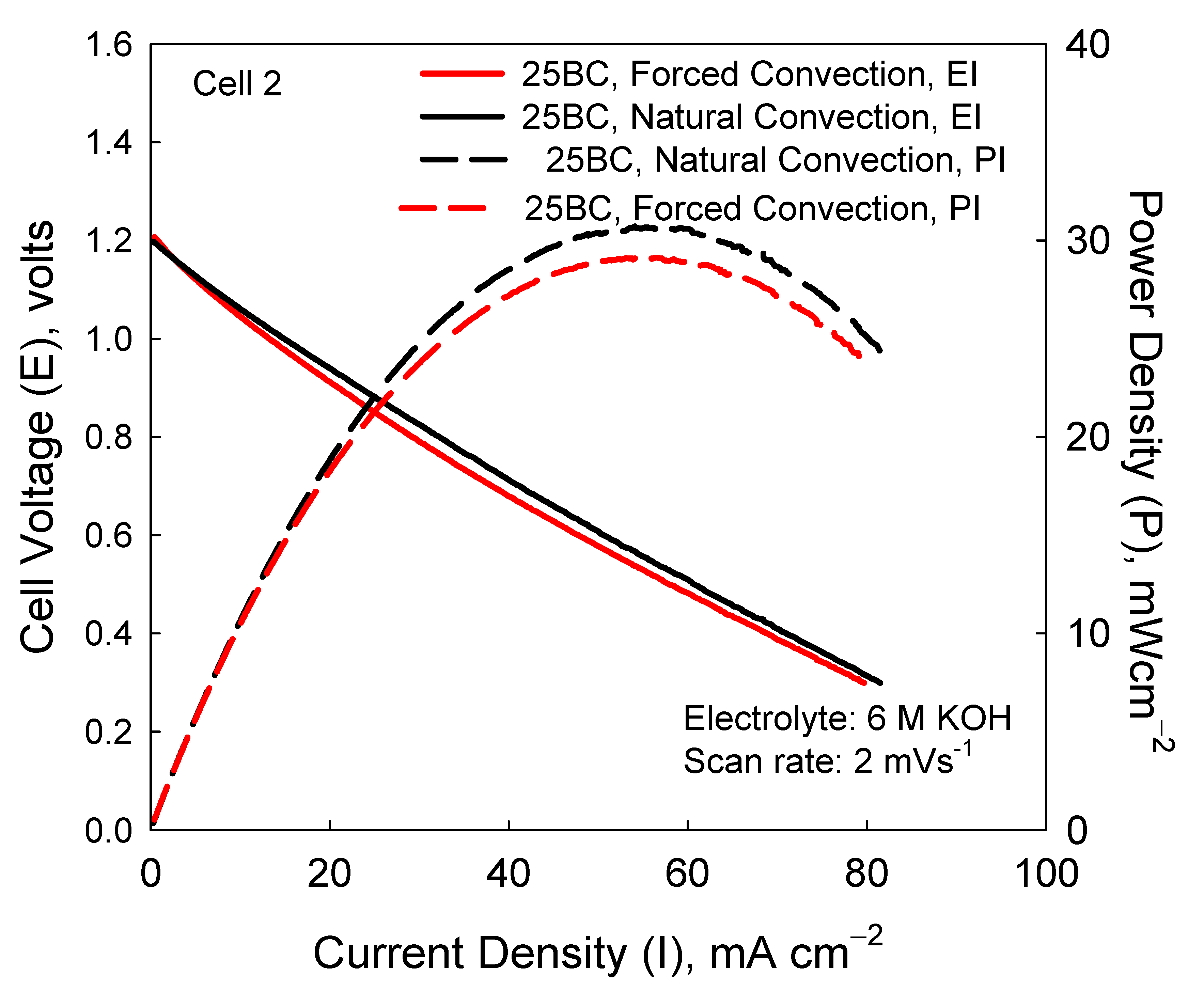
3.2. Cell 2
3.3. Cell 3
3.4. Cells 4a–c
3.5. Volumetric Power Density Versus Power Density Based on Cathode Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wang, X.-G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum-air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.-B.; Yan, C.; Huang, J.-Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Shkolnikov, E.I.; Zhuk, A.Z.; Vlaskin, M.S. Aluminum as energy carrier: Feasibility analysis and current technologies overview. Renew. Sustain. Energy Rev. 2011, 15, 4611–4623. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Guo, Z.; Ren, J.; Wang, Y.; Peng, H. Flexible, stretchable, and rechargeable fiber-shaped zinc- air battery based on cross-stacked carbon nanotube sheets. Angew. Chem. 2015, 127, 15610–15614. [Google Scholar] [CrossRef]
- Suren, S.; Kheawhom, S. Development of a High Energy Density Flexible Zinc-Air Battery. J. Electrochem. Soc. 2016, 163, A846–A850. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, D.; Zhang, C.; Shao, Q.; Murugadoss, V.; Guo, Z.; Jiang, Q.; Yang, X. An Overview of Oxygen Reduction Electrocatalysts for Rechargeable Zinc-Air Batteries Enabled by Carbon and Carbon Composites. Eng. Sci. 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Sherman, S.B.; Cano, Z.P.; Fowler, M.; Chen, Z. Range-extending Zinc-air battery for electric vehicle. AIMS Energy 2018, 6, 121–145. [Google Scholar] [CrossRef]
- Goldstein, J.; Brown, I.; Koretz, B. New developments in the Electric Fuel Ltd. zinc/air system. J. Power Source 1999, 80, 171–179. [Google Scholar] [CrossRef]
- Georgious, R.; Refaat, R.; Garcia, J.; Daoud, A.A. Review on Energy Storage Systems in Microgrids. Electronics 2021, 10, 2134. [Google Scholar] [CrossRef]
- Chakkaravarthy, C.; Waheed, A.K.A.; Udupa, H.V.K. Zn–air alkaline batteries—A review. J. Power Sources 1981, 6, 203–228. [Google Scholar] [CrossRef]
- Caramia, V.; Bozzini, B. Materials science aspects of Zn–air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3, 28. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in Zn–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; Meatza, I.d.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary Zn–air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–Air Batteries: From Static to Flow System. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline Zn–air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Grande, H.J.; Blázquez, J.A. Enhancing the cycle life of a Zn–air battery by means of electrolyte additives and zinc surface protection. Batteries 2018, 4, 46. [Google Scholar] [CrossRef]
- Li, P.C.; Hu, C.C.; You, T.H.; Chen, P.Y. Development and characterization of bi-functional air electrodes for rechargeable Zn–air batteries: Effects of carbons. Carbon 2017, 111, 813–821. [Google Scholar] [CrossRef]
- Xu, N.; Qiao, J.; Zhang, X.; Ma, C.; Jian, S.; Liu, Y.; Pei, P. Morphology controlled La2O3/Co3O4/MnO2-CNTs hybrid nanocomposites with durable bi-functional air electrode in high- performance Zn–air energy storage. Appl. Energy 2016, 175, 495–504. [Google Scholar] [CrossRef]
- Ti, X.; Liu, Z.; Song, L.; Wang, D.; Zhang, Z. Three-dimensional graphene network supported ultrathin CeO2 nanoflakes for oxygen reduction reaction and rechargeable metal-air batteries. Electrochim. Acta 2018, 263, 561–569. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Leonet, O.; Alcaide, F.; Iruin, J.J.; Weinberger, S.; Hacker, V.; Iruin, E.; Urdanpilleta, I.; Blazquez, J.A. Manganese oxide catalysts for secondary zinc air batteries: From electrocatalytic activity to bifunctional air electrode performance. Electrochim. Acta 2016, 217, 80–91. [Google Scholar] [CrossRef]
- Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Mesoporous MnCo2O4 spinel oxide for a highly active and stable air electrode for Zn–air rechargeable battery. Electrochim. Acta 2019, 300, 455–460. [Google Scholar] [CrossRef]
- Lu, C.T.; Chiu, Y.W.; Li, M.J.; Hsueh, K.L.; Hung, J.S. Reduction of the electrode overpotential of the oxygen evolution reaction by electrode surface modification. Int. J. Electrochem. Sci. 2017, 2017, 7494571. [Google Scholar] [CrossRef]
- Wang, H.; Min, Y.; Li, P.; Yang, J.; Li, J. In situ integration of ultrathin PtRuCu alloy overlayer on copper foam as an advanced free-standing bifunctional cathode for rechargeable Zn–air batteries. Electrochim. Acta 2018, 283, 54–62. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Cheng, G.; Han, S.; Zhou, J.; Yuan, J.; Sun, M.; Yu, L. Flower-like NiCo2O4eCN as efficient bifunctional electrocatalyst for Zn–air battery. Electrochim. Acta 2020, 341, 135997. [Google Scholar] [CrossRef]
- Xu, N.; Wilsonc, J.A.; Wang, Y.D.; Suc, T.; Weia, Y.; Qiaoa, J.; Zhou, X.D.; Zhang, Y.; Sun, S. Flexible self-supported bi-metal electrode as a highly stable carbon- and binder-free cathode for large-scale solid-state Zn–air batteries. Appl. Catal. B 2020, 272, 118953. [Google Scholar] [CrossRef]
- Han, S.; Hao, Y.; Guo, Z.; Yu, D.; Huang, H.; Hu, F.; Li, L.; Chen, H.Y.; Penga, S. Self-supported N-doped NiSe2 hierarchical porous nanoflake arrays for efficient oxygen electrocatalysis in flexible Zn–air batteries. Chem. Eng. J. 2020, 401, 126088. [Google Scholar] [CrossRef]
- Jiao, D.; Ma, Z.; Li, J.; Han, Y.; Mao, J.; Ling, T.; Qiao, S. Test factors affecting the performance of zinc–air battery. J. Energy Chem. 2020, 44, 1–7. [Google Scholar] [CrossRef]
- Xu, K.; Loh, A.; Wang, B.; Li, X. Enhancement of Oxygen Transfer by Design Nickel Foam Electrode for Zinc−Air Battery. J. Electrochem. Soc. 2018, 165, A809–A818. [Google Scholar] [CrossRef]
- Mohamad, A.A. Zn/gelled 6 M KOH/O2 zinc—Air battery. J. Power Sources 2006, 159, 752–757. [Google Scholar] [CrossRef]
- Park, J.E.; Lim, M.S.; Kim, J.K.; Choi, H.J.; Sung, Y.S.; Cho, Y.-H. Optimization of cell components and operating conditions in primary and rechargeable zinc–air battery. J. Ind. Eng. Chem. 2019, 69, 161–170. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Ji, S.; Pollet, B.G.; Wang, R. Toward high performance of Zn–air battery using hydrophobic carbon foam-based diffusion electrode. J. Ind. Eng. Chem. 2019, 71, 284–292. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Li, Y.; Liang, D.; Hu, Y.; Li, C. In-situ enriching active sites on co-doped Fe-Co4N@N-C nanosheet array as air cathode for flexible rechargeable Zn–air batteries. Appl. Catal. B 2019, 256, 117893. [Google Scholar] [CrossRef]
- Ji, D.; Fan, L.; Li, L.L.; Mao, N.; Qinm, X.; Peng, S.; Ramakrishna, S. Hierarchical catalytic electrodes of cobalt-embedded carbon nanotube / carbon flakes array for flexible solid-state Zn–air batteries. Carbon 2019, 142, 379–387. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Wang, B.; Li, B.Q.; Cui, X.; Zhang, Q. Defect-rich carbon fiber electrocatalysts with porous graphene skin for flexible solid-state zinc–air batteries. Energy Storage Mater. 2018, 15, 124–130. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Cai, R.; Chen, C.; Xia, Y.; Zhang, H.; Yang, D.; Yao, X. Air cathode of zinc–air batteries: A highly efficient and durable aerogel catalyst for oxygen reduction. Nanoscale 2019, 11, 826–832. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Srijaroenpramong, N.; Arpavate, W.; Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. Effects of anode orientation and flow channel design on performance of refuelable Zn–air fuel cells. J. Appl. Electrochem. 2014, 44, 1205–1218. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, G.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for Zn–air batteries: Recent progress, challenges and perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Bockelmann, M.; Kunz, U.; Turek, T. Electrically rechargeable zinc-oxygen flow battery with high power density. Electrochem. Commun. 2016, 69, 24–27. [Google Scholar] [CrossRef]
- Pei, P.; Ma, Z.; Wang, K.; Wang, X.; Song, M.; Xu, H. High performance zinc air fuel cell stack. J. Power Sources 2014, 249, 13–20. [Google Scholar] [CrossRef]
- Han, J.J.; Li, N.; Zhang, T.Y. Ag/C nanoparticles as an cathode catalyst for a Zn–air battery with a flowing alkaline electrolyte. J. Power Sources 2009, 193, 885–889. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Olaru, S.; Arpornwichanop, A.; Kheawhom, S. Discharge performance and dynamic behavior of refuellable Zn–air battery. Sci. Data 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.H.; Olmo, D.d.; Fischer, P.; Pinkwart, K.; Tübke, J. Development of Flow Fields for Zinc Slurry Air Flow Batteries. Batteries 2021, 6, 15. [Google Scholar] [CrossRef]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef]


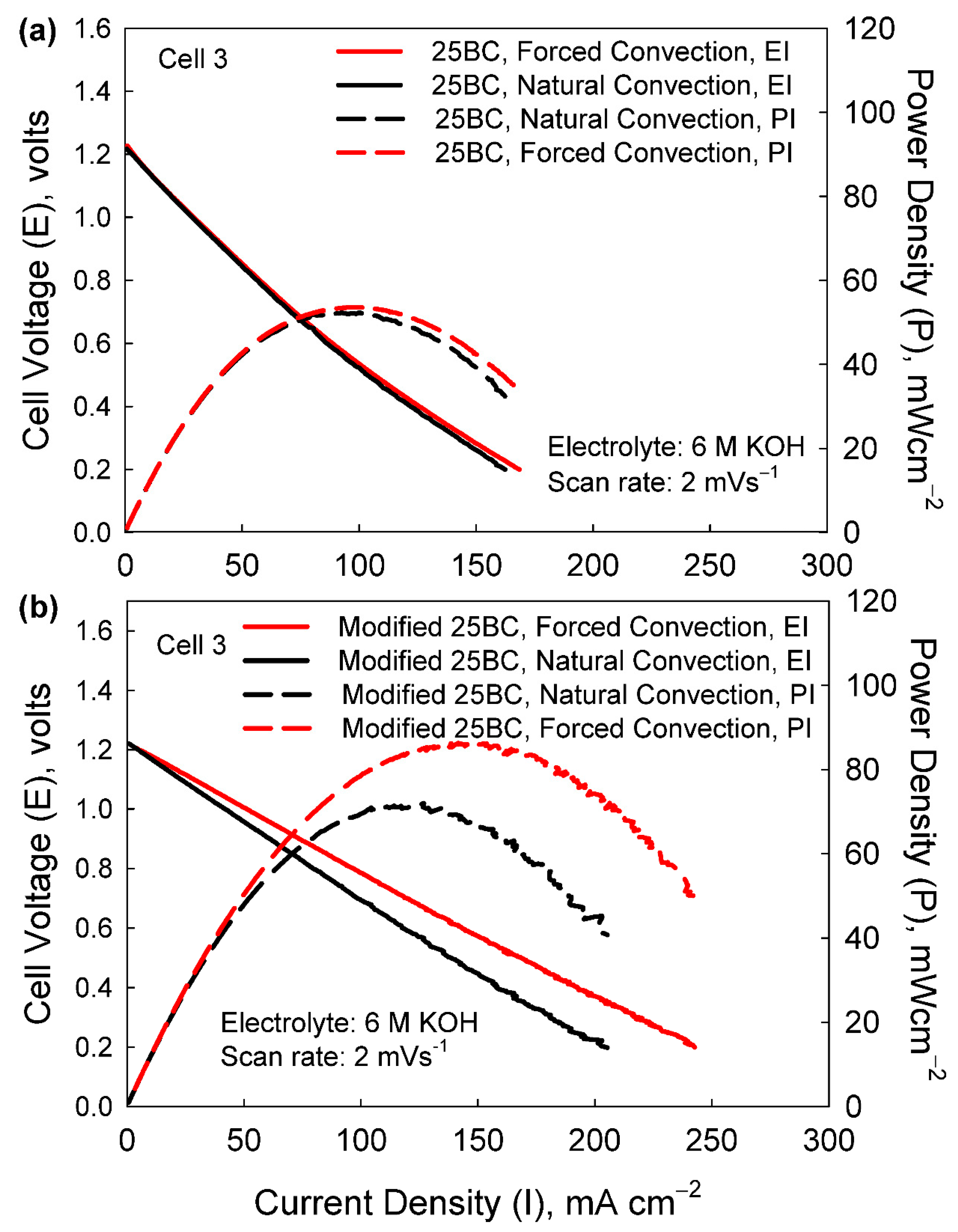
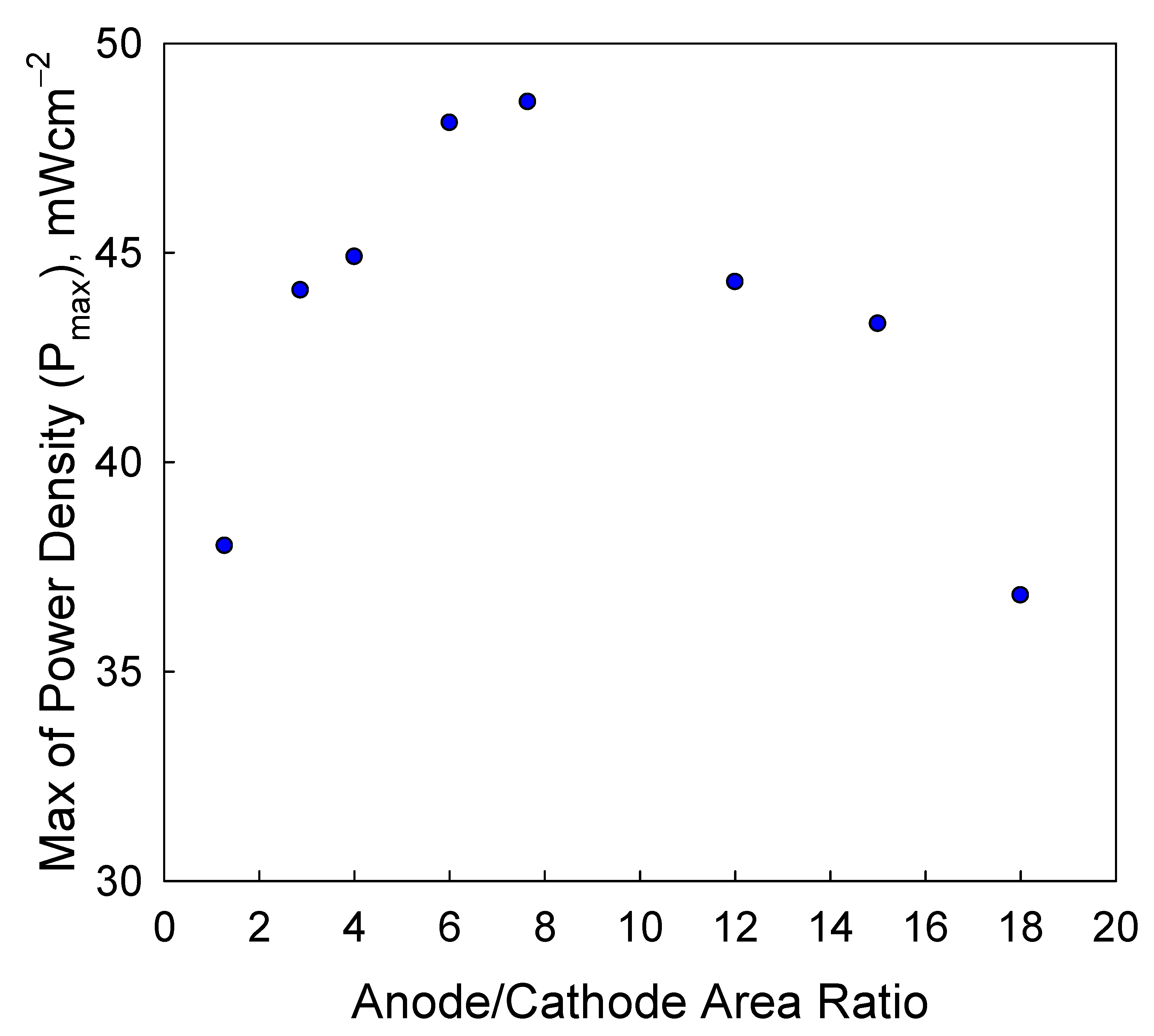


| Factor | Modified ORR Electrode | Forced Air Convection | Anode/Cathode Area Ratio | Anode/Cathode Distance | |
|---|---|---|---|---|---|
| Cell Type | |||||
| Cell 1 | ○ | ○ | △ | ||
| Cell 2 | ○ | ○ | △ | ||
| Cell 3 | ○ | ○ | ○ | △ | |
| Cell 4 | ○ | ○ & △ | |||
| c_bulk | Reactant bulk concentration | 1600 [mmol/L] |
| Cdl | Double layer capacitance | 0.5 [F/m^2] |
| DA | Reactant diffusion coefficient | 3.5 × 10−9 [m^2/s] |
| DB | Product diffusion coefficient | 3.5 × 10−9 [m^2/s] |
| E_eqref1 | Equilibrium potential of RXN 1 | 1.25 [V] |
| E_vertex1 | Start potential | 1.3 [V] |
| E_vertex2 | Switching potential | 0.4 [V] |
| i0ref1 | Reference exchange current density of RXN 1 | 1.0 × 10 [A/m^2] |
| K0 | Reaction rate (dimensionless) | 1.00 × 1010 |
| T | Temperature | 298.15 [K] |
| v | Voltametric scan rate | 0.002 [V/s] |
| L | Outer bound on diffusion layer | 6 × sqrt(DA × 2 × abs(E_vertex1 − E_vertex2)/v) |
| cB0 | Initial product concentration at electrode | c_bulk/(1 + exp(−E_vertex1 × F_const/(R_const × T))) |
| Alpha_a | Anodic charge transfer coefficient | 0.7 |
| sigma_l | Electrolyte conductivity 65 S/m 6M KOH | 5.0 × 102 [S/m] |
| T | Temperature | 25 + 273 [K] |
| disp | Cathode displacement in parametric sweep | 0 [mm] |
| a_H | Proton activity | 0.5 × 2 × 180 [g/dm3]/98.1 [g/mol]/(1 [mol/dm3]) |
| c_Zn | Zinc concentration | 50 [g/dm3]/65.4 [g/mol] |
| E_O2 | Equilibrium potential, Oxygen oxidation reaction | 1.229 [V] − R_const × T/(2 × F_const) × log(0.9/(1 × a_H^2)) |
| E_Zn | Equilibrium potential, Zinc reaction | −0.763 [V] − R_const × T/(2 × F_const) × log(1/(0.1 × c_Zn/1 [mol/dm3])) |
| E_H | Equilibrium potential, Hydrogen evolution | 0-R_const × T/(F_const) × log(1/a_H) |
| i0_O2 | Exchange current density, Oxygen oxidation reaction | 0.1 × i0_Zn |
| i0_Zn | Exchange current density, Zinc reaction | −1.0 × 10−7 [A/m^2] |
| i0_H | Exchange current density, Hydrogen evolution | −1 × 10−11 [A/m^2] |
| alphaa_O2 | Anodic transfer coefficient, Oxygen oxidation reaction | 2-alphac_O2 |
| alphac_O2 | Cathodic transfer coefficient, Oxygen oxidation reaction | 0.6 |
| alphaa_Zn | Anodic transfer coefficient, Zinc reaction | 1.1 |
| alphac_Zn | Cathodic transfer coefficient, Zinc reaction | 2-alphaa_Zn |
| phisext | External electric potential | 0.9 [V] |
| Cell | Pmax, mW | Cathode Area (Acathode) cm2 | Pmax/Acathode | Velectrolyte | Pmax/Velectolyte | |||
|---|---|---|---|---|---|---|---|---|
| 25BC | Modified 25BC | 25BC | Modified 25BC | cm3 | 25BC | Modified 25BC | ||
| 1 | 32.0 | 52.6 | 4.0 | 8 | 13.15 | 0.4 | 320.0 | 131.5 |
| 2 | 29.1 | 30.6 | 25.0 | 1.16 | 1.22 | 12.5 | 58.0 | 2.45 |
| 3 | 72.0 | 88.3 | 1.0 | 72.0 | 88.3 | 42.0 | 2.0 | 2.10 |
| 4a | 41.9 | 10.0 | 4.19 | 5.1 | 82.0 | |||
| 4b | 38.5 | 10.0 | 3.85 | 10.2 | 38.0 | |||
| 4c | 28.6 | 10.0 | 2.86 | 15.4 | 19.0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-T.; Zhu, Z.-Y.; Chen, S.-W.; Chang, Y.-L.; Hsueh, K.-L. Effects of Cell Design Parameters on Zinc-Air Battery Performance. Batteries 2022, 8, 92. https://doi.org/10.3390/batteries8080092
Lu C-T, Zhu Z-Y, Chen S-W, Chang Y-L, Hsueh K-L. Effects of Cell Design Parameters on Zinc-Air Battery Performance. Batteries. 2022; 8(8):92. https://doi.org/10.3390/batteries8080092
Chicago/Turabian StyleLu, Cian-Tong, Zhi-Yan Zhu, Sheng-Wen Chen, Yu-Ling Chang, and Kan-Lin Hsueh. 2022. "Effects of Cell Design Parameters on Zinc-Air Battery Performance" Batteries 8, no. 8: 92. https://doi.org/10.3390/batteries8080092





