Model for Rating a Vanadium Redox Flow Battery Stack through Constant Power Charge–Discharge Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Model Construction
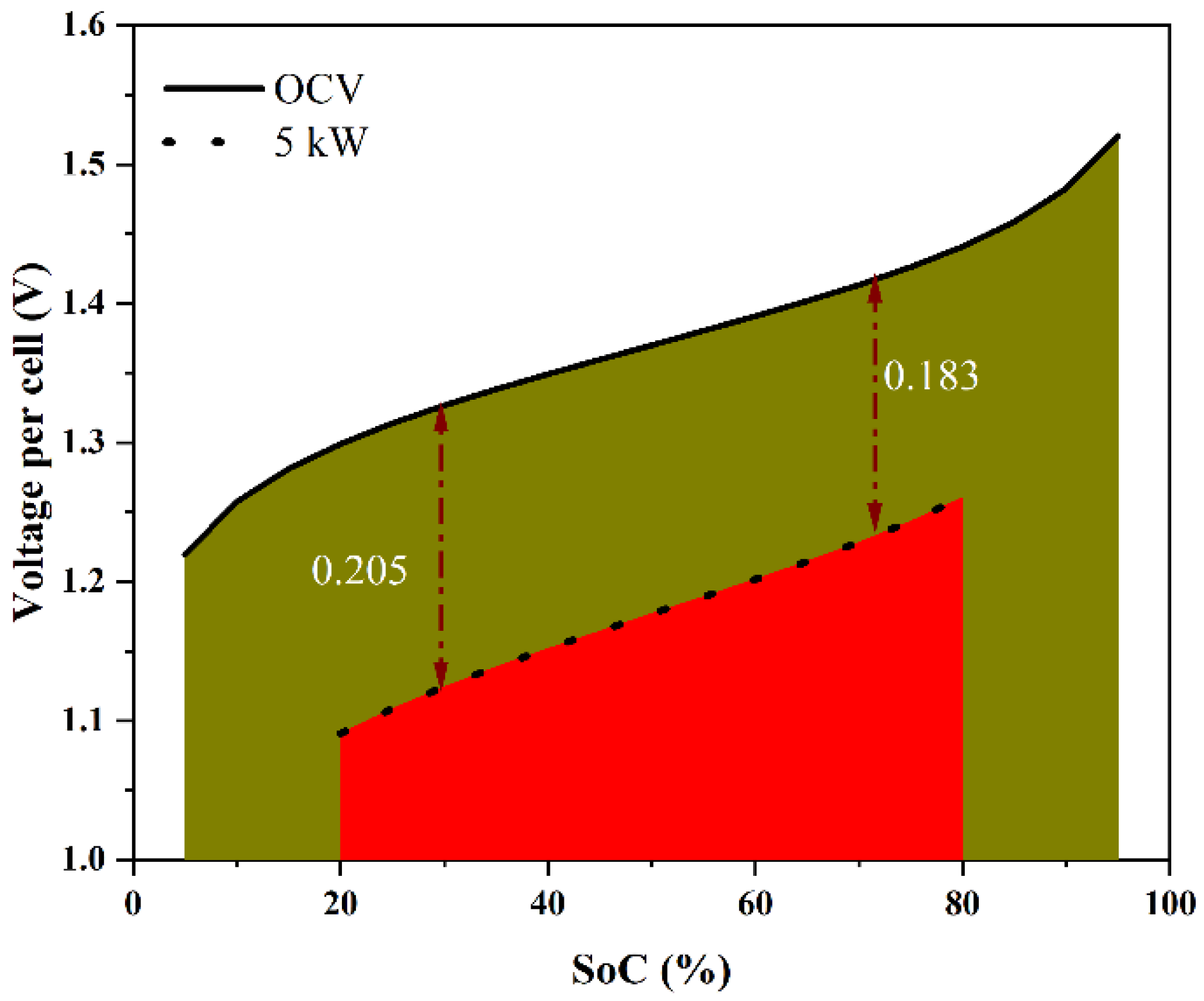
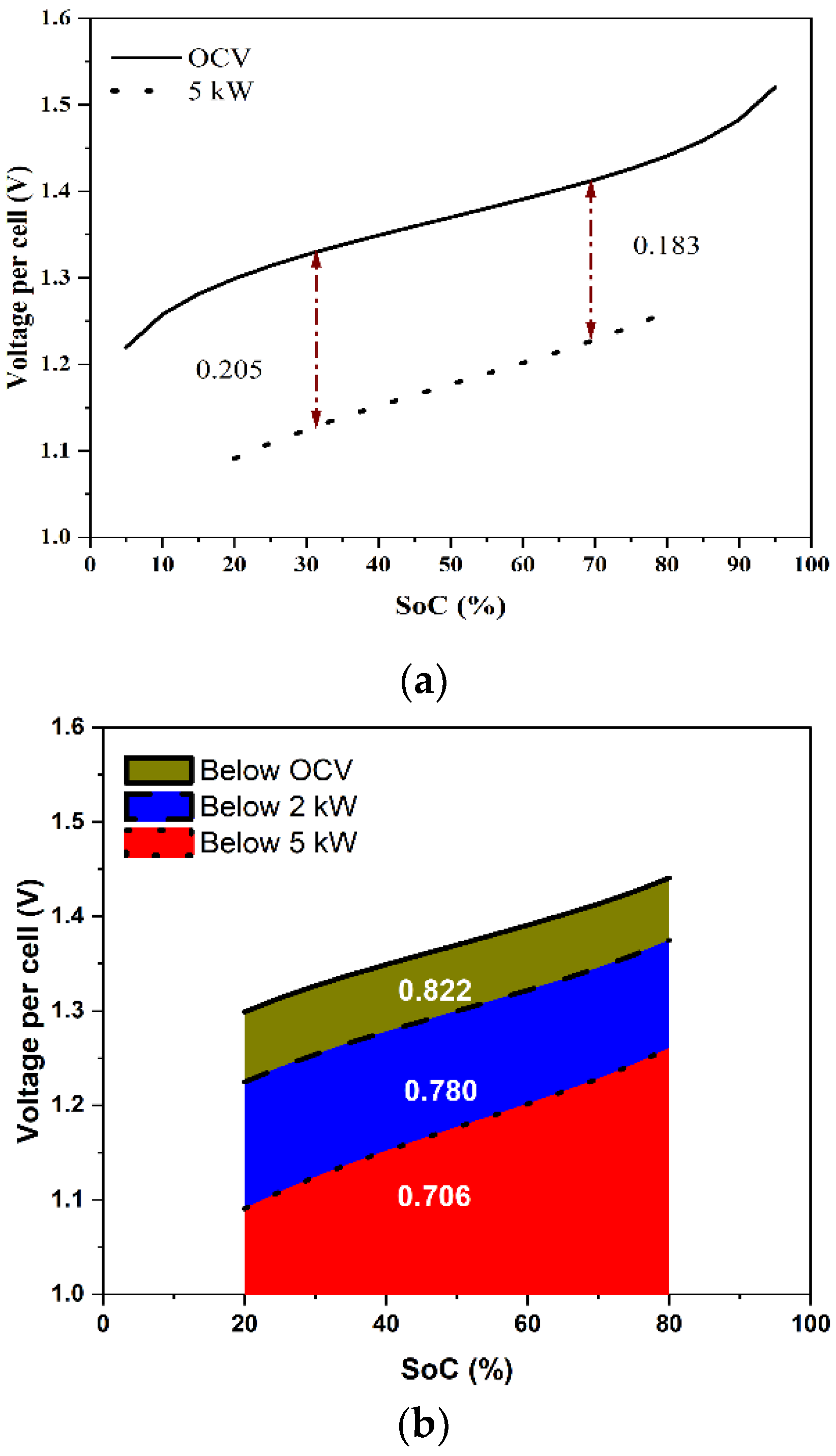
- The VRFB system is operated primarily in the SoC range of 20 to 80% under normal conditions to maintain the electrolyte in good condition by preventing the occurrence of side reactions. Under these conditions, it is expected that no irreversible damage occurs to the electrolyte due to crossover.
- The range of operation of the battery is limited by the SoC and not by voltage limits. This is justified for the limited purposes of the present model for the stack rating in which the rated power is expected to be much less than the peak power obtained from a short duration test.
- Concentration overpotential can be neglected when considering cell overpotential. This possibility arises from the first two assumptions, which are likely to keep the system sufficiently far from the point where mass transfer limits the performance of the stack.
- Over the 20 to 80% SoC range, the overpotential can be expected to vary primarily as a function of the current density and is a weak function of the SoC in this range. This assumption is justified based on empirical observations from the present data (see below), as well as stack data from the literature [29].
3. Results and Discussion
3.1. Polarization Curve
3.2. Constant Power Charge–Discharge Cycles
3.3. Model Predictions

4. Stack Rating
5. Conclusions
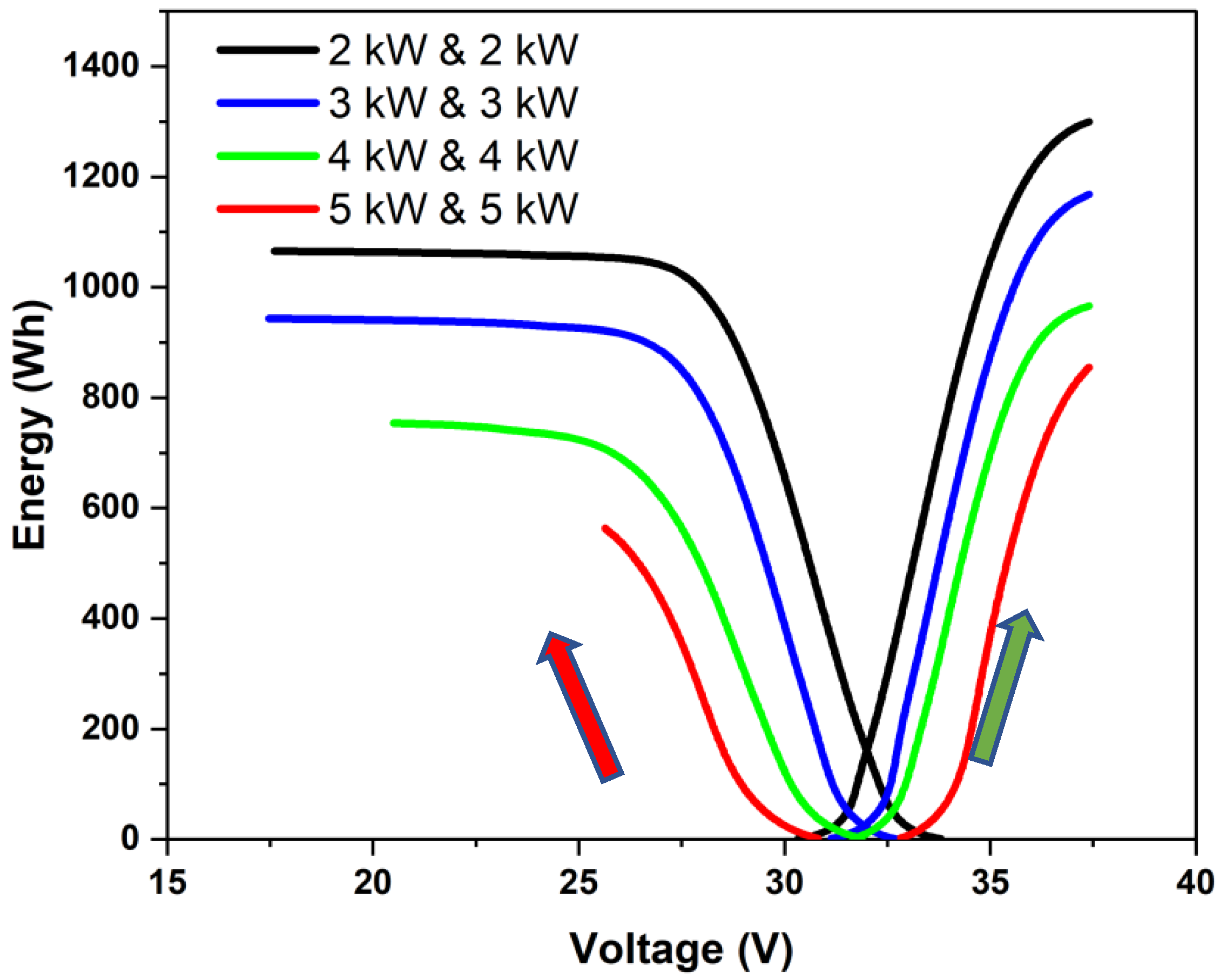
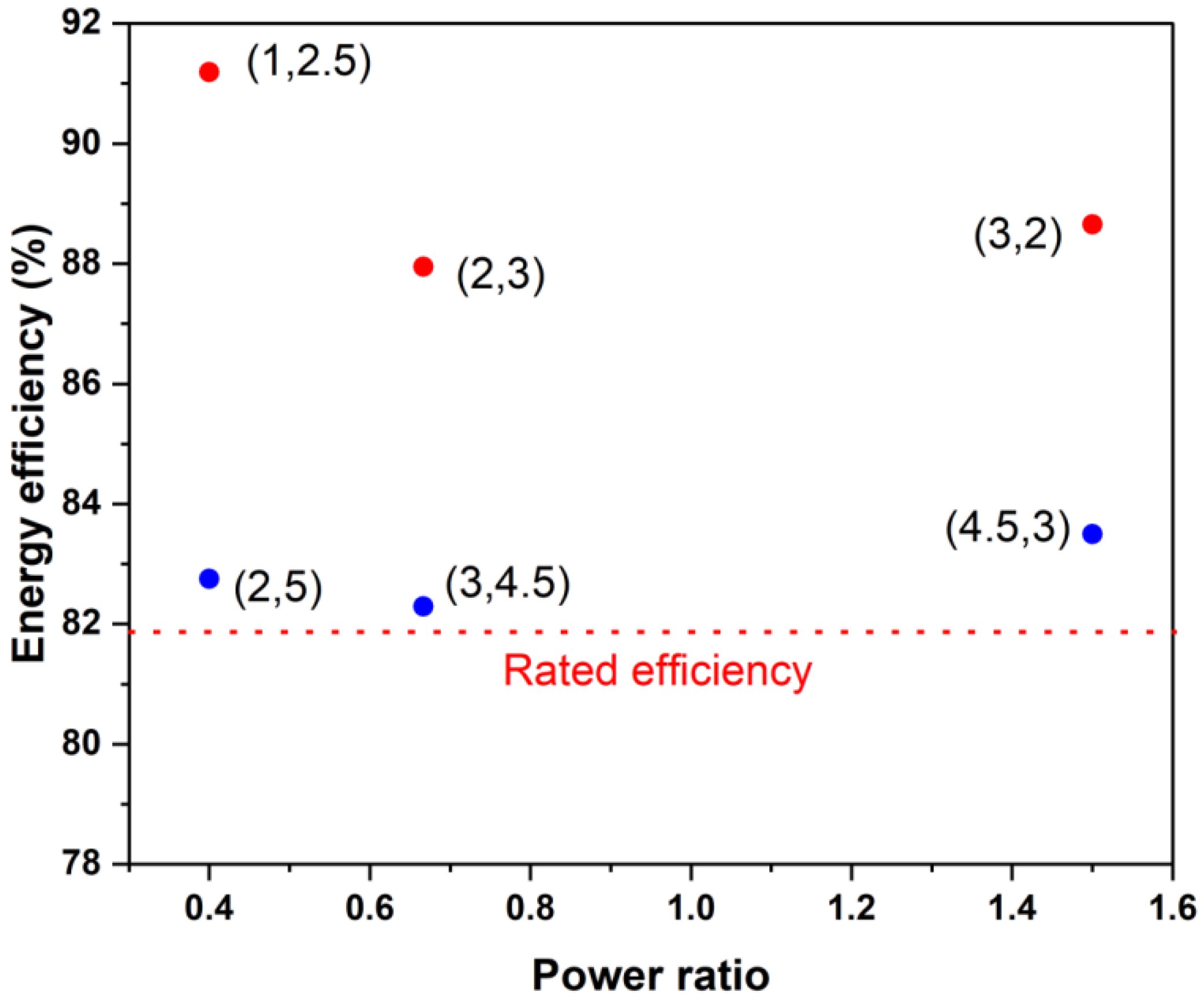
- It was found that the discharge energy varied linearly with varying power and that high-power operations resulted in less energy extraction due to higher overpotentials. Round-trip energy efficiencies were found to vary from 75% to 82%, which depended primarily on the power rather than on the power ratio.
- It was observed that the stack overpotential was a strong function of the current density in the healthy SoC range (30–80%) and was governed by an effective ohmic potential type of variation. The area-specific resistance in this SoC range was estimated to be 1.48 mΩ-cm2.
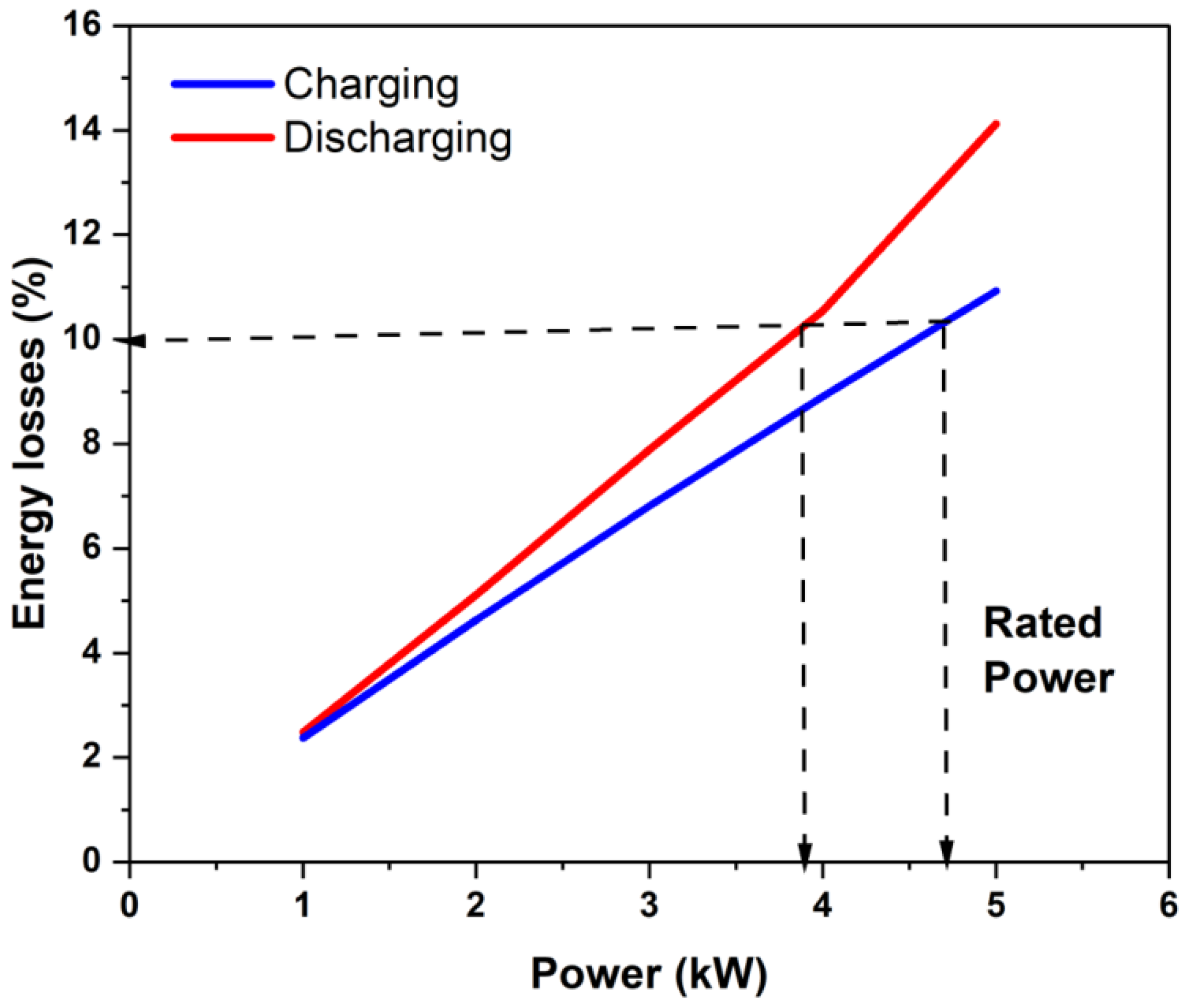
- A protocol was developed for determining the power rating of a stack based on the anticipated energy losses when the stack is run at constant power over the SoC range of 20 to 80%. It was found that using 10% energy loss in each charging and discharging process led to a reasonable power rating of the stack, as well as reasonably high capacity utilization of the electrolyte while maintaining a stack round-trip energy efficiency of over 80% (without counting pumping and other auxiliary losses).
- The power rating of the stack using the proposed criterion gave different power ratings under charging and discharging conditions. Operation under different power ratios did not strongly influence the energy efficiency if the operating power was about the same or lower than the rated power.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Vizcaíno, R.; Mena, E.; Millán, M.; Rodrigo, M.A.; Lobato, J. Performance of a vanadium redox flow battery for the storage of electricity produced in photovoltaic solar panels. Renew. Energy 2017, 114, 1123–1133. [Google Scholar] [CrossRef]
- Jia, H.; Fu, Y.; Zhang, Y.; He, W. Design of hybrid energy storage control system for wind farms based on flow battery and electric double-layer capacitor. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010. [Google Scholar]
- Parmeshwarappa, P.; Gundlapalli, R.; Jayanti, S. Power and Energy Rating Considerations in Integration of Flow Battery with Solar PV and Residential Load. Batteries 2021, 7, 62. [Google Scholar] [CrossRef]
- Kumar, A.; Jayanti, S. A land-use-constrained, generation–transmission model for electricity generation through solar photovoltaic technology: A case study of south India. Clean Technol. Environ. Policy 2021, 23, 2757–2774. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications–A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef] [Green Version]
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent Advancements in All-Vanadium Redox Flow Batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Sun, C.; Zou, Y.; Zhang, T. An optimal strategy of electrolyte flow rate for vanadium redox flow battery. J. Power Sources 2012, 203, 153–158. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Zhou, H.; Chen, J.; Gao, S.; Yi, B. Characteristics and performance of 10 kW class all-vanadium redox-flow battery stack. J. Power Sources 2006, 162, 1416–1420. [Google Scholar] [CrossRef]
- Kim, S.; Thomsen, E.; Xia, G.; Nie, Z.; Bao, J.; Recknagle, K.; Wang, W.; Viswanathan, V.; Luo, Q.; Wei, X.; et al. 1 kW/1 kWh advanced vanadium redox flow battery utilizing mixed acid electrolytes. J. Power Sources 2013, 237, 300–309. [Google Scholar] [CrossRef]
- Park, D.J.; Jeon, K.S.; Ryu, C.H.; Hwang, G.J. Performance of the all-vanadium redox flow battery stack. J. Ind. Eng. Chem. 2017, 45, 387–390. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Comparative study of kilowatt-scale vanadium redox flow battery stacks designed with serpentine flow fields and split manifolds. Batteries 2021, 7, 30. [Google Scholar] [CrossRef]
- Guarnieri, M.; Trovò, A.; D’Anzi, A.; Alotto, P. Developing vanadium redox flow technology on a 9-kW 26-kWh industrial scale test facility: Design review and early experiments. Appl. Energy 2018, 230, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Trovo, A.; Picano, F.; Guarnieri, M. Maximizing Vanadium Redox Flow Battery Efficiency: Strategies of Flow Rate Control. In Proceedings of the 2019 IEEE 28th International Symposium on Industrial Electronics (ISIE), Vancouver, BC, Canada, 12–14 June 2019. [Google Scholar]
- Shah, A.A.; Watt-Smith, M.J.; Walsh, F.C. A dynamic performance model for redox-flow batteries involving soluble species. Electrochim. Acta 2008, 53, 8087–8100. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Zhang, H.; Chen, J. A simple model for the vanadium redox battery. Electrochim. Acta 2009, 54, 6827–6836. [Google Scholar] [CrossRef]
- Knehr, K.W.; Kumbur, E.C. Open circuit voltage of vanadium redox flow batteries: Discrepancy between models and experiments. Electrochem. Commun. 2011, 13, 342–345. [Google Scholar] [CrossRef]
- Vynnycky, M. Analysis of a model for the operation of a vanadium redox battery. Energy 2011, 36, 2242–2256. [Google Scholar] [CrossRef]
- Chen, C.L.; Yeoh, H.K.; Chakrabarti, M.H. An enhancement to Vynnycky’s model for the all-vanadium redox flow battery. Electrochim. Acta 2014, 120, 167–179. [Google Scholar] [CrossRef]
- Shah, A.A.; Tangirala, R.; Singh, R.; Wills, R.G.A.; Walsh, F.C. A Dynamic Unit Cell Model for the All-Vanadium Flow Battery. J. Electrochem. Soc. 2011, 158, A671. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.W.; He, Y.L.; Li, Y.S. Performance Modeling of a Vanadium Redox Flow Battery during Discharging. Electrochim. Acta 2015, 155, 279–287. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Pugach, M.; Kondratenko, M.; Briola, S.; Bischi, A. Zero dimensional dynamic model of vanadium redox fl ow battery cell incorporating all modes of vanadium ions crossover. Appl. Energy 2018, 226, 560–569. [Google Scholar] [CrossRef]
- Pugach, M.; Vyshinsky, V.; Bischi, A. Energy efficiency analysis for a kilo-watt class vanadium redox flow battery system. Appl. Energy 2019, 253, 113533. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Effect of electrolyte convection velocity in the electrode on the performance of vanadium redox flow battery cells with serpentine flow fields. J. Energy Storage 2020, 30, 101516. [Google Scholar] [CrossRef]
- Guarnieri, M.; Trovò, A.; Marini, G.; Sutto, A.; Alotto, P. High current polarization tests on a 9 kW vanadium redox flow battery. J. Power Sources 2019, 431, 239–249. [Google Scholar] [CrossRef]
- Langner, J.; Melke, J.; Ehrenberg, H.; Roth, C. Determination of Overpotentials in All Vanadium Redox Flow Batteries. ECS Trans. 2014, 58, 1–7. [Google Scholar] [CrossRef]





| S No. | Redox Species | OCV (V) | Energy Cost (USD/kWh) |
|---|---|---|---|
| 1 | Fe-Cr | 1.2 | 250–450 |
| 2 | All-Vanadium | 1.2–1.6 | 175–400 |
| 3 | Zn-Br | 1.8 | 200–400 |
| 4 | Zn-Ce | 2.3 | 750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vudisi, P.K.; Jayanti, S.; Chetty, R. Model for Rating a Vanadium Redox Flow Battery Stack through Constant Power Charge–Discharge Characterization. Batteries 2022, 8, 85. https://doi.org/10.3390/batteries8080085
Vudisi PK, Jayanti S, Chetty R. Model for Rating a Vanadium Redox Flow Battery Stack through Constant Power Charge–Discharge Characterization. Batteries. 2022; 8(8):85. https://doi.org/10.3390/batteries8080085
Chicago/Turabian StyleVudisi, Pavan Kumar, Sreenivas Jayanti, and Raghuram Chetty. 2022. "Model for Rating a Vanadium Redox Flow Battery Stack through Constant Power Charge–Discharge Characterization" Batteries 8, no. 8: 85. https://doi.org/10.3390/batteries8080085






