Influence of the Ambient Storage of LiNi0.8Mn0.1Co0.1O2 Powder and Electrodes on the Electrochemical Performance in Li-ion Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Aging of the Electrodes/Materials
2.3. Assembly of Coin Cells
2.4. Electrochemical Testing
2.5. Postmortem Characterization
3. Results and Discussion
3.1. Electrochemical Experiments
3.1.1. Galvanostatic Cycling
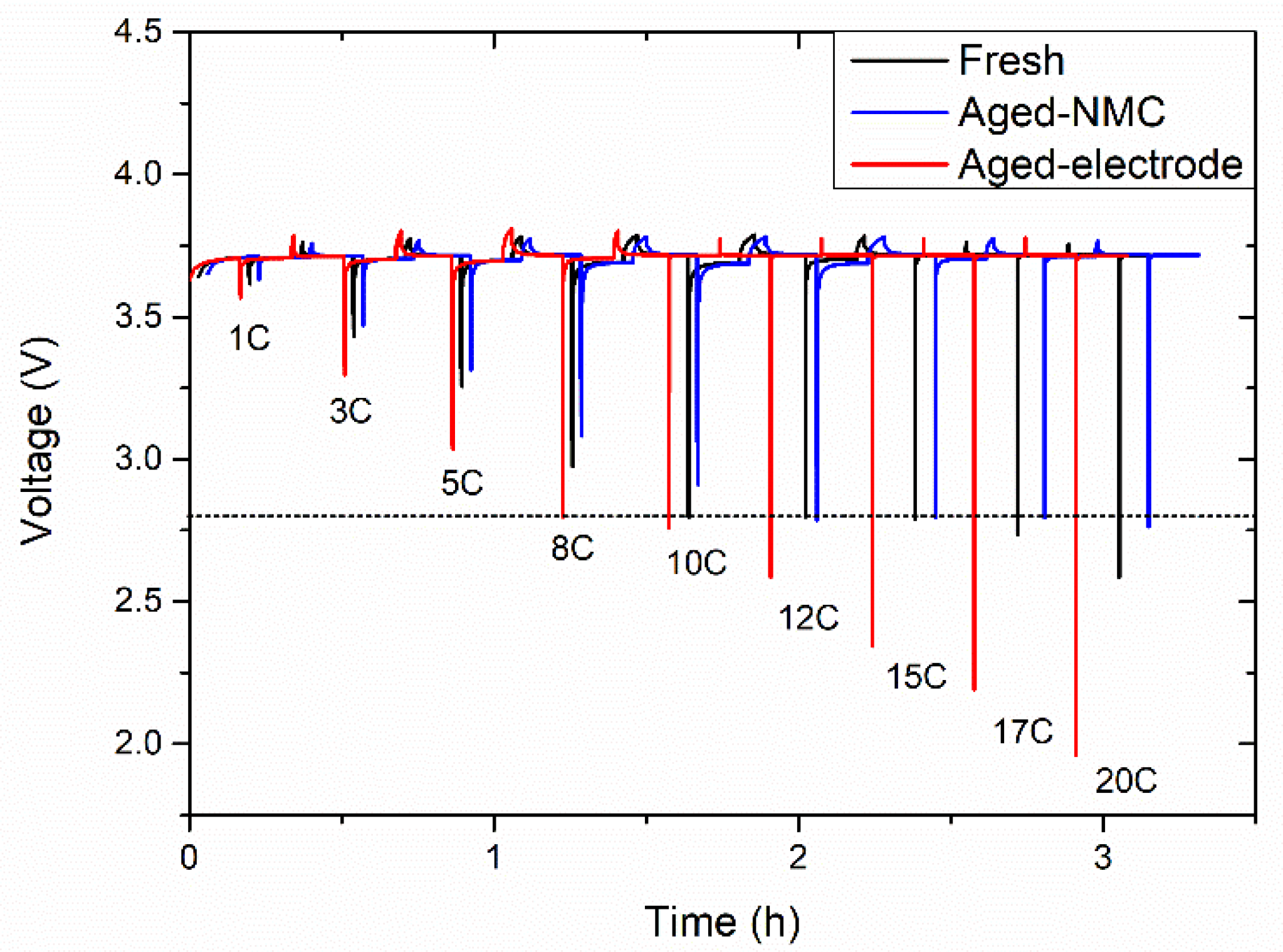
3.1.2. Power Tests
3.2. Materials Characterization
3.2.1. X-ray Diffraction
3.2.2. Field Emission Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
3.2.3. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Armand, M.; Rojo, T. Innovative Polymeric Materials for Better Rechargeable Batteries: Strategies from CIC Energigune. J. Electrochem. Soc. 2019, 166, A679–A686. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dai, F.; Cai, M. Opportunities and Challenges of High-Energy Lithium Metal Batteries for Electric Vehicle Applications. ACS Energy Lett. 2020, 5, 3140–3151. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- International Energy Agency. Global EV Outlook 2020: Entering the Decade of Electric Drive? OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Wetjen, M.; Pritzl, D.; Jung, R.; Solchenbach, S.; Ghadimi, R.; Gasteiger, H.A. Differentiating the Degradation Phenomena in Silicon-Graphite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2840–A2852. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Busà, C.; Belekoukia, M.; Loveridge, M.J. The effects of ambient storage conditions on the structural and electrochemical properties of NMC-811 cathodes for Li-ion batteries. Electrochim. Acta 2021, 366, 137358. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Röser, S.; Börner, M.; Winter, M. Do Increased Ni Contents in LiNixMnyCozO2 (NMC) Electrodes Decrease Structural and Thermal Stability of Li Ion Batteries? A Thorough Look by Consideration of the Li+ Extraction Ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Teichert, P.; Eshetu, G.G.; Jahnke, H.; Figgemeier, E. Degradation and aging routes of Ni-rich cathode based Li-ion batteries. Batteries 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Ely, D.R.; García, R.E. Heterogeneous Nucleation and Growth of Lithium Electrodeposits on Negative Electrodes. J. Electrochem. Soc. 2013, 160, 662–668. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, B.; Cao, H.; Xia, Y.; Liu, Z. Electrochemical properties of 0.6Li[Li1/3Mn2/3]O2-0.4LiNixMnyCo1−x−yO2 cathode materials for lithium-ion batteries. J. Power Sources 2012, 218, 128–133. [Google Scholar] [CrossRef]
- Ghatak, K.; Basu, S.; Das, T.; Sharma, V.; Kumar, H.; Datta, D. Effect of cobalt content on the electrochemical properties and structural stability of NCA type cathode materials. Phys. Chem. Chem. Phys. 2018, 20, 22805–22817. [Google Scholar] [CrossRef] [Green Version]
- Bondarchuk, O.; LaGrow, A.P.; Kvasha, A.; Thieu, T.; Ayerbe, E.; Urdampilleta, I. On the X-ray photoelectron spectroscopy analysis of LiNixMnyCozO2 material and electrodes. Appl. Surf. Sci. 2021, 535, 147699. [Google Scholar] [CrossRef]
- Jung, R.; Morasch, R.; Karayaylali, P.; Phillips, K.; Stinner, C.; Maglia, F.; Shao-Horn, Y.; Gasteiger, H.A. Effect of Ambient Storage on the Degradation of Ni-Rich Positive Electrode Materials (NMC811) for Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A132–A141. [Google Scholar] [CrossRef]
- Sicklinger, J.; Metzger, M.; Beyer, H.; Pritzl, D.; Gasteiger, H.A. Ambient Storage Derived Surface Contamination of NCM811 and NCM111: Performance Implications and Mitigation Strategies. J. Electrochem. Soc. 2019, 166, A2322–A2335. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; Eguia-Barrio, A.; Sananes-Israel, S.; Lijó-Pando, S.; Boyano, I.; Alcaide, F.; Urdampilleta, I.; De Meatza, I. In Situ Analysis of NMC ∣ graphite Li-Ion Batteries by Means of Complementary Electrochemical Methods. J. Electrochem. Soc. 2020, 167, 090528. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Phys. Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Plattard, T.; Barnel, N.; Assaud, L.; Franger, S.; Duffault, J.-M. Combining a Fatigue Model and an Incremental Capacity Analysis on a Commercial NMC/Graphite Cell under Constant Current Cycling with and without Calendar Aging. Batteries 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Raj, T.; Wang, A.A.; Monroe, C.W.; Howey, D.A. Investigation of Path-Dependent Degradation in Lithium-Ion Batteries. Batter. Supercaps 2020, 3, 1377–1385. [Google Scholar] [CrossRef]
- Dose, W.M.; Xu, C.; Grey, C.P.; De Volder, M.F.L. Effect of Anode Slippage on Cathode Cutoff Potential and Degradation Mechanisms in Ni-Rich Li-Ion Batteries. Cell Reports Phys. Sci. 2020, 1, 100253. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, C.H.; Shen, S.Y.; Xu, Y.F.; Shi, C.G.; Huang, L.; Li, J.T.; Sun, S.G. Origin of Structural Evolution in Capacity Degradation for Overcharged NMC622 via Operando Coupled Investigation. ACS Appl. Mater. Interfaces 2017, 9, 24731–24742. [Google Scholar] [CrossRef]
- Song, C.; Wang, W.; Peng, H.; Wang, Y.; Zhao, C.; Zhang, H.; Tang, Q.; Lv, J.; Du, X.; Dou, Y. Improving the electrochemical performance of LiNi0.80Co0.15Al0.05O2 in lithium ion batteries by LiAlO2 surface modification. Appl. Sci. 2018, 8, 378. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Phillip, N.D.; Daniel, C.; Veith, G.M. Influence of Binder Coverage on Interfacial Chemistry of Thin Film LiNi0.6Mn0.2Co0.2O2 Cathodes. J. Electrochem. Soc. 2020, 167, 040521. [Google Scholar] [CrossRef]
- Renfrew, S.E.; McCloskey, B.D. Quantification of Surface Oxygen Depletion and Solid Carbonate Evolution on the First Cycle of LiNi0.6Mn0.2Co0.2O2 Electrodes. ACS Appl. Energy Mater. 2019, 2, 3762–3772. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Lin, R.; Bak, S.M.; Shin, Y.; Zhang, R.; Wang, C.; Kisslinger, K.; Ge, M.; Huang, X.; Shadike, Z.; Pattammattel, A.; et al. Hierarchical nickel valence gradient stabilizes high-nickel content layered cathode materials. Nat. Commun. 2021, 12, 2350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Colclasure, A.M.; Finegan, D.P.; Ren, D.; Shi, Y.; Feng, X.; Cao, L.; Yang, Y.; Smith, K. Degradation mechanisms of high capacity 18,650 cells containing Si-graphite anode and nickel-rich NMC cathode. Electrochim. Acta 2019, 297, 1109–1120. [Google Scholar] [CrossRef]
- Raccichini, R.; Amores, M.; Hinds, G. Critical review of the use of reference electrodes in Li-ion batteries: A diagnostic perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Le, H.T.; Kalubarme, R.S.; Ngo, D.T.; Jang, S.Y.; Jung, K.N.; Shin, K.H.; Park, C.J. Citrate gel synthesis of aluminum-doped lithium lanthanum titanate solid electrolyte for application in organic-type lithium–oxygen batteries. J. Power Sources 2015, 274, 1188–1199. [Google Scholar] [CrossRef]
- Huang, L.H.; Chen, D.; Li, C.C.; Chang, Y.L.; Lee, J.T. Dispersion homogeneity and electrochemical performance of Si anodes with the addition of various water-based binders. J. Electrochem. Soc. 2018, 165, A2239–A2246. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Meatza, I.; Landa-Medrano, I.; Sananes-Israel, S.; Eguia-Barrio, A.; Bondarchuk, O.; Lijó-Pando, S.; Boyano, I.; Palomares, V.; Rojo, T.; Grande, H.-J.; et al. Influence of the Ambient Storage of LiNi0.8Mn0.1Co0.1O2 Powder and Electrodes on the Electrochemical Performance in Li-ion Technology. Batteries 2022, 8, 79. https://doi.org/10.3390/batteries8080079
de Meatza I, Landa-Medrano I, Sananes-Israel S, Eguia-Barrio A, Bondarchuk O, Lijó-Pando S, Boyano I, Palomares V, Rojo T, Grande H-J, et al. Influence of the Ambient Storage of LiNi0.8Mn0.1Co0.1O2 Powder and Electrodes on the Electrochemical Performance in Li-ion Technology. Batteries. 2022; 8(8):79. https://doi.org/10.3390/batteries8080079
Chicago/Turabian Stylede Meatza, Iratxe, Imanol Landa-Medrano, Susan Sananes-Israel, Aitor Eguia-Barrio, Oleksandr Bondarchuk, Silvia Lijó-Pando, Iker Boyano, Verónica Palomares, Teófilo Rojo, Hans-Jürgen Grande, and et al. 2022. "Influence of the Ambient Storage of LiNi0.8Mn0.1Co0.1O2 Powder and Electrodes on the Electrochemical Performance in Li-ion Technology" Batteries 8, no. 8: 79. https://doi.org/10.3390/batteries8080079





