Identification of Two Bacillus Strains with Antimicrobial Activity and Preliminary Evaluation of Their Biocontrol Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Condition
2.2. Determination of Antifungal Activity
2.3. Growth Condition of Pathogenic Fungi on Culture Medium Containing Different Concentrations of Bacillus
2.4. Identification of B5 and B6
2.5. The Growth Promotion Ability of B5 and B6 in Cabbage Seedlings
2.6. Biocontrol Efficacy of B5 and B6 in Potted Cabbage Seedlings Inoculated with F. oxysporum
2.7. Colonization Characteristics of B6 in Cabbage Seedlings and Soil
2.8. Statistical Analysis
3. Results
3.1. Determining the Antimicrobial Spectrum of Strains B5 and B6
3.2. Inhibition Effect of B5 and B6 Strains at Different Concentrations on Plant Pathogens
3.3. Identification of B5 and B6
3.4. Growth Promoting Ability of B5 and B6 in Cabbage Seedlings
3.5. Biocontrol Efficacy of B5 and B6 in Cabbage Seedlings Inoculated with F. oxysporum
3.6. Colonization Characteristics of B6 in Cabbage Seedlings and Soil
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, E.F. The fungus infestation of agricultural soils in the United States. Sci. Am. Suppl. 1899, 48, 1981–1982. [Google Scholar]
- Li, M.Y.; Zhang, T.T.; Li, X.H.; Yan, H. Wilt disease of cruciferous vegetables and its pathogen identification. Plant Prot. 2003, 29, 44–45. (In Chinese) [Google Scholar]
- Liu, X.; Ling, J.; Xiao, Z.L.; Xie, B.Y.; Fang, Z.Y.; Yang, L.M.; Zhang, Y.Y.; Lv, H.H.; Yang, Y.H. Characterization of emerging populations of Fusarium oxysporum f. sp. conglutinans causing cabbage wilt in China. J. Phytopathol. 2017, 165, 813–821. [Google Scholar]
- Gava, C.A.T.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control. 2016, 97, 13–20. [Google Scholar]
- Phong, N.H.; Pongnak, W.; Soytong, K. Antifungal activities of Chaetomium spp. against Fusarium wilt of tea. Plant Protect. Sci. 2016, 52, 10–17. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Zhang, J.X.; Gu, Y.B.; Chi, F.M.; Ji, Z.R.; Wu, J.Y.; Dong, Q.L.; Zhou, Z.S. Bacillus amyloliquefaciens GB1 can effectively control apple valsa canker. Biol. Control 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Alam, S.S.; Sakamoto, K.; Inubushi, K. Biocontrol efficiency of Fusarium wilt diseases by a root-colonizing fungus Penicillium sp. Soil Sci. Plant Nutr. 2011, 57, 204–212. [Google Scholar] [CrossRef]
- Rania, A.B.A.; Jabnoun-Khiareddine, H.; Nefzi, A.; Mokni-Tlili, S.; Daami-Remadi, M. Endophytic bacteria from Datura metel for plant growth promotion and bioprotection against Fusarium wilt in tomato. Biocontrol Sci. Technol. 2016, 26, 1139–1165. [Google Scholar] [CrossRef]
- Wang, J.; Cai, B.Y.; Li, K.; Zhao, Y.K.; Li, C.Y.; Liu, S.W.; Xiang, D.D.; Zhang, L.; Xie, J.H.; Wang, W. Biological control of Fusarium oxysporum f. sp. cubense tropical race 4 in banana plantlets using newly isolated Streptomyces sp. WHL7 from marine soft coral. Plant Dis. 2022, 106, 254–259. [Google Scholar]
- Obagwu, J.; Korsten, L. Integrated control of citrus green and blue molds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biol. Technol. 2003, 28, 187–194. [Google Scholar] [CrossRef]
- Li, H.Q.; Yue, H.W.; Li, L.; Liu, Y.; Zhang, H.Y.; Wang, J.H.; Jiang, X.W. Seed biostimulant Bacillus sp. MGW9 improves the salt tolerance of maize during seed germination. AMB Expr. 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Galeano, B. UV Resistance of Bacillus anthracis spores revisited: Validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis sterne. Appl. Environ. Microb. 2003, 69, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Roberts, D.P.; Maul, J.E.; Emche, S.E.; Liao, X.; Guo, X.L.; Liu, Y.Y.; McKenna, L.F.; Buyer, J.S.; Liu, S.Y. Formulations of the endophytic bacterium Bacillus subtilis Tu-100 suppress Sclerotinia sclerotiorum on oilseed rape and improve plant vigor in field trials conducted at separate locations. Can. J. Microbiol. 2011, 57, 539–546. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiraishi, S.; Suzuki, S. Are cyclic lipopeptides produced by Bacillus amyloliquefaciens S13-3 responsible for the plant defence response in strawberry against Colletotrichum gloeosporioides? Lett. Appl. Microbiol. 2015, 60, 379–386. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M.S. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Schofield, B.J.; Skarshewski, A.; Lachner, N.; Ouwerkerk, D.; Klieve, A.V.; Dart, P.; Hugenholtz, P. Near complete genome sequence of the animal feed probiotic, Bacillus amyloliquefaciens H57. Stand. Genom. Sci. 2016, 11, 60. [Google Scholar] [CrossRef]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef]
- Han, Q.; Wu, F.L.; Wang, X.N.; Qi, H.; Shi, L.; Ren, A.; Liu, Q.H.; Zhao, M.W.; Tang, C.M. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ. Microbiol. 2015, 17, 1166–1188. [Google Scholar] [CrossRef]
- Farzaneh, M.; Shi, Z.Q.; Ahmadzadeh, M.; Hu, L.B.; Ghassempour, A. Inhibition of the Aspergillus flavus growth and Aflatoxin B1 contamination on pistachio nut by fengycin and surfactin-producing Bacillus subtilis UTBSP1. Plant Pathol. J. 2016, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microb. 2005, 71, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Wu, Q.; Wang, J.L.; Liu, W.C.; Ren, J.H.; Zhang, D.P.; Zhao, J.; Liu, D.W.; Rao, Y.H.; Lu, C.G. Identification and comprehensive evaluation of a novel biocontrol agent Bacillus atrophaeus JZB120050. J. Environ. Sci. Health B 2018, 53, 777–785. [Google Scholar] [CrossRef]
- Pei, J.J.; Yue, T.L.; Jin, W.G. Application of bacteriocin RC20975 in apple juice. Food Sci. Technol. Int. 2016, 23, 166–173. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.W.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.Y.; Zhou, L.; Yi, Y.H.; Kuipers, O.P. Biocontrol properties from phyllospheric bacteria isolated from Solanum lycopersicum and Lactuca sativa and genome mining of antimicrobial gene clusters. BMC Genom. 2022, 23, 152. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.L.; Humza, M.; Geng, H.R.; Wang, G.; Zhang, C.X.; Gao, S.; Xing, F.G.; Liu, Y. A novel strain Lactobacillus brevis 8-2B inhibiting Aspergillus carbonarius growth and ochratoxin a production. LWT 2021, 136, 110308. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.L.; Zhang, M.F.; Xue, J.; Zhang, X.H. Pseudomonas aeruginosa Ld-08 isolated from Lilium davidii exhibits antifungal and growth-promoting properties. PLoS ONE 2022, 17, e0269640. [Google Scholar] [CrossRef]
- Dong, X.Z.; Cai, M.Y.B. Systematic Determinative Manual of General Bacteria; Science Press: Beijing, China, 2001; pp. 353–370. [Google Scholar]
- Wang, B.B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.X.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Chun, J.; Bae, K.S. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 2000, 78, 123–127. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Xi, N.; Lang, D.Y.; Zhou, L.; Zhang, Y.J.; Zhang, X.H. Potential biocontrol and plant growth promotion of an endophytic bacteria isolated from Glycyrrhiza uralensis seeds. Egypt. J. Biol. Pest Control 2022, 32, 55. [Google Scholar] [CrossRef]
- Li, E.F.; Wang, G.; Xiao, J.L.; Ling, J.; Yang, Y.H.; Xie, B.Y. A SIX1 homolog in Fusarium oxysporum f. sp. conglutinans is required for full virulence on cabbage. PLoS ONE 2016, 11, 0152273. [Google Scholar]
- Jing, C.L.; Zhao, J.; Han, X.B.; Huang, R.H.; Cai, D.S.; Zhang, C.S. Essential oil of Syringa oblata Lindl. As a potential biocontrol agent against tobacco brown spot caused by Alternaria alternata. Crop Prot. 2018, 104, 41–46. [Google Scholar] [CrossRef]
- Ding, T.; Su, B.; Chen, X.J.; Xie, S.S.; Gu, S.Y.; Wang, Q.; Huang, D.Y.; Jiang, H.Y. An endophytic bacterial strain isolated from Eucommia ulmoides inhibits Southern corn leaf blight. Front. Microbiol. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Liu, Z.P.; Wang, H.X.; Xu, W.H.; Wang, Z.G. Isolation and evaluation of the plant growth promoting rhizobacterium Bacillus methylotrophicus (DD-1) for growth enhancement of rice seedling. Arch. Microbiol. 2020, 202, 2169–2179. [Google Scholar] [CrossRef]
- Liu, X.M.; Li, Q.; Li, Y.B.; Guan, G.H.; Chen, S.F. Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ 2019, 7, e7445. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of plant growth-promoting bacteria Bacillus cereus YN917 for biocontrol of rice blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, J.X.; Tan, T.M.; Xu, J.P.; Shen, A.R.; Yang, X.B.; Li, J.L.; Zeng, L.B.; Wei, L. Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against Fusarium wilt of cucumber seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009, 140, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.M.M.; Gomaa, N.M.; Essa, A.M.M. Application of endophytic bacteria for the biocontrol of Rhizoctonia solani (Cantharellales: Ceratobasidiaceae) damping-off disease in cotton seedlings. Biocontrol Sci. Technol. 2017, 27, 81–95. [Google Scholar] [CrossRef]
- Beauregard, P.B. Chapter One—Not just sweet talkers: How roots stimulate their colonization by beneficial bacteria. Adv. Bot. Res. 2015, 75, 1–20. [Google Scholar]
- Raaijmakers, J.M.; Bruijn, I.D.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 346, 1037–1062. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Coy, R.M.; Held, D.W.; Kloepper, J.W. Rhizobacterial colonization of bermudagrass by Bacillus spp. in a Marvyn loamy sand soil. Appl. Soil Ecol. 2019, 141, 10–17. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Steinkellner, S.; Mammerler, R.; Vierheilig, H. Germination of Fusarium oxysporum in root exudates from tomato plants challenged with different Fusarium oxysporum strains. Eur. J. Plant Pathol. 2008, 122, 398–401. [Google Scholar] [CrossRef]
- Fan, B.; Borriss, R.; Bleiss, W.; Wu, X.Q. Gram-positive rhizobacterium Bacillus amyloliquefaciens FZB42 colonizes three types of plants in different patterns. J. Microbiol. 2012, 50, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pandic, C.; Coq, D.L.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Rueckert, C.; Niu, B.; Wang, Q.; Borriss, R. The complete genome of Bacillus amyloliquefaciens subsp. plantarum CAU B946 contains a gene cluster for nonribosomal synthesis of Iturin A. J. Bacteriol. 2012, 194, 1845–1846. [Google Scholar] [PubMed]
- Posada, L.F.; Álvarez, J.C.; Romero-Tabarez, M.; de-Bashan, L.; Villegas-Escobar, V. Enhanced molecular visualization of root colonization and growth promotion by Bacillus subtilis EA-CB0575 in different growth systems. Microbiol. Res. 2018, 217, 69–80. [Google Scholar] [CrossRef]
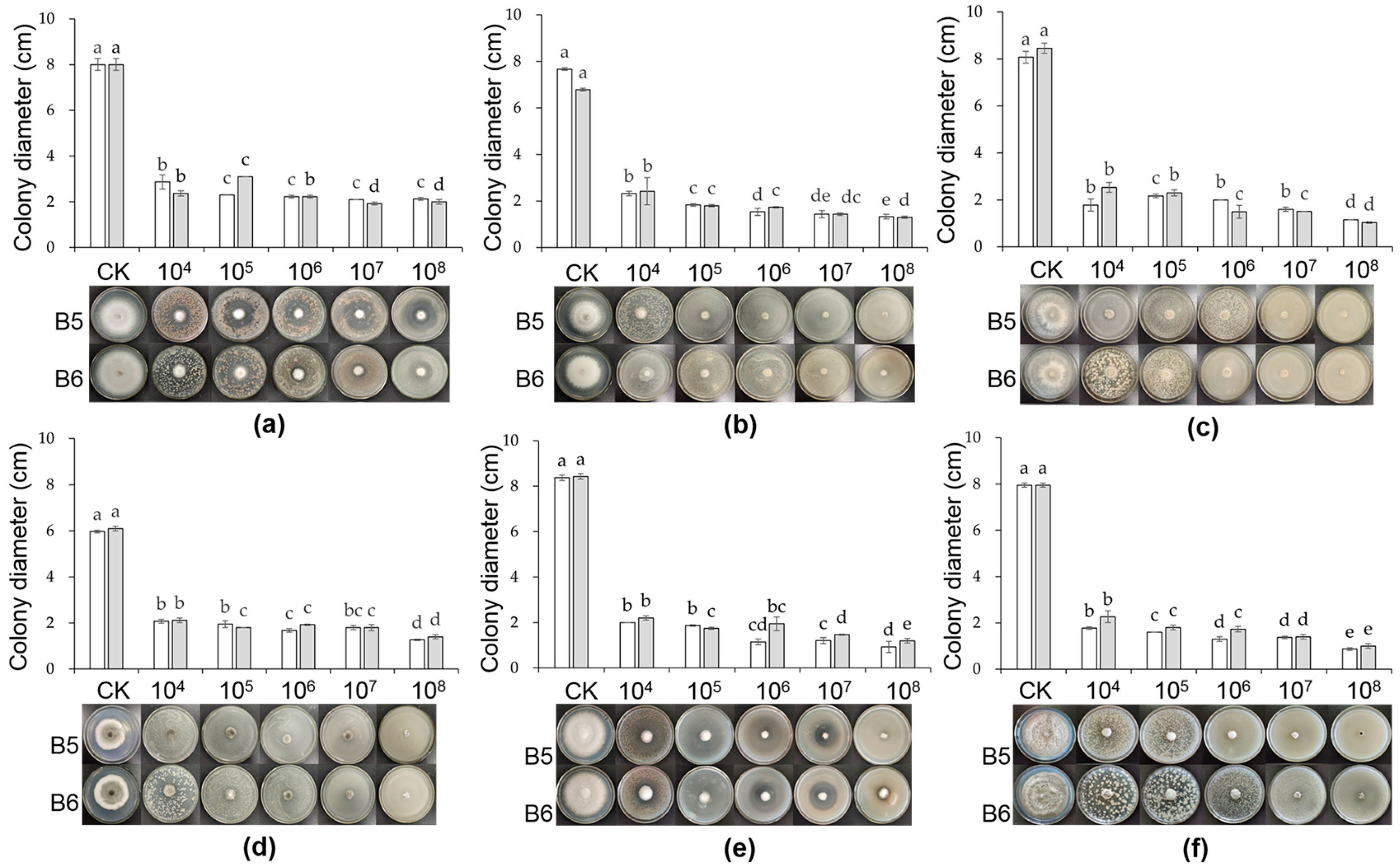

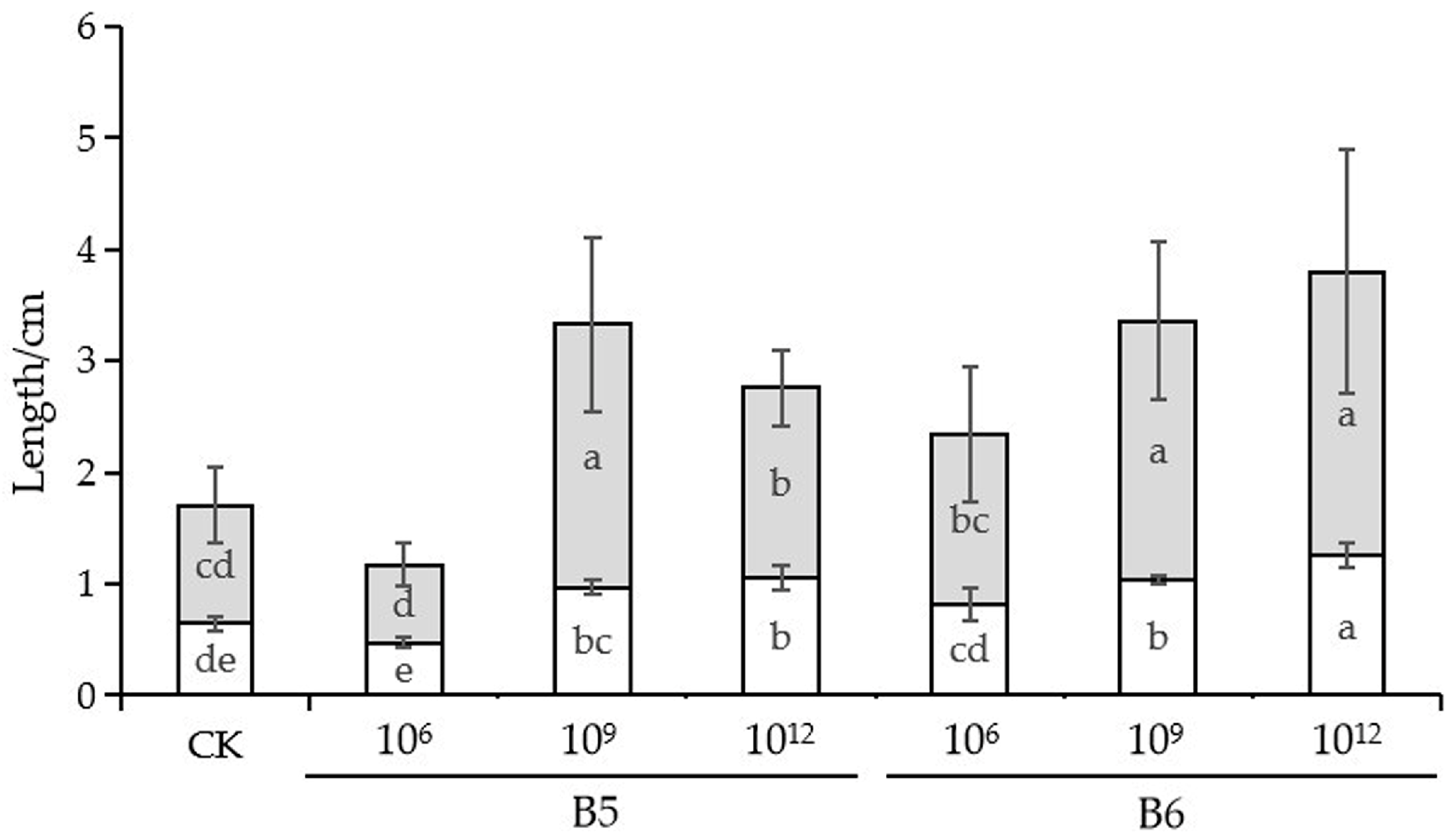
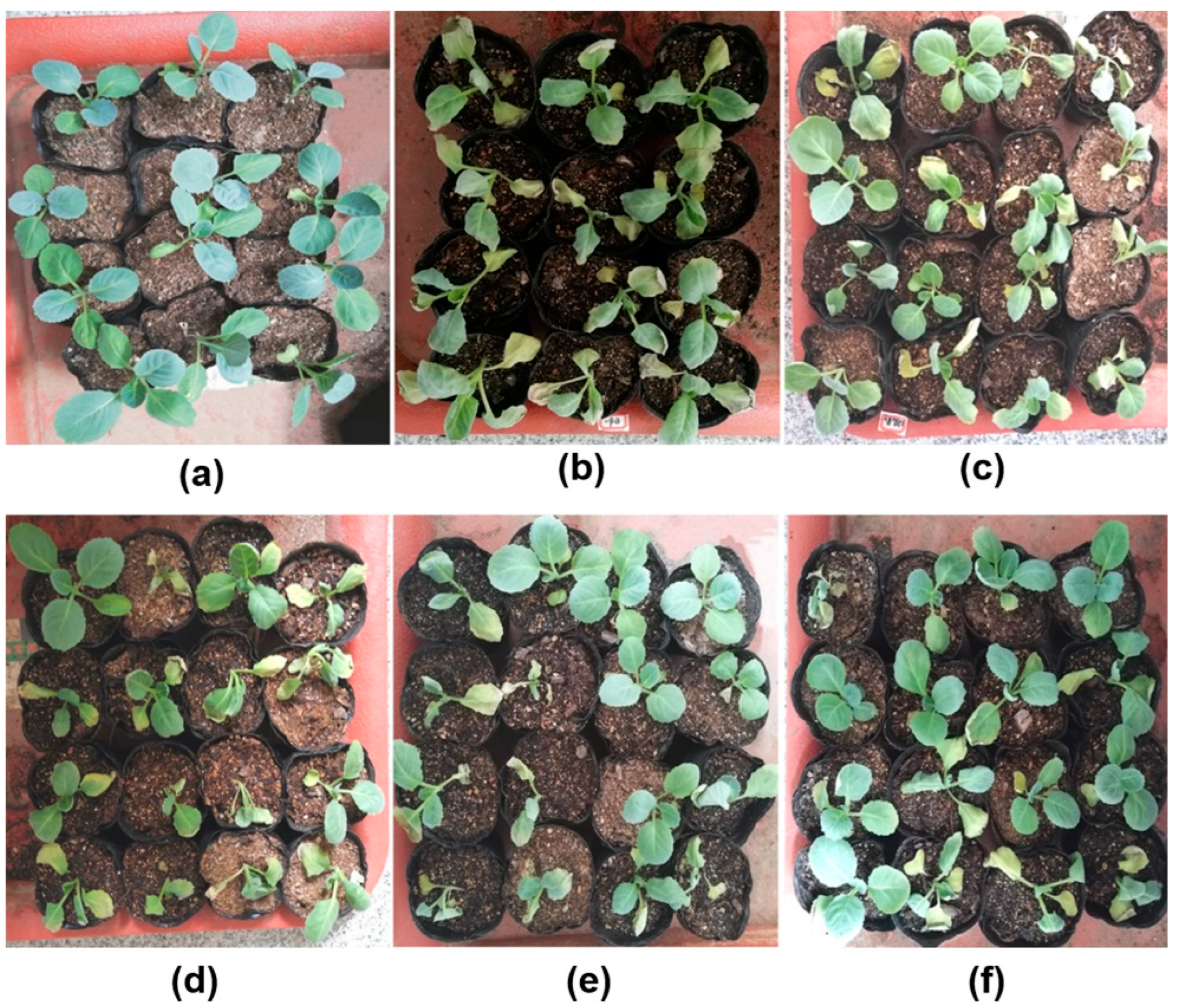
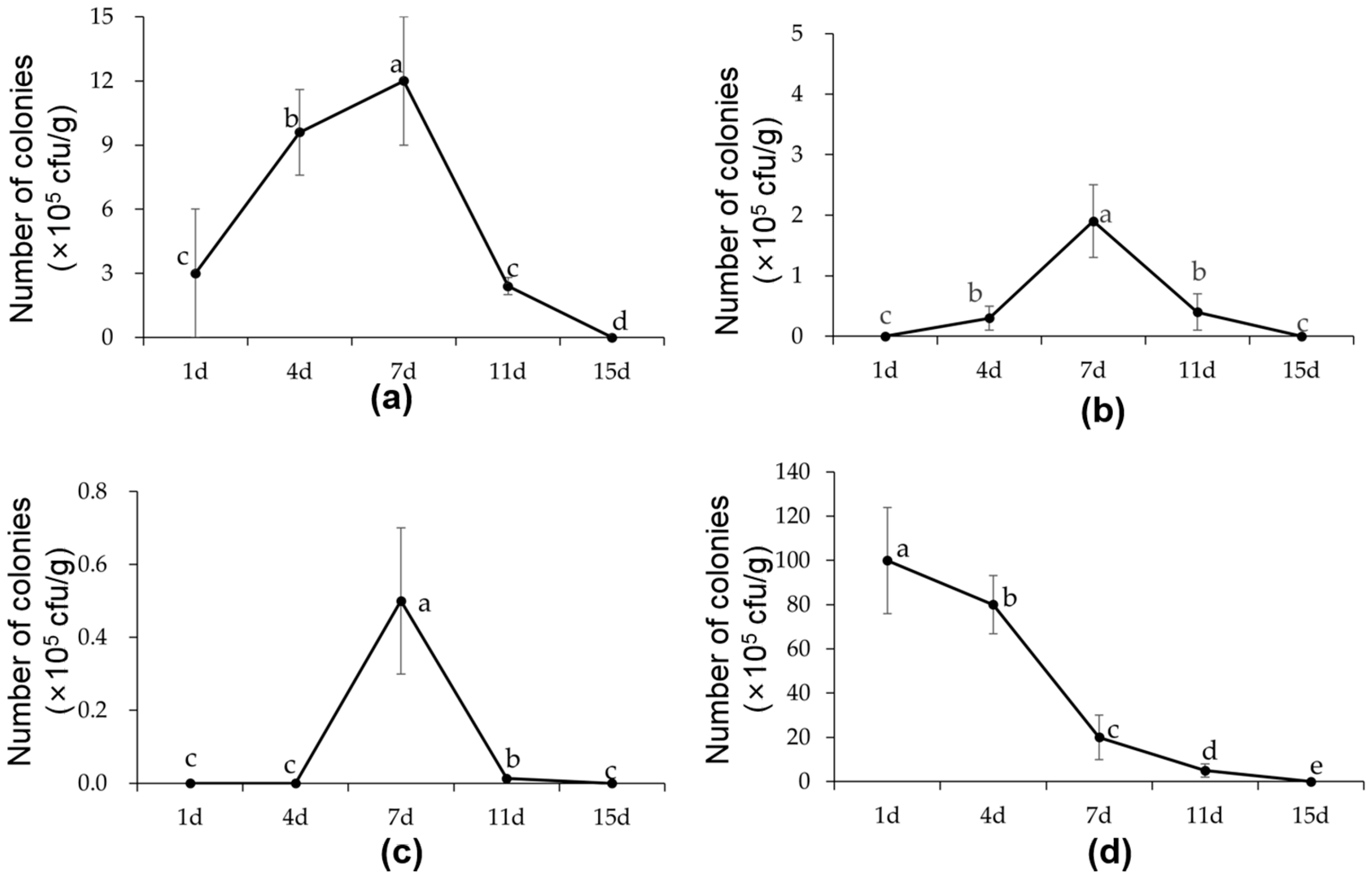
| Strains | Inhibition Effects 1 | ||||||
|---|---|---|---|---|---|---|---|
| F. oxysporum f. sp. niveum | F. oxysporum f. sp. vasinfectum | F. oxysporum f. sp. cucumerinum | F. oxysporum f. sp. conglutinans Race 1 | F. oxysporum f. sp. conglutinans Race 2 | Botrytis cinerea | Altenaria alternata | |
| B5 | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| B6 | +++ | +++ | +++ | ++ | ++ | + | + |
| Treatment (CFU/mL) | |||||||
|---|---|---|---|---|---|---|---|
| CK | B5 | B6 | |||||
| 106 | 109 | 1012 | 106 | 109 | 1012 | ||
| Germination rate | 85% a | 90% a | 100% b | 100% b | 90% a | 100% b | 100% b |
| Treatments 1 | Disease Index | Control Effect/% |
|---|---|---|
| A | 0 | − |
| B | 60.42 a | − |
| C | 39.58 b | 34.49 a |
| D | 47.92 b | 20.69 a |
| E | 43.75 b | 27.59 a |
| F | 27.08 c | 55.18 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Li, Y.; Dai, X.; Yan, W.; Wang, G. Identification of Two Bacillus Strains with Antimicrobial Activity and Preliminary Evaluation of Their Biocontrol Efficiency. Horticulturae 2022, 8, 744. https://doi.org/10.3390/horticulturae8080744
Li E, Li Y, Dai X, Yan W, Wang G. Identification of Two Bacillus Strains with Antimicrobial Activity and Preliminary Evaluation of Their Biocontrol Efficiency. Horticulturae. 2022; 8(8):744. https://doi.org/10.3390/horticulturae8080744
Chicago/Turabian StyleLi, Erfeng, Yuxin Li, Xinyu Dai, Wanrong Yan, and Gang Wang. 2022. "Identification of Two Bacillus Strains with Antimicrobial Activity and Preliminary Evaluation of Their Biocontrol Efficiency" Horticulturae 8, no. 8: 744. https://doi.org/10.3390/horticulturae8080744






