Substituted Poly(Vinylphosphonate) Coatings of Magnetite Nanoparticles and Clusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Syntheses
2.3. Characterization
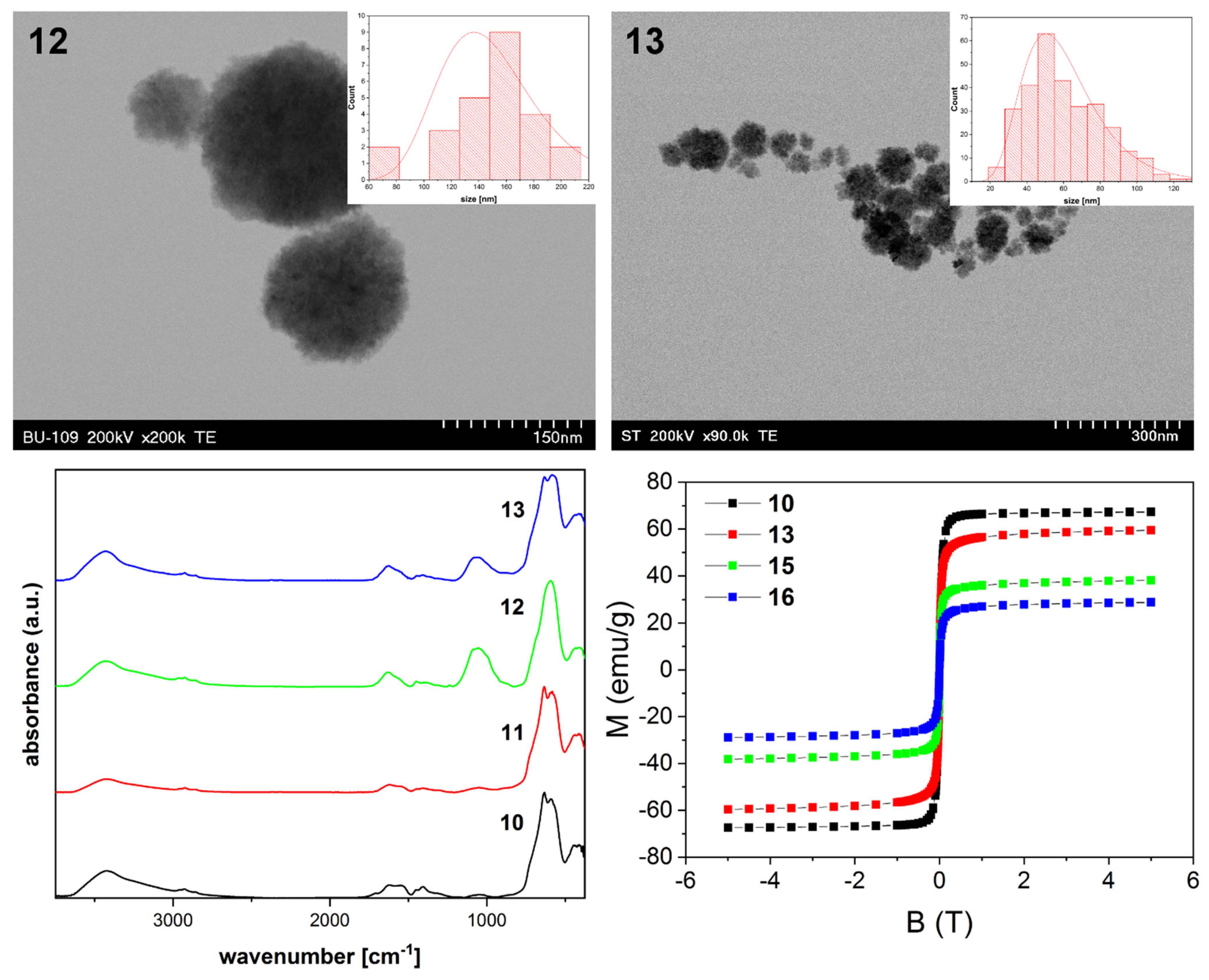
3. Results and Discussion
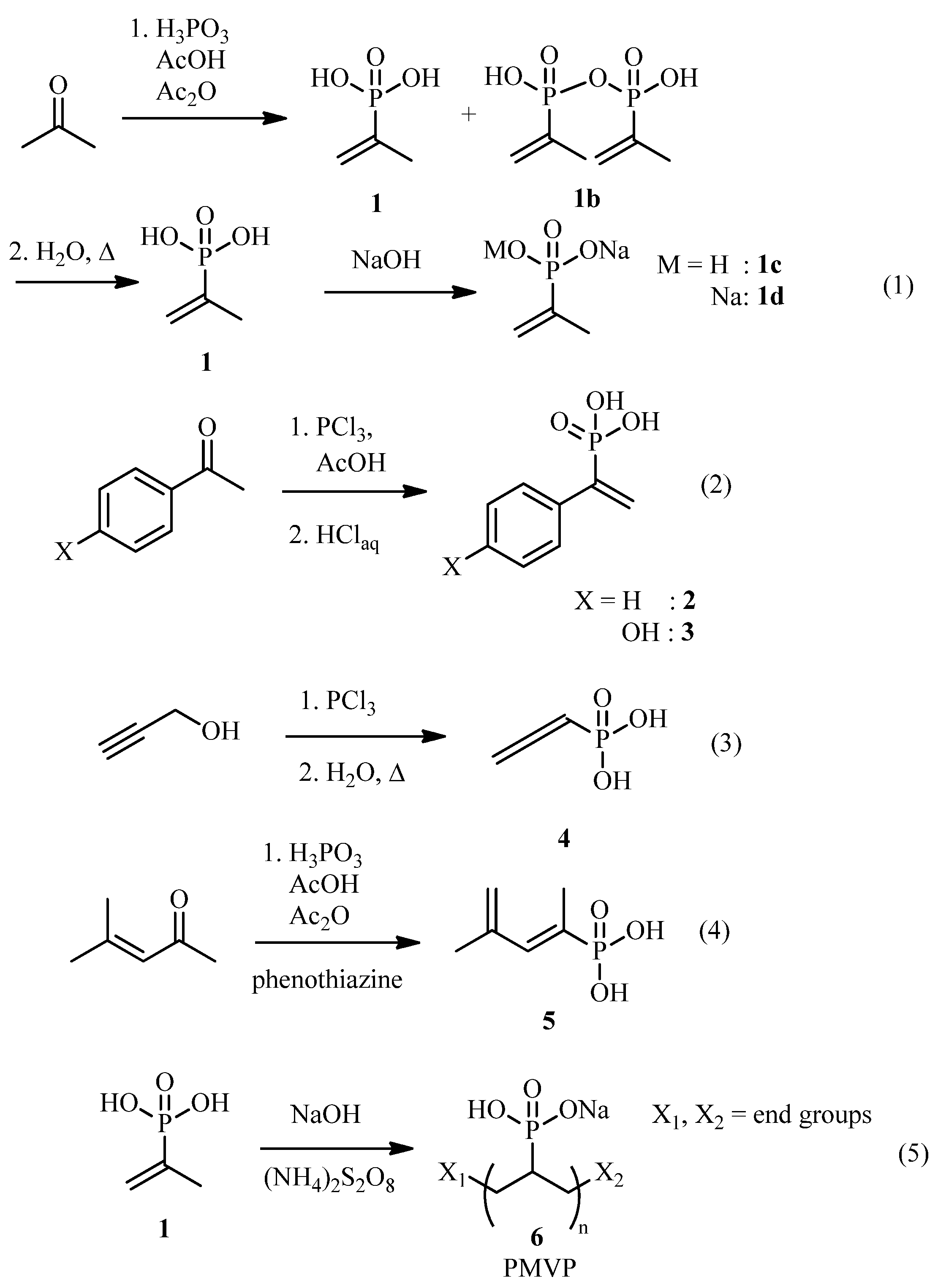
3.1. Synthesis and Polymerization of Vinylphosphonates
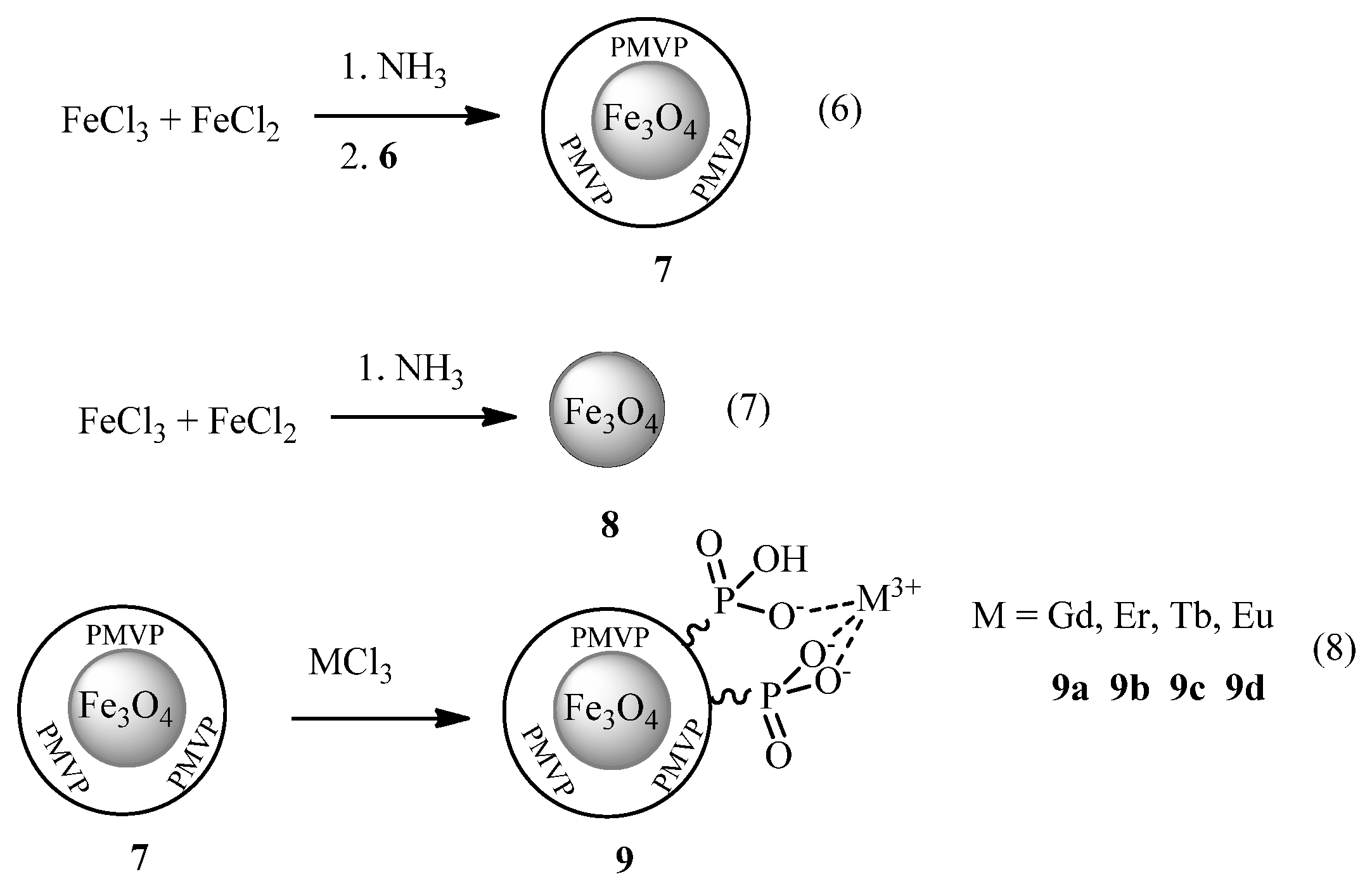
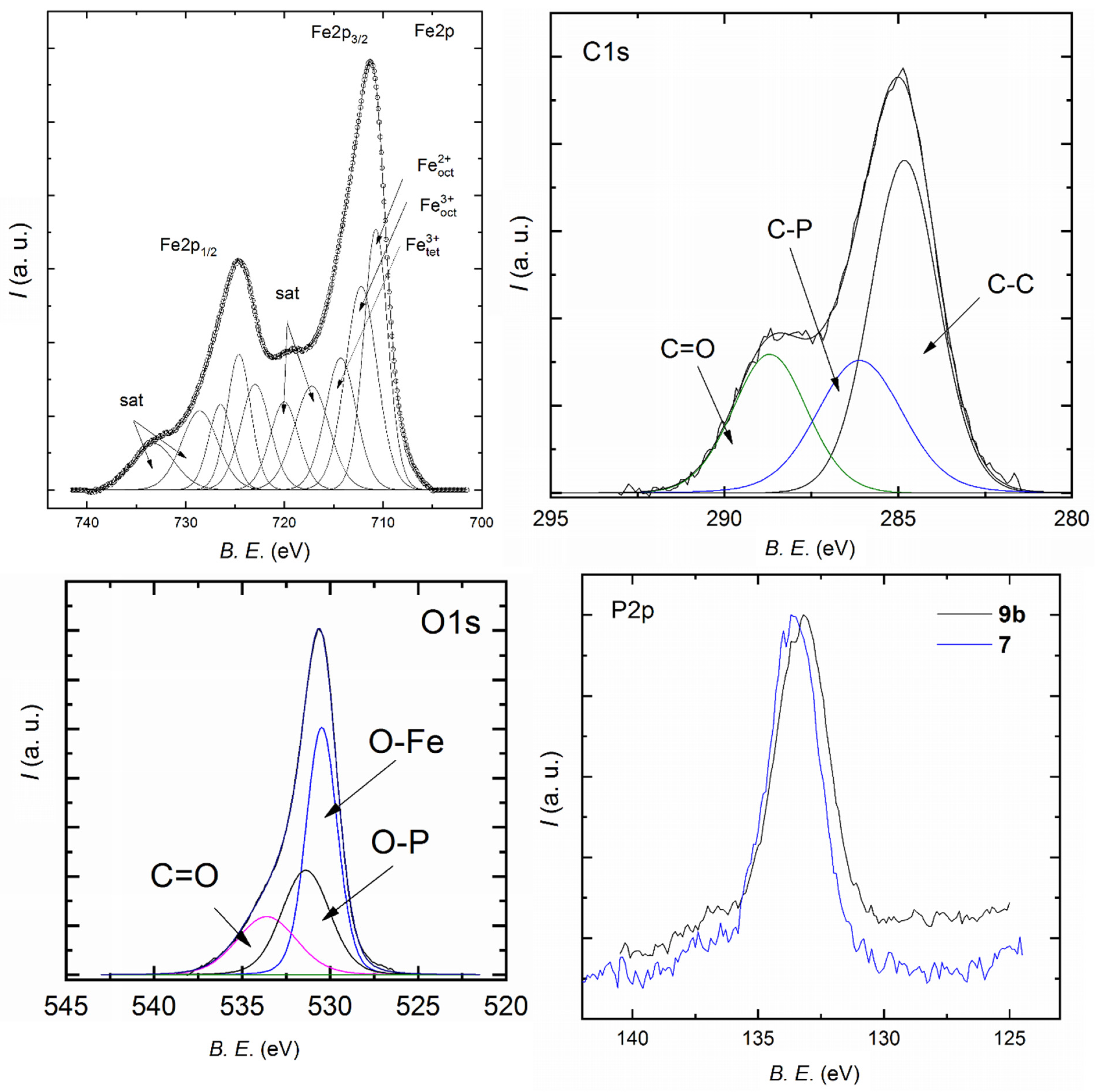
3.2. Synthesis and Characterization of Magnetite Nanoparticles
3.3. Synthesis and Characterization of Magnetite Clusters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. Nanoparticles and nanotechnology research. J. Nanopart. Res. 1999, 1, 1–6. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Nan, A.; Liebscher, J. Magnetic nanoparticle-supported organocatalysts—An efficient way of recycling and reuse. RSC Adv. 2014, 4, 5927–5952. [Google Scholar] [CrossRef]
- Terris, B.D.; Thomson, T. Nanofabricated and self-assembled magnetic structures as data storage media. J. Phys. D Appl. Phys. 2005, 38, R199–R222. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.T.; Wei, W.Z. Industry applications of magnetic separation based on nanoparticles: A review. Int. J. Appl. Electromagn. Mech. 2019, 60, 281–297. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, P.; Gupta, C.; Goel, P.; Jain, A.; Anand, S.; Pundir, A. Engineered magnetic nanoparticles as efficient sorbents for wastewater treatment: A review. Mater. Res. Innov. 2018, 22, 434–450. [Google Scholar] [CrossRef]
- Kumar, J.S.; Paul, P.S.; Raghunathan, G.; Alex, D.G. A review of challenges and solutions in the preparation and use of magnetorheological fluids. Int. J. Mech. Mater. Eng. 2019, 14, 13. [Google Scholar] [CrossRef]
- Lee, H.; Shin, T.H.; Cheon, J.; Weissleder, R. Recent Developments in Magnetic Diagnostic Systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. A review: Synthetic strategy control of magnetite nanoparticles production. Adv. Nano Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Nan, A.; Bunge, A.; Turcu, R. Hybride Magnetic Nanostructure Based on Amino Acids Functionalized Polypyrrole. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1700, p. 060007. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.F.; Neacsu, I.A.; Vasile, B.S.; Oprea, O.C.; Croitoru, A.M.; Trusca, R.D.; Andronescu, E.; Sorescu, I.; Barbuceanu, F. Iron Oxide-Silica Core-Shell Nanoparticles Functionalized with Essential Oils for Antimicrobial Therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, I.; Palade, P.; Iacob, N.; Ispas, G.M.; Stanciu, A.E.; Kuncser, V.; Turcu, R.P. High-Performance Functionalized Magnetic Nanoparticles with Tailored Sizes and Shapes for Localized Hyperthermia Applications. J. Phys. Chem. C 2021, 125, 11132–11146. [Google Scholar] [CrossRef]
- Rafienia, M.; Bigham, A.; Hassanzadeh-Tabrizi, S. Solvothermal synthesis of magnetic spinel ferrites. J. Med. Signals Sens. 2018, 8, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Upadhyay, C. Fine Tuning of Size and Morphology of Magnetite Nanoparticles Synthesized by Microemulsion. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1953, p. 030051. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moaca, E.A.; Watz, C.G.; Pinzaru, S.C.; Tudoran, L.B.; Nekvapil, F.; Avram, S.; Dehelean, C.A.; Cretu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef]
- Lu, Z.D.; Yin, Y.D. Colloidal nanoparticle clusters: Functional materials by design. Chem. Soc. Rev. 2012, 41, 6874–6887. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, W.L.; Wang, C.C. Magnetic Colloidal Supraparticles: Design, Fabrication and Biomedical Applications. Adv. Mater. 2013, 25, 5196–5214. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Tian, R.; Wang, Z.Y.; Yang, Z.; Liu, Y.J.; Liu, G.; Wang, R.F.; Gao, J.H.; Song, J.B.; Nie, L.M.; et al. Artificial local magnetic field inhomogeneity enhances T-2 relaxivity. Nat. Commun. 2017, 8, 15468. [Google Scholar] [CrossRef] [Green Version]
- Kostopoulou, A.; Lappas, A. Colloidal magnetic nanocrystal clusters: Variable length-scale interaction mechanisms, synergetic functionalities and technological advantages. Nanotech. Rev. 2015, 4, 595–624. [Google Scholar] [CrossRef]
- Li, Z.H.; Ma, Y.R.; Qi, L.M. Formation of nickel-doped magnetite hollow nanospheres with high specific surface area and superior removal capability for organic molecules. Nanotechnology 2016, 27, 485601. [Google Scholar] [CrossRef]
- Ge, J.P.; Hu, Y.X.; Biasini, M.; Beyermann, W.P.; Yin, Y.D. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Tsiaoussis, I.; Lappas, A. Magnetic iron oxide nanoclusters with tunable optical response. Photonics Nanostruct. Fundam. Appl. 2011, 9, 201–206. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, T.T.; Li, Y.N.; Zhao, Y.T.; Ye, Y.C.; Wu, W.H.; Tong, G.X. Effects of crystal size and sphere diameter on static magnetic and electromagnetic properties of monodisperse Fe3O4 microspheres. Mater. Chem. Phys. 2016, 173, 152–160. [Google Scholar] [CrossRef]
- Wang, W.T.; Tang, B.T.; Wu, S.L.; Gao, Z.M.; Ju, B.Z.; Teng, X.X.; Zhang, S.F. Controllable 5-sulfosalicylic acid assisted solvothermal synthesis of monodispersed superparamagnetic Fe3O4 nanoclusters with tunable size. J. Magn. Magn. Mater. 2017, 423, 111–117. [Google Scholar] [CrossRef]
- Weiss, C.K.; Landfester, K. Miniemulsion Polymerization as a Means to Encapsulate Organic and Inorganic Materials. Adv. Polym. Sci. 2010, 233, 185–236. [Google Scholar] [CrossRef]
- Fiévet, F.; Brayner, R. The Polyol Process. In Nanomaterials: A Danger or a Promise? A Chemical and Biological Perspective; Brayner, R., Fiévet, F., Coradin, T., Eds.; Springer: London, UK, 2013; pp. 1–25. [Google Scholar]
- Bunge, A.; Porav, A.S.; Borodi, G.; Radu, T.; Pirnau, A.; Berghian-Grosan, C.; Turcu, R. Correlation between synthesis parameters and properties of magnetite clusters prepared by solvothermal polyol method. J. Mater. Sci. 2019, 54, 2853–2875. [Google Scholar] [CrossRef]
- Xuan, S.H.; Wang, F.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094. [Google Scholar] [CrossRef]
- Jean, M.; Nachbaur, V.; Le Breton, J.M. Synthesis and characterization of magnetite powders obtained by the solvothermal method: Influence of the Fe3+ concentration. J. Alloys Compd. 2012, 513, 425–429. [Google Scholar] [CrossRef]
- Susan-Resiga, D.; Socoliuc, V.; Bunge, A.; Turcu, R.; Vekas, L. From high colloidal stability ferrofluids to magnetorheological fluids: Tuning the flow behavior by magnetite nanoclusters. Smart Mater. Struct 2019, 28, 115014. [Google Scholar] [CrossRef]
- Petran, A.; Radu, T.; Nan, A.; Olteanu, D.; Filip, A.; Clichici, S.; Baldea, I.; Suciu, M.; Turcu, R. Synthesis, characterization, and cytotoxicity evaluation of high-magnetization multifunctional nanoclusters. J. Nanopart. Res. 2016, 19, 10. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.K.; Deng, Y.H.; Zou, Y.; Li, C.Y.; Guo, X.H.; Xiong, L.Q.; Gao, Y.; Li, F.Y.; Zhao, D.Y. Highly Water-Dispersible Biocompatible Magnetite Particles with Low Cytotoxicity Stabilized by Citrate Groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Bao, J.F.; Wang, D.; Wang, Y.X.; Chen, Z.W.; Ren, L.; Zhou, X.; Ke, X.B.; Chen, M.; Yang, A.Q. One-step synthesis of monodisperse, water-soluble ultra-small Fe3O4 nanoparticles for potential bio-application. Nanoscale 2013, 5, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.H.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Tuning the Grain Size and Particle Size of Superparamagnetic Fe3O4 Microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Wang, W.T.; Tang, B.T.; Ju, B.Z.; Zhang, S.F. Size-controlled synthesis of water-dispersible superparamagnetic Fe3O4 nanoclusters and their magnetic responsiveness. RSC Adv. 2015, 5, 75292–75299. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [Green Version]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W. A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy 2020, 10, 1018. [Google Scholar] [CrossRef]
- Almahfood, M.; Bai, B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J. Pet. Sci. Eng. 2018, 171, 196–210. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Boer, J.C.; Selomulya, C.; Plebanski, M. Amino Acid Functionalized Inorganic Nanoparticles as Cutting-Edge Therapeutic and Diagnostic Agents. Bioconjug. Chem. 2018, 29, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Kostevsek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T-2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Bunge, A.; Magerusan, L.; Morjan, I.; Turcu, R.; Borodi, G.; Liebscher, J. Diazonium salt-mediated synthesis of new amino, hydroxy, propargyl, and maleinimido-containing superparamagnetic Fe@C nanoparticles as platforms for linking bio-entities or organocatalytic moieties. J. Nanopart. Res. 2015, 17, 379. [Google Scholar] [CrossRef]
- Sulek, F.; Drofenik, M.; Habulin, M.; Knez, Z. Surface functionalization of silica-coated magnetic nanoparticles for covalent attachment of cholesterol oxidase. J. Magn. Magn. Mater. 2010, 322, 179–185. [Google Scholar] [CrossRef]
- Kallay, N.; Matijevic, E. Adsorption at Solid-Solution Interfaces. 1. Interpretation of Surface Complexation of Oxalic and Citric Acids with Hematite. Langmuir 1985, 1, 195–201. [Google Scholar] [CrossRef]
- Fresnais, J.; Yan, M.; Courtois, J.; Bostelmann, T.; Bee, A.; Berret, J.F. Poly(acrylic acid)-coated iron oxide nanoparticles: Quantitative evaluation of the coating properties and applications for the removal of a pollutant dye. J. Colloid Interface Sci. 2013, 395, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.R.; Li, X.; Li, H.B.; Zhang, W.K. Quantifying thiol-gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [Green Version]
- Miles, W.C.; Huffstetler, P.P.; Goff, J.D.; Chen, A.Y.; Riffle, J.S.; Davis, R.M. Design of Stable Polyether-Magnetite Complexes in Aqueous Media: Effects of the Anchor Group, Molecular Weight, and Chain Density. Langmuir 2011, 27, 5456–5463. [Google Scholar] [CrossRef]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: A comparison with fatty acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Demin, A.M.; Mekhaev, A.V.; Esin, A.A.; Kuznetsov, D.K.; Zelenovskiy, P.S.; Shur, V.Y.; Krasnov, V.P. Immobilization of PMIDA on Fe3O4 magnetic nanoparticles surface: Mechanism of bonding. Appl. Surf. Sci. 2018, 440, 1196–1203. [Google Scholar] [CrossRef]
- Muthukumaran, T.; Philip, J. Effect of phosphate and oleic acid capping on structure, magnetic properties and thermal stability of iron oxide nanoparticles. J. Alloys Compd. 2016, 689, 959–968. [Google Scholar] [CrossRef]
- Yee, C.; Kataby, G.; Ulman, A.; Prozorov, T.; White, H.; King, A.; Rafailovich, M.; Sokolov, J.; Gedanken, A. Self-assembled monolayers of alkanesulfonic and -phosphonic acids on amorphous iron oxide nanoparticles. Langmuir 1999, 15, 7111–7115. [Google Scholar] [CrossRef]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.J.; Jallet, P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci. 2001, 238, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shafi, K.V.P.M.; Ulman, A.; Yan, X.Z.; Yang, N.L.; Estournes, C.; White, H.; Rafailovich, M. Sonochemical synthesis of functionalized amorphous iron oxide nanoparticles. Langmuir 2001, 17, 5093–5097. [Google Scholar] [CrossRef]
- Goff, J.D.; Huffstetler, P.P.; Miles, W.C.; Pothayee, N.; Reinholz, C.M.; Ball, S.; Davis, R.M.; Riffle, J.S. Novel Phosphonate-Functional Poly(ethylene oxide)-Magnetite Nanoparticles Form Stable Colloidal Dispersions in Phosphate-Buffered Saline. Chem. Mater. 2009, 21, 4784–4795. [Google Scholar] [CrossRef]
- Oleksa, V.; Bernatova, I.; Patsula, V.; Liskova, S.; Balis, P.; Radosinska, J.; Micurova, A.; Kluknavsky, M.; Jasenovec, T.; Radosinska, D.; et al. Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials 2021, 11, 1238. [Google Scholar] [CrossRef]
- Tombácz, E.; Tóth, I.Y.; Nesztor, D.; Illés, E.; Hajdú, A.; Szekeres, M.; Vékás, L. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Carroll, M.R.J.; Huffstetler, P.P.; Miles, W.C.; Goff, J.D.; Davis, R.M.; Riffle, J.S.; House, M.J.; Woodward, R.C.; St Pierre, T.G. The effect of polymer coatings on proton transverse relaxivities of aqueous suspensions of magnetic nanoparticles. Nanotechnology 2011, 22, 325702. [Google Scholar] [CrossRef]
- Pothayee, N.; Balasubramaniam, S.; Davis, R.M.; Riffle, J.S.; Carroll, M.R.J.; Woodward, R.C.; Pierre, T.G.S. Synthesis of ‘ready-to-adsorb’ polymeric nanoshells for magnetic iron oxide nanoparticles via atom transfer radical polymerization. Polymer 2011, 52, 1356–1366. [Google Scholar] [CrossRef]
- Baldim, V.; Bia, N.; Graillot, A.; Loubat, C.; Berret, J.F. Monophosphonic versus Multiphosphonic Acid Based PEGylated Polymers for Functionalization and Stabilization of Metal (Ce, Fe, Ti, Al) Oxide Nanoparticles in Biological Media. Adv. Mater. Interfaces 2019, 6, 1801814. [Google Scholar] [CrossRef]
- Golas, P.L.; Louie, S.; Lowry, G.V.; Matyjaszewski, K.; Tilton, R.D. Comparative Study of Polymeric Stabilizers for Magnetite Nanoparticles Using ATRP. Langmuir 2010, 26, 16890–16900. [Google Scholar] [CrossRef]
- Chen, Y.; He, L.; Chen, Z.Y.; Zhao, L.R.; Liang, J.Y.; Liu, G.J. Under-oil superhydrophilic TiO2/poly(sodium vinylphosphonate) nanocomposite for the separation of water from oil. Sep. Purif. Technol. 2020, 251, 117397. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Mounsamy, M.; Lauth-de Viguerie, N.; Coppel, Y.; Harrisson, S.; Destarac, M.; Mingotaud, C.; Kahn, M.L.; Marty, J.D. Luminescent zinc oxide nanoparticles: From stabilization to slow digestion depending on the nature of polymer coating. Polym. Chem. 2019, 10, 145–154. [Google Scholar] [CrossRef]
- Dolan, C.; Naysmith, B.; Hinkley, S.F.R.; Sims, I.M.; Brimble, M.A.; Williams, D.E.; Jin, J.Y. Synthesis of Novel Triazole-Containing Phosphonate Polymers. Aust. J. Chem. 2015, 68, 680–686. [Google Scholar] [CrossRef]
- Bal’tser, A.E.; Zaitsev, D.A.; Ivanova, T.V.; Babenko, T.G.; Barskova, E.N. Addition of morpholine and pyrrolidine to isopropenylphosphonic acid in situ. Russ. J. Org. Chem. 2013, 49, 627–628. [Google Scholar] [CrossRef]
- Gubnitskaya, E.S.; Peresypina, L.P. Esters Of N-Protected β-Aminoethylphosphonic Acid. J. Gen. Chem. USSR 1989, 59, 492–499. [Google Scholar]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Goettingen Math. Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Kenyon, G.L.; Westheimer, F.H. The Stereochemistry of Unsaturated Phosphonic Acids1. J. Am. Chem. Soc. 1966, 88, 3557–3561. [Google Scholar] [CrossRef]
- Dong, K.W.; Wang, Z.; Ding, K.L. Rh(I)-Catalyzed Enantioselective Hydrogenation of alpha-Substituted Ethenylphosphonic Acids. J. Am. Chem. Soc. 2012, 134, 12474–12477. [Google Scholar] [CrossRef]
- Kosolapoff, G.M. The Synthesis of Phosphonic and Phosphinic Acids. Org. React. 2011, 6, 273–338. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. [Google Scholar] [CrossRef]
- David, G.; Negrell-Guirao, C.; Iftene, F.; Boutevin, B.; Chougrani, K. Recent progress on phosphonate vinyl monomers and polymers therefore obtained by radical (co)polymerization. Polym. Chem. 2012, 3, 265–274. [Google Scholar] [CrossRef]
- Dabdoub, A.M. Methods for Synthesizing Phosphonic Compounds and Compounds Thereof. U.S. Patent US2007287858A1, 3 May 2007. [Google Scholar]
- Li, C.Y.; Saga, Y.; Onozawa, S.Y.; Kobayashi, S.; Sato, K.; Fukaya, N.; Han, L.B. Wet and Dry Processes for the Selective Transformation of Phosphonates to Phosphonic Acids Catalyzed by Bronsted Acids. J. Org. Chem. 2020, 85, 14411–14419. [Google Scholar] [CrossRef]
- Bondarenko, G.N.; Ganina, O.G.; Sharma, R.K.; Beletskaya, I.P. Catalytic activity of Pd catalysts on different supports in hydrogenation of 1-phenylethenylphosphonic acid. Russ. Chem. B 2014, 63, 1856–1859. [Google Scholar] [CrossRef]
- Zupancic, B.; Mohar, B.; Stephan, M. Impact on Hydrogenation Catalytic Cycle of the R Groups’ Cyclic Feature in “R-SMS-Phos”. Org. Lett. 2010, 12, 3022–3025. [Google Scholar] [CrossRef]
- Gulyukina, N.S.; Dolgina, T.M.; Bondarenko, G.N.; Beletskaya, I.P.; Bondarenko, N.A.; Henry, J.C.; Lavergne, D.; Ratovelomanana-Vidal, V.; Genet, J.P. Synthesis of biologically active 1-arylethylphosphonates. Russ. J. Org. Chem. 2002, 38, 573–587. [Google Scholar] [CrossRef]
- Macomber, R.S.; Kennedy, E.R. Phosphorus-containing products from the reaction of propargyl alcohols with phosphorus trihalides. 4. Alkyl substituent effects on oxaphospholene formation. J. Org. Chem. 1976, 41, 3191–3197. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Fallon, L.; Lever, D.C.; Shuey, S.W. Inverse phosphotriester DNA synthesis using photochemically-removable dimethoxybenzoin phosphate protecting groups. J. Org. Chem. 1996, 61, 2129–2136. [Google Scholar] [CrossRef]
- Liu, Z.; De Campo, F.; Guy, L.; Wilson, D.J.; Jost, P. New Coupling Agents for Elastomer Compositions. World Patent WO2012146198 (A1), 11 November 2012. [Google Scholar]
- Liu, Z.; De Campo, F. Method for Preparing Conjugated Diene Phosphonate Compounds. World Patent WO2011050533 (A1), 30 October 2009. [Google Scholar]
- Circu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of Magnetite Nanoparticles by Coating with Organic Stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenzi, P.; Bettinelli, M.; Boffi, A.; Botta, M.; De Simone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare earth elements (REE) in biology and medicine. Rend. Lincei. Sci. Fis. E Nat. 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Nieciecka, D.; Rekorajska, A.; Cichy, D.; Konska, P.; Zuk, M.; Krysinski, P. Synthesis and Characterization of Magnetic Drug Carriers Modified with Tb3+ Ions. Nanomaterials 2022, 12, 795. [Google Scholar] [CrossRef]
- Gotić, M.; Koščec, G.; Musić, S. Study of the reduction and reoxidation of substoichiometric magnetite. J. Mol. Struct. 2009, 924–926, 347–354. [Google Scholar] [CrossRef]
- Hu, L.; Percheron, A.; Chaumont, D.; Brachais, C.-H. Microwave-assisted one-step hydrothermal synthesis of pure iron oxide nanoparticles: Magnetite, maghemite and hematite. J. Sol. Gel. Sci. Technol. 2011, 60, 198. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Fleet, M.E. The Structure of Magnetite. Acta Cryst. B 1981, 37, 917–920. [Google Scholar] [CrossRef]
- Fluck, E.; Weber, D. P2p-Bindungsenergien in Phosphor(III)—Verbindungen, Phosphoniumsalzen und Sauerstoffsäuren des Phosphors. Z. Naturforsch. B 1974, 29b, 603–607. [Google Scholar] [CrossRef]
- Mercier, F.; Alliot, C.; Bion, L.; Thromat, N.; Toulhoat, P. XPS study of Eu(III) coordination compounds: Core levels binding energies in solid mixed-oxo-compounds EumXxOy. J. Electron. Spectrosc. 2006, 150, 21–26. [Google Scholar] [CrossRef]
- Sudakar, C.; Subbanna, G.N.; Kutty, T.R.N. Effect of anions on the phase stability of gamma-FeOOH nanoparticles and the magnetic properties of gamma-ferric oxide derived from lepidocrocite. J. Phys. Chem. Solids 2003, 64, 2337–2349. [Google Scholar] [CrossRef]
- Thomas, G.; Demoisson, F.; Boudon, J.; Millot, N. Efficient functionalization of magnetite nanoparticles with phosphonate using a one-step continuous hydrothermal process. Dalton Trans. 2016, 45, 10821–10829. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Q.; Yuan, R.T.; Zhang, C.Y.; Wu, N.; Yan, F.Y.; Yu, S.S.; Chen, K.Z. Stable and Biocompatible Colloidal Dispersions of Superparamagnetic Iron Oxide Nanoparticles with Minimum Aggregation for Biomedical Applications. J. Phys. Chem. C 2016, 120, 23799–23806. [Google Scholar] [CrossRef]
- Kinoshita, K.; Takano, Y.; Ohkouchi, N.; Deguchi, S. Free-Radical Polymerization of Acrylic Acid under Extreme Reaction Conditions Mimicking Deep-Sea Hydrothermal Vents. ACS Omega 2017, 2, 2765–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
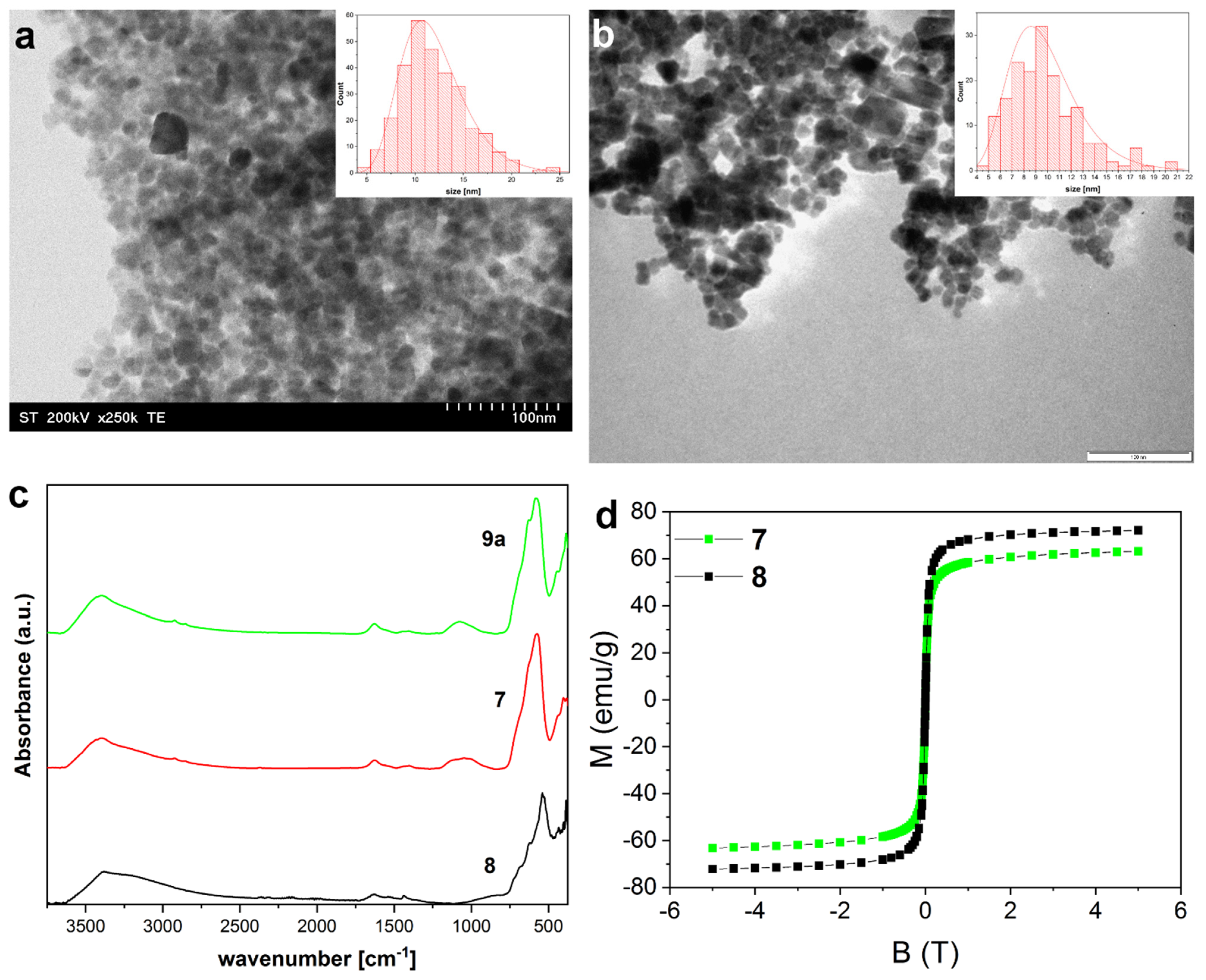

| Entry | Size (TEM) [nm] | Crystallite Size (XRD) [nm] |
|---|---|---|
| 7 | 9.8 ± 3 | 11.3 |
| 8 | 12.0 ± 3.4 | 26.9 |
| 9a | 10.4 ± 2.9 | |
| 9b | 12.5 ± 3.4 | |
| 10 | 300 ± 102 | |
| 11 | 299 ± 79 | 13.7 |
| 12 | 149 ± 32 | 19.4 |
| 13 | 61 ± 21 | 10.9 |
| 14 | 41 ± 9 | |
| 15 | 52 ± 11 | |
| 16 | 59 ± 12 | 19.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunge, A.; Leoștean, C.; Radu, T.; Tripon, S.C.; Borodi, G.; Turcu, R. Substituted Poly(Vinylphosphonate) Coatings of Magnetite Nanoparticles and Clusters. Magnetochemistry 2022, 8, 79. https://doi.org/10.3390/magnetochemistry8080079
Bunge A, Leoștean C, Radu T, Tripon SC, Borodi G, Turcu R. Substituted Poly(Vinylphosphonate) Coatings of Magnetite Nanoparticles and Clusters. Magnetochemistry. 2022; 8(8):79. https://doi.org/10.3390/magnetochemistry8080079
Chicago/Turabian StyleBunge, Alexander, Cristian Leoștean, Teodora Radu, Septimiu Cassian Tripon, Gheorghe Borodi, and Rodica Turcu. 2022. "Substituted Poly(Vinylphosphonate) Coatings of Magnetite Nanoparticles and Clusters" Magnetochemistry 8, no. 8: 79. https://doi.org/10.3390/magnetochemistry8080079







