1. Introduction
Plant propagation is an essential component of the horticulture and agriculture industries, used to increase the number of plants. Vegetative propagation is one of the most common methods for reproducing ornamental and herbaceous plants, usually through cuttings. In addition to being cost-effective, it also allows for the large-scale multiplication of new plantlets genetically identical to the mother plant, thereby retaining the desirable genetic characteristics [
1,
2]. Cuttings must possess adventitious roots to thrive. Several researchers and industry professionals have implemented techniques and methods such as misting, fogging, and the use of rooting hormones and biostimulants to increase the survival and rooting rate for cutting propagation [
3,
4,
5,
6]. However, optimal environmental conditions must be provided until the establishment of adventitious roots.
Successful propagation via stem cutting relies on optimal environmental factors such as light, substrate, air humidity, and water availability within the substrate [
7]. Among these, air humidity and substrate moisture content are particularly important for the survival of cuttings, especially because it relates to the formation of adventitious roots [
8]. Insufficient substrate moisture can lead to desiccation of cuttings, and adequate moisture is, therefore, applied to the substrate until the emergence of adventitious roots. This is usually done by applying mist via a misting system [
9]. The system works by periodically providing water as fine particles through nozzles to maintain high humidity and provide sufficient moisture to the plant materials. However, issues may occasionally arise where excess irrigation can lead to a lack of aeration within the rooting substrate, thereby affecting the growth processes of the cuttings [
10].
The development of soil moisture sensors has provided various advantages for monitoring and controlling the water content in growing media. In particular, frequency domain reflectometry (FDR) sensors with a data logger allow for a greater understanding of plant responses to a specific substrate moisture condition [
11,
12]. When combined with automated irrigation systems, these sensors control substrate moisture content by providing irrigation when the moisture level falls below a pre-determined irrigation threshold level. These systems not only allow for more precise irrigation but also produce plants of better quality while increasing the water use efficiency [
13].
Although this technology is effective in its precision and accuracy when used for potted plants, little research has been conducted on its effectiveness in propagation. Several studies related to propagation have been carried out using varying irrigation methods (misting, hand watering, and sub-irrigation) in
Colutea istria,
Veronica dahurica, V. pusanensis,
Myrica gale,
Rhododendron canadense, and
Ilex mucronata [
14,
15,
16]. However, there is limited information on the specific substrate moisture level used. Therefore, the optimal substrate moisture level related to propagation should be investigated.
Lavender is an aromatic and melliferous perennial flowering plant belonging to the Lamiaceae family, with English lavender (
Lavandula angustifolia) being one of the most popular cultivars, best known for its high essential oil content [
17]. Lavender may be propagated by seeds or by cuttings. However, when propagated by seeds, the method is prolonged, and germination is low and sporadic. Lack of homogeneity is also an issue that may arise, with plants not retaining the desirable traits. The preferred propagation method is via stem cuttings because it allows for the quick establishment of plants while retaining the genetic traits of the stock plant [
18].
Although cutting propagation has been well documented and is one of the standard protocols within the industry [
6,
19], the utilization of soil-moisture-sensor-based automated irrigation has been insufficiently investigated. The objectives of this study were, therefore, to investigate the effects of varying irrigation thresholds on the survival, rooting rates, and growth of English lavender cuttings, and to determine the proper substrate moisture level for the successful cutting propagation of English lavender.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
The experiment was conducted in a glass greenhouse at Korea University, Seoul, Korea (latitude 37° N, longitude 127° E) from 24 May to 6 July 2021. Apical cuttings of English lavender were obtained on 24 May 2021 from the Korea University Farm (Namyangju, Korea). The cuttings had 5.96 ± 0.28 leaves and were 4.02 ± 0.36 cm in stem length (mean ± SD). For the cutting propagation media, a soilless propagation substrate (Sunshine Mix #5; Sun Gro Horticulture, Agawam, MA, USA) with fine particle sizes was used following the industrial practice. Before placing the cuttings into the substrate, the basal parts of the cuttings were dipped in an indole-3-acetic acid solution (Sigma Aldrich, St. Louis, MO, USA) at 1000 mg·L−1 using the quick-dip method to improve rooting.
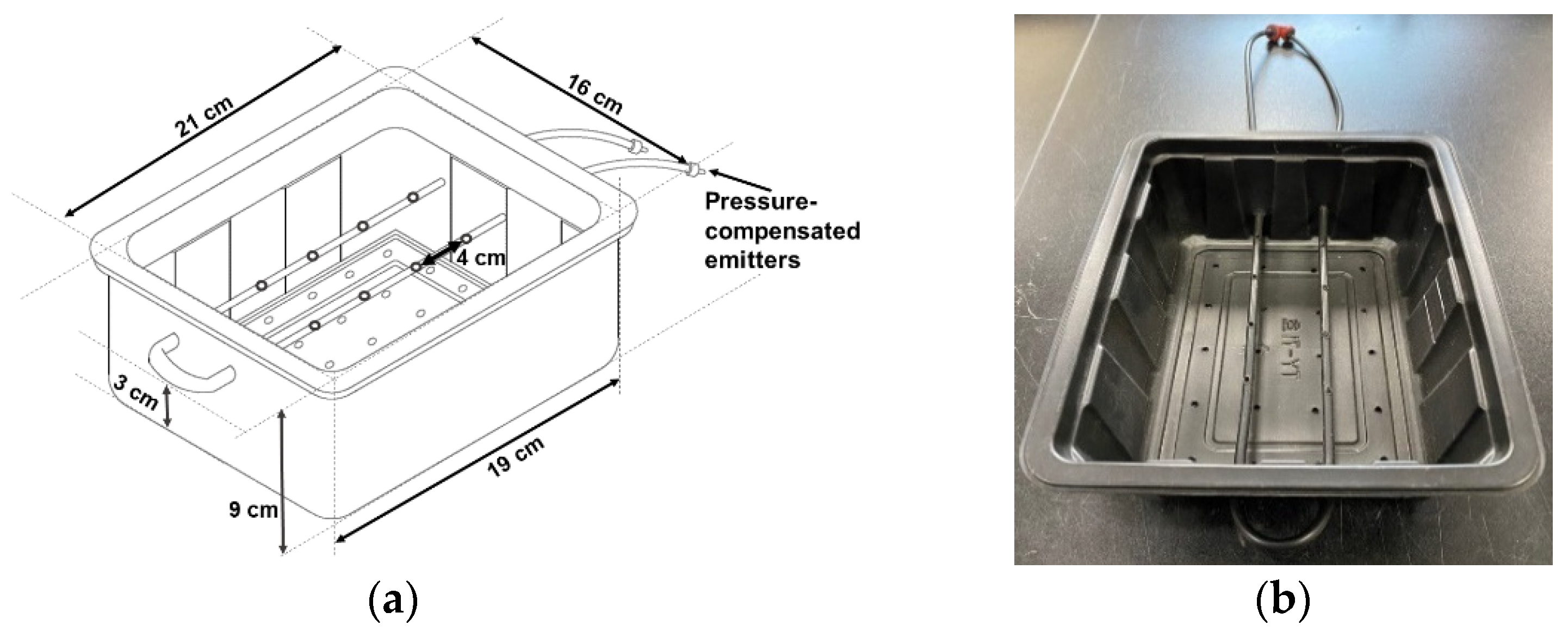
The cuttings were placed in a customized container with a subsurface drip irrigation tubing connected to two pressure-compensated emitters (2 L·h
−1; Netafim, Tel Aviv, Israel) (
Figure 1). The containers were placed on a modified propagation bench fitted with covering film and shade cloth to increase the relative humidity (RH) and reduce the light intensity. A misting system composed of misters (CoolNet™ Pro super-fine misters, Netafim) and air circulators (630; Vornado, Andover, KS, USA) were installed underneath the bench to maintain high RH above 75%.
Environmental conditions such as temperature, RH, and daily light integral (DLI) inside the propagation bench were monitored using a temperature and humidity sensor (VP-3; Meter Group, Pullman, WA, USA) and a photosynthetic active radiation sensor (SQ-110-SS; Apogee Instruments, Logan, UT, USA) connected to a data logger (CR1000; Campbell Scientific, Logan, UT, USA). The daily average temperature, RH, and DLI during the experiment were 26.0 ± 0.4 °C, 79.5 ± 1.3%, and 1.3 ± 0.4 mol·m−2·d−1 (mean ± SD), respectively.
2.2. Determining the Irrigation Thresholds
The irrigation threshold treatments for the substrate used for the cuttings were determined at −1.0, −2.5, −5.0, and −10.0 kPa, ranging from easily available water (EAW, −1.0 to −5.0 kPa) to water buffering capacity (WBC, −5.0 to −10.0 kPa) [
20]. A moisture retention curve of the substrate was determined using a Hyprop (Meter Group) to determine the proper volumetric water content (VWC). The VWC treatments were determined at 0.62, 0.48, 0.35, and 0.26 m
3·m
−3 for the −1.0, −2.5, −5.0, and −10.0 kPa treatments, respectively (
Figure 2a).
2.3. Automated Irrigation System
An FDR-sensor-based automated irrigation system was used to maintain specific irrigation threshold treatments of the substrates. A total of 16 FDR sensors (EC-5; Meter Group) were connected to a data logger (CR1000; Campbell Scientific) via a multiplexer (AM 16/32B; Campbell Scientific) and powered via 2.5 V excitation. The FDR sensors were calibrated to the specific substrate used for the experiment [VWC (m3·m−3) = 0.001749 × sensor output (mV) − 0.5622, r2 = 0.96]. The irrigation was controlled by a relay drive (SDM-16AC/DC; Campbell Scientific) connected to the data logger that would switch the solenoid valve actuators (Bermad S-390T-2-R; Bermad, Kibbutz Evron, Israel).
One FDR sensor was inserted diagonally for each experimental unit at a 45° angle in the middle containers among the three replicates. The VWC was measured every 10 s for the containers with sensors, whereas the irrigation thresholds were checked every 20 min using the datalogger. If the VWC fell below the set irrigation threshold level, the specific solenoid valve was opened, and the experimental unit was irrigated for 10 s (approximately 11.1 mL). The VWC data of the experimental units were recorded hourly via the data logger, and the system maintained the VWCs of the treatment as programmed throughout the experimental period (
Figure 2b).
2.4. Cutting Survival, Rooting, and Growth Parameters
The survival rate of the cuttings (number of surviving cuttings/numbers of used cuttings × 100) was recorded weekly after the cuttings were stuck in the substrate. The first rooting was observed two weeks after sticking (WAS); therefore, one container from each experimental unit was harvested biweekly at 2, 4, and 6 WAS. Rooting percentage (rooted cuttings/numbers of cutting inserted × 100) was recorded biweekly. The number of adventitious roots, root length, root fresh/dry weight, shoot fresh/dry weight, the number of leaves, and shoot height were measured at each harvest of the cuttings that were alive. After measuring the fresh weights, the samples were placed in a drying oven at 80 °C for 72 h to determine the dry weights of the shoots and roots.
2.5. Experimental Design and Statistical Analysis
The experimental design was a randomized complete block design with four irrigation thresholds and four blocks. Three containers were used as replicates for harvest at 2, 4, and 6 WAS, each containing nine cuttings that served as sub-replicates. SAS 9.4 statistical software (SAS Institute, Cary, NC, USA) was used to perform the analysis of variance (ANOVA). Two-way ANOVA was conducted to test the effect of irrigation thresholds, WAS, and their interaction. When there was a significant difference at p ≤ 0.05 among the irrigation threshold treatments within WAS, mean separation was conducted using Duncan’s multiple range test to compare the means among the treatments.
4. Discussion
Sufficient substrate moisture conditions are essential for cutting survival until the emergence of adventitious roots. The high mortality rate of the cuttings in the −10.0 kPa threshold was mostly attributed to insufficient moisture levels within the substrate. Due to this water deficit, an increase in water loss from the cuttings could lead to leaf desiccation and finally wilting, with death occurring in the more severe cases. Similarly, treatments with a substrate moisture content ≤ 0.2 m
3·m
−3 resulted in high mortality rates of hydrangea cuttings as demonstrated in previous research [
21].
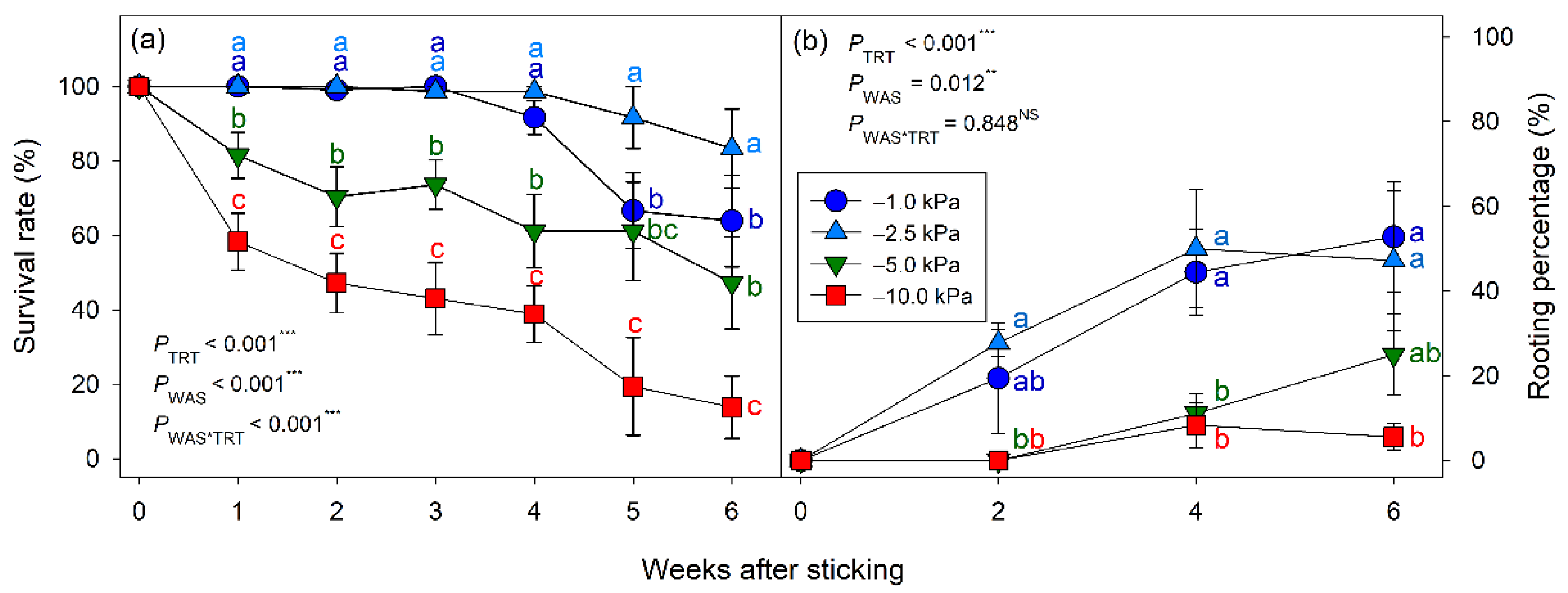
Conversely, an increase in the mortality rate of cuttings grown under −1.0 kPa irrigation thresholds could be attributed to excess moisture within the propagation substrate (
Figure 3a). Excessive substrate moisture can significantly affect the rooting media, leading to a lack of aeration and hypoxic conditions unsuitable for rootless cuttings. Other effects include a reduction in water uptake by the cuttings within the substrate before the formation of proper adventitious roots. This can inhibit aerobic respiration in the rhizosphere and impair plant growth [
22]. Additionally, unrooted cuttings under the −1.0 kPa treatment were found to have a brown-colored basal area, indicating the occurrence of basal rot caused by fungi most likely due to too-wet substrate conditions [
23]. A decrease in the substrate moisture will be followed by a decrease in the rooting percentage [
24]. Similarly, we observed an expectedly low rooting percentage of the cuttings grown below −5.0 kPa, which corresponded to their low survival rate (
Figure 3b). In contrast, cuttings grown in the ranges above −2.5 kPa demonstrated a higher rooting percentage as these irrigation levels provided sufficient substrate moisture to increase rooting. Rein et al. [
25] also reported an increased rooting percentage for woody cuttings of juniper, azalea, and holly under wet substrate moisture conditions. Information on the precise and quantitative substrate moisture level was, however, limited at the time. Overall, it may be concluded that, for a sufficient substrate moisture condition, the middle range of available water in substrate as −2.5 kPa is adequate to secure a higher survival rate and rooting of English lavender cuttings. Too wet (−1.0 kPa) or too dry (≤−5.0 kPa) substrate moisture conditions should be avoided to ensure the optimal survival of the cuttings.
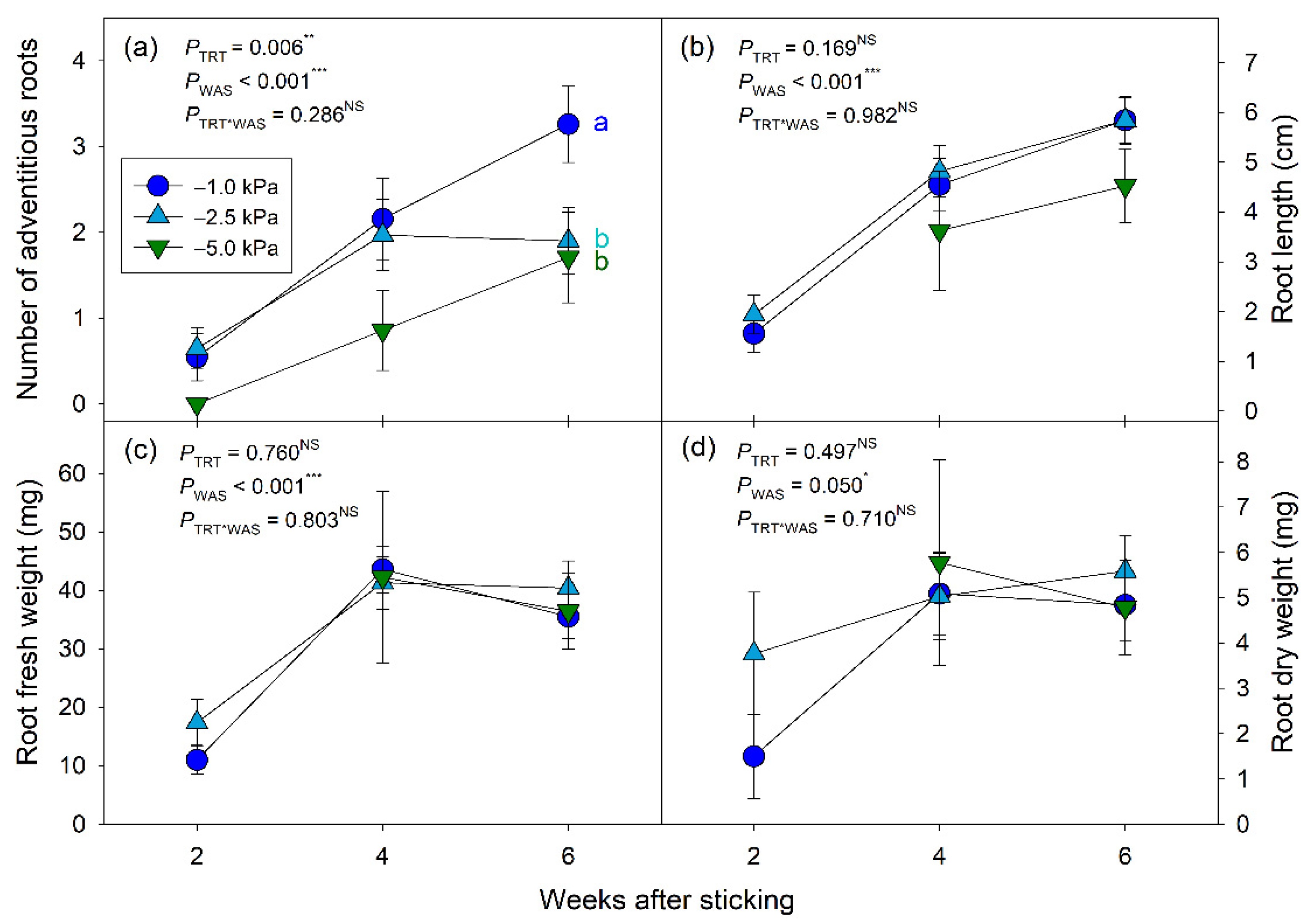
Establishing adventitious roots is an important process of cutting propagation to provide water and nutrient uptake for the cuttings [
1]. The results of this study suggest that high substrate moisture conditions favor faster development and a greater number of adventitious roots in English lavender cuttings. Previous studies showed that the initial formation of adventitious roots is regulated by the amount of moisture present in a given substrate [
26]. Puri and Thompson [
27] reported that, in cuttings of
Populus × euramericana (Dode) Guinier cv. ‘Robusta’, as substrate moisture levels increased, adventitious root number increased and the length of adventitious roots increased. However, there was no significant difference in other root growth parameters at the final harvest among the substrate moisture treatments (
Figure 4), indicating that high substrate moisture content may not be critically required for enhanced root growth after the formation of adventitious roots. In this case, more efficient irrigation practice can be applied for efficient root management of cuttings with saving water. Likewise, previous research with FDR-sensor-based automated irrigation systems reported improved water use efficiency by providing adequate moisture for the plants [
11,
28].
Previous studies with potted plants reported that higher threshold levels with sufficient irrigation produced more leaves and stronger shoot growth in several species, mostly due to sufficient turgor pressure in plant cells [
21,
24,
28]. Although the present study discussed cutting propagation, the trends in shoot growth under different irrigation threshold levels were similar to those reported in previous studies. However, the adventitious roots could assume the function in moisture absorption only after the rooting establishment of the cuttings. The fresh weight of the shoots of the cuttings was proportional to the irrigation threshold levels within a given propagation substrate [
25]; therefore, cuttings grown at lower moisture conditions would exhibit reduced turgor pressure and thereby a declining shoot fresh weight. This indicates that higher moisture conditions are required to promote shoot growth of English lavender cuttings than for rooting.
During the experimental period, the growth and development of roots and shoots varied over time and their optimum irrigation threshold levels were different. English lavender cuttings demonstrated intense root growth between 2 and 4 WAS, and the irrigation threshold level at –2.5 kPa provided sufficient moisture for rooting while avoiding substrate conditions that were too wet. Subsequently, the root growth was maintained, with the fresh and dry weight of the roots barely increasing. In contrast, the shoot growth of the English lavender cutting barely increased during the rooting process. However, after the roots were formed, the shoot growth markedly increased under sufficient moisture conditions. Contrary to the first rooting period, the irrigation threshold level at –1.0 kPa resulted in greater shoot growth, which was a higher moisture level than the optimum level for rooting.
5. Conclusions
In the present study, implementing an FDR-sensor-based sub-surface automated irrigation system allowed for a precise and quantitative investigation of the optimal substrate moisture level required for the cutting propagation of English lavender. Sufficient moisture (wetter than –5 kPa) secured the survival and rooting of English lavender cuttings. However, maintaining the substrate moisture level at –2.5 kPa (not too wet) avoided basal rot of unrooted cuttings, thereby increasing the survival rate. However, a wet substrate condition (−1.0 kPa) promoted shoot growth after the new root systems of the cuttings were established. Securing more cuttings with successful rooting is critical for cutting propagation. The results of this study indicated that maintaining the substrate moisture at –2.5 kPa is optimal for English lavender propagation from stem cuttings. The optimal substrate moisture levels for cutting propagation may differ between the period of adventitious root formation and after rooting. When a precise irrigation system is applied for the cutting propagation, the optimal substrate moisture levels would depend on the developmental stages of the cuttings, allowing for better growth of root and shoot parts, respectively. These findings are likely to apply to most other species that are propagated by cuttings, and further study to find the best irrigation management practice for cutting propagation of various species would be required.