Discovering the Health Promoting Potential of Fermented Papaya Preparation—Its Future Perspectives for the Dietary Management of Oxidative Stress During Diabetes
Abstract
:1. Introduction
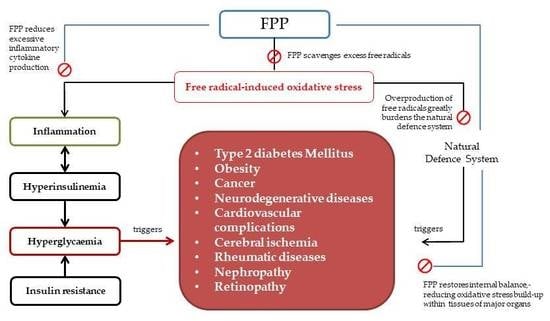
2. The Concept of Oxidative Stress as a Unique Therapeutic Pathway by Nutraceuticals for the Management of Type 2 Diabetes
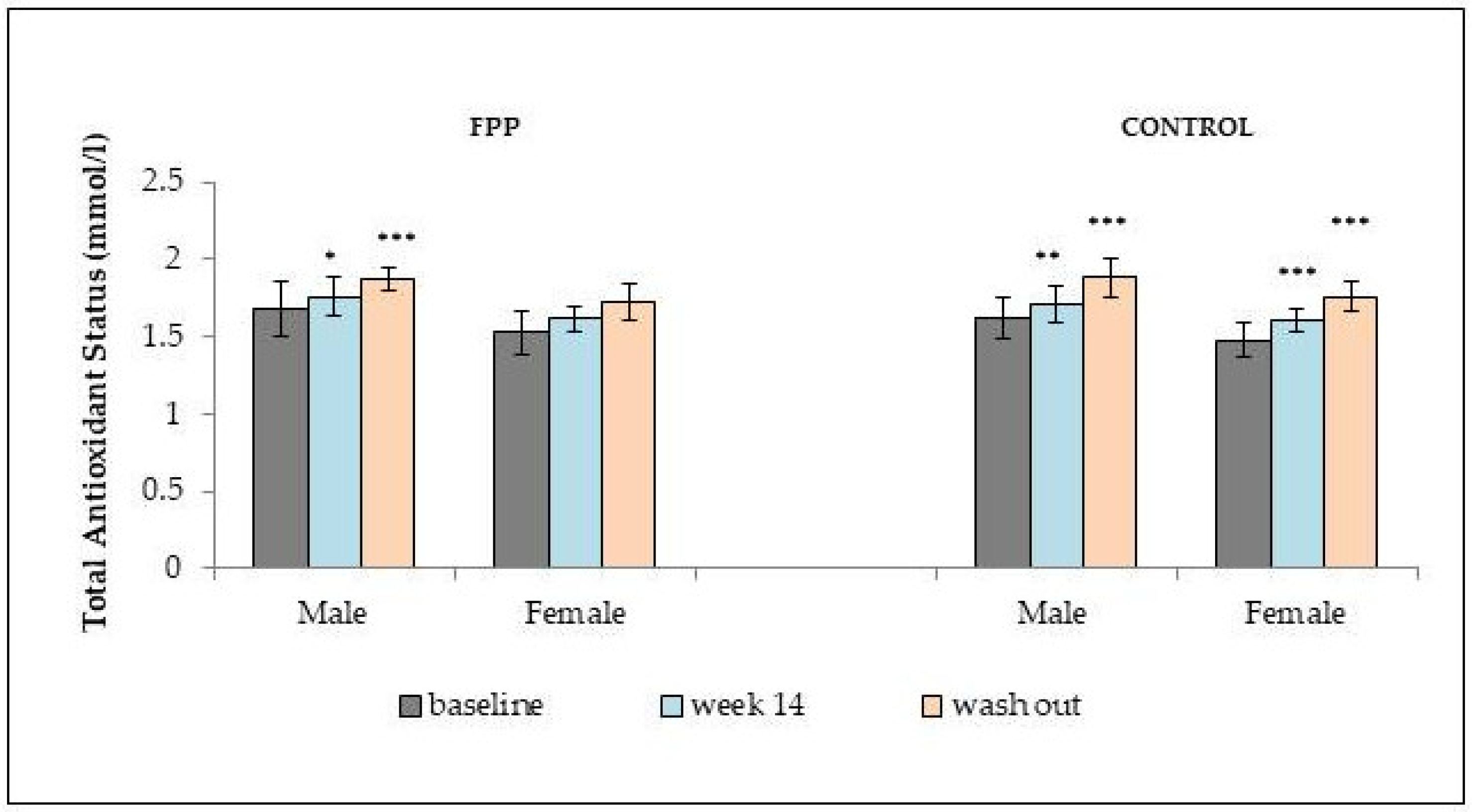
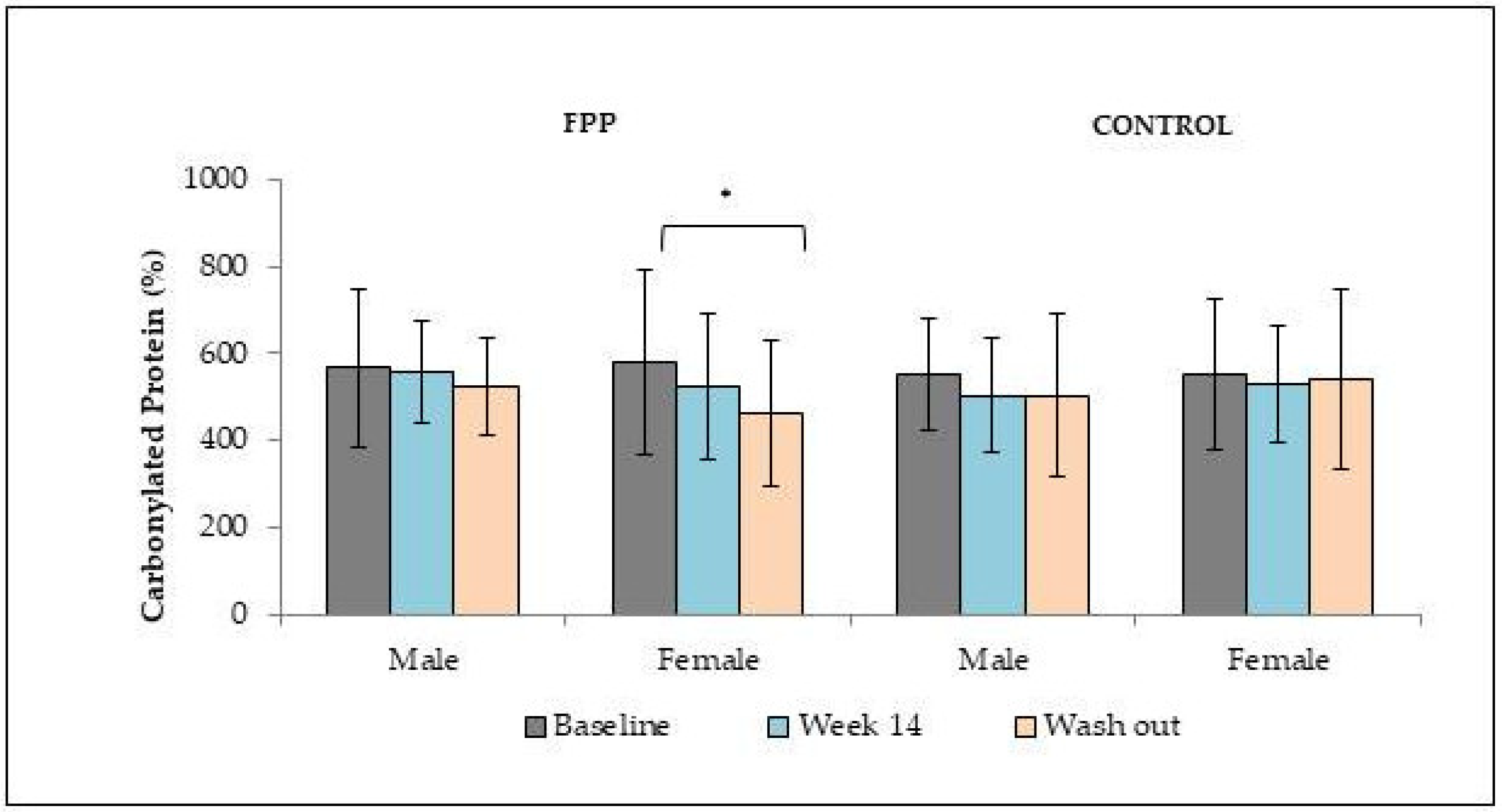
2.1. Interaction of FPP at the Physiological and Organ System Levels in Diabetes
2.2. Anti-Inflammatory and Immuno-Modulatory Effects of FPP in Diabetic Conditions
3. Attenuating Type 2 Diabetes Associated Diseases Using the Anti-Oxidant Properties of FPP
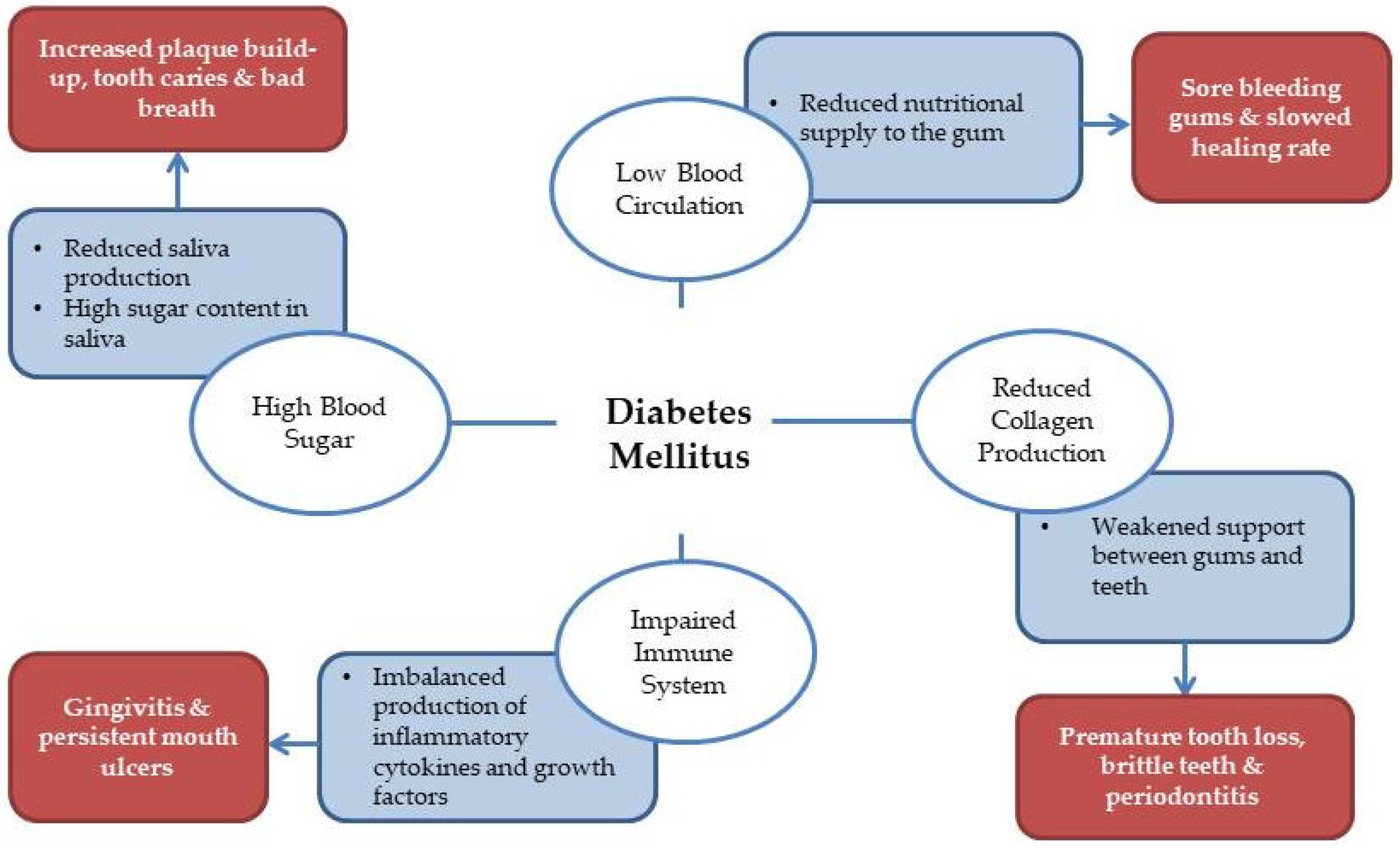
Oral Health Challenges Amongst the Diabetic Community: Examining the Anti-Cariogenic Potential of FPP
4. Appreciating the Anti-Cancer Effects Precipitated by FPP
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, D.; Chattopadhyay, P. Preparation of idli batter, its properties and nutritional improvement during fermentation. J. Food Sci. Technol. 2011, 48, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Ichise, H.; Kakuda, H.; Yamaguchi, M. Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and gamma-carboxylated osteocalcin concentration in normal individuals. J. Bone Miner. Metab. 2000, 18, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Babu, P.; Bhakyaraj, R.; Vidhyalakshmi, R. A low-cost nutritious food “Tempeh”—A review. World J. Diary Food Sci. 2009, 4, 22–27. [Google Scholar]
- Montagnac, J.; Davis, C.; Tanumihardjo, S. Processing Techniques to Reduce Toxicity and Anti nutrients of Cassava for Use as a Staple Food. Compr. Rev. Food Sci. Food Saf. 2009, 8, 17–27. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Yuki, H.; Marotta, F.; Mantello, P.; Rachmilewitz, E.A.; Montagnier, L. Applications and bioefficacy of the functional food supplement fermented papaya preparation. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Park, Y.C.; Guo, Q.; Moini, H.; Qureshi, N.; Saliou, C.; Takayama, K.; Virgili, F.; Packer, L. Nitric oxide synthesis and TNF-alpha secretion in RAW 264.7 macrophages: Mode of action of a fermented papaya preparation. Life Sci. 2000, 67, 679–694. [Google Scholar] [CrossRef]
- Fibach, E.; Ginsburg, I. The Antioxidant Effect of Fermented Papaya Preparation in the Oral Cavity. Phytother. Res. 2015, 29, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Tsuno, H.; Nakayama, J. Fermented Papaya Preparation Restores Age-Related Reductions in Peripheral Blood Mononuclear Cell Cytolytic Activity in Tube-Fed Patients. PLoS ONE 2017, 12, e0169240. [Google Scholar] [CrossRef] [PubMed]
- Maisarah, A.M.; Asmah, R.; Fauziah, O. Proximate analysis, antioxidant and antiproliferative activities of different parts of Carica papaya. J. Nutr. Food Sci. 2014, 4, 267. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J. Sci. Food Agric. 2003, 83, 496–502. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography–UV detection–mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Moussa, S.A. Oxidative stress in diabetes mellitus. Rom. J. Biophys. 2008, 18, 225–236. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Arch. Biochem. Biophys. 2002, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, K.; Puri, S. Free radicals and antioxidants in health and disease. East Mediterr. Health J. 1998, 4, 350–360. [Google Scholar]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2010, 52, 102–110. [Google Scholar] [CrossRef]
- Finegood, D.T.; Mcarthur, M.D.; Kojwang, D.; Thomas, M.J.; Topp, B.G.; Leonard, T.; Buckingham, R.E. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 2001, 50, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kirwan, J.P. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.S.; Little, J.P.; Tarnopolsky, M.A.; Myslik, F.; Gibala, M.J. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med. Sci. Sports Exerc. 2011, 43, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Mshelia, D.S. Role of free radicals in pathogenesis of diabetes nephropathy. Ann. Afr. Med. 2004, 3, 55–62. [Google Scholar]
- Oloffson, E.A.; Marlund, S.L.; Behnoig, A. Enhanced diabetes induced cataract in copper zinc superoxide dismutase-null mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil. Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Pandeya, K.B.; Tripathi, I.P.; Mishra, M.K.; Dwivedi, N.; Pardhi, Y.; Kamal, A.; Gupta, P.; Dwivedi, N.; Mishra, C. A Critical Review on Traditional Herbal Drugs: An Emerging Alternative Drug for Diabetes. Int. J. Org. Chem. 2013, 3, 1–22. [Google Scholar] [CrossRef]
- Oboh, G.; Olabiyi, A.A.; Akinyemi, A.J.; Ademiluyi, A.O. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water-extractable phytochemicals from unripe pawpaw fruit (Carica papaya). J. Basic Clin. Physiol. Pharmacol. 2013, 30, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Danese, C.; Espoisto, D.; D’alfonso, V.; Civene, M.; Ambrosino, M.; Colotto, M. Plasma glucose level decreases as a collateral effect of fermented papaya preparation use. Clin. Ther. 2006, 157, 195–198. [Google Scholar]
- Collard, E.; Roy, S. Improved function of diabetic wound site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid. Redox. Signal 2010, 13, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Lakey, W.C.; Barnard, K.; Batch, B.C.; Chiswel, L.K.; Tasneem, A.; Green, J.B. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia 2013, 56, 1226–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somanah, J.; Bourdon, E.; Bahorun, T.; Aruoma, O.I. The inhibitory effect of a fermented papaya preparation on growth, hydrophobicity, and acid production of Streptococcus mutans, Streptococcus mitis, and Lactobacillus acidophilus: Its implications in oral health improvement of diabetics. Food Sci. Nutr. 2013, 1, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T.; Aruoma, O.I. Relationship between fermented papaya preparation supplementation, erythrocyte integrity and antioxidant status in pre-diabetics. Food Chem. Toxicol. 2014, 65, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Rosenbaum, H.; Fibach, E.; Rachmilewitz, E.A. Decreased hemolysis following administration of antioxidant—fermented papaya preparation (FPP) to a patient with PNH. Ann. Hematol. 2010, 89, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Aruoma, O.I.; Gunness, T.K.; Kowelssur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Bourdon, E.; et al. Effects of a short term supplementation of a fermented papaya preparation on biomarkers of diabetes mellitus in a randomized Mauritian population. J. Prev. Med. 2012, 54, S90–S97. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Uno, K.; Kishida, T.; Miyagawa, F.; Osato, J.A.; Mori, A. Effect of Immun’Age on serum components and immunological functions in humans. Neurosciences 1994, 20, 149–152. [Google Scholar]
- Trojak, A. Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes- Gender Differentiation in Determinants. J. Diabetes Metab. 2015, 6, 476. [Google Scholar] [CrossRef]
- Holmes, M.V.; Lange, L.A.; Palmer, T.; Lanktree, M.B.; North, K.E.; Almoguera, B.; Buxbaum, S.; Chandrupatla, H.R.; Elbers, C.C.; GUO, Y.; et al. Causal effects of body mass index on cardiometabolic traits and events: A Mendelian randomization analysis. Am. J. Hum. Genet. 2014, 94, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Morinaga, H.; Talukdar, S.; Bae, E.J.; Olefsky, J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012, 61, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Bourdon, E.; Bahorun, T. Extracts of Mauritian Carica papaya (var. solo) protect SW872 and HepG2 cells against hydrogen peroxide induced oxidative stress. J. Food Sci. Technol. 2017, 54, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabetic Med. 2006, 23, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a casual role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, F.; Nanetti, L.; Montecchiani, G.; Borroni, F.; Salvolini, E.; Faloia, E.; Ferretti, G.; Mazzanti, L.; Vignini, A. In vitro effects of fermented papaya (Carica papaya, L.) on platelets obtained from patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Colognato, R.; Fontana, I.; Gartlon, J.; Migliore, L.; Koike, K.; Coecke, S.; Lamy, E.; Mersch-Sundernann, V.; Laurenz, I.; et al. Molecular effects of a fermented papaya preparation on oxidative damage, MAP kinase activation and modulation of the benzo [a] pyrene mediated genotoxicity. Biofactors 2006, 26, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Lee, M.C.I.; Kobayashi, K.; Hayashi, Y.; Aruoma, O.I. Assessment of the effect of a fermented papaya preparation on oxidative damage in spontaneously hypertensive rat (SHR) brain using electron spin resonance (ESR) imaging and L-band spectroscopy. J. Funct. Food 2009, 1, 375–380. [Google Scholar] [CrossRef]
- Marotta, F.; Koike, K.; Lorenzetti, A.; Jain, S.; Signorelli, P.; Metugriachuk, Y.; Mantello, P.; Locorotondo, N. Regulating redox balance gene expression in healthy individuals by nutraceuticals: A pilot study. Rejuv. Res. 2010, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Calvache, J.; Cueto, M.; Farroni, A.; De Escalada, P.M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from Papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Scheen, A.J. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 1996, 30, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; Fuente, L.F.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Cao, H.; Wang, Y.; Xiao, J. Dietary Polyphenols and Type 2 Diabetes: Human Study and Clinical Trial. Free Radic. Biol. Med. 2017, 112, 158. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo’, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlur, K.R.; Carani, V.A. Polyphenols activate energy sensing network in insulin resistant models. Chem. Biol. Interact. 2017, 275, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Shivavedi, N.; Kumar, M.; Tej, G.N.V.C.; Nayak, P.K. Metformin and ascorbic acid combination therapy ameliorates type 2 diabetes mellitus and comorbid depression in rats. Brain Res. 2017, 1674, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Prasad, S.; Gupta, A.; Singh, V.B. Oral manifestations in type 2 diabetes and related complications. Indian J. Endocrinol. Metab. 2012, 16, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Sano, T.; Kodama, Y.; Ozaki, K.; Matsura, T. Allozan-induced hyoerglycemia causes rapid onset and progressive dental caries and periodontitis in F344 rats. Histol. Histopathol. 2012, 27, 1297–1306. [Google Scholar] [PubMed]
- Kodama, Y.; Matsurura, M.; Sanyo, T.; Nakaraha, Y.; Ozaki, K.; Narama, I.; Matsuura, T. Diabetes enhances dental caries and apical periodontitis in caries susceptible WBN/KobSIc rats. J. Complement. Med. 2011, 61, 53–59. [Google Scholar]
- Sano, T.; Matsuura, T.; Ozaki, K.; Narama, I. Dental caries and caries-related periodontitis in type 2 diabetic mice. Vet. Pathol. 2011, 48, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.A. Glucose in the saliva of the non-diabetic and the diabetic patient. Arch. Oral Biol. 1965, 10, 197–205. [Google Scholar] [CrossRef]
- Loë, H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hotwani, K.; Baliga, S.; Sharma, K. Phytodentistry: Use of medicinal plants. J. Complement. Integr. Med. 2014, 11, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Clinical dental plaque fermentation and its role in caries risk assessment. Int. J. Dent. 2006, 8, 34–40. [Google Scholar]
- Marotta, F.; Naito, Y.; Jain, S.; Lorenzetti, A.; Soresi, V.; Kumari, A.; Carrera Bastos, P.; Tomella, C.; Yadav, H. Is there a potential application of a fermented nutraceutical in acute respiratory illnesses? An in-vivo placebo-controlled, cross-over clinical study in different age groups of healthy subjects. J. Biol. Regul. Homeost. Agents 2012, 26, 285–294. [Google Scholar] [PubMed]
- Kharaeva, Z.F.; Zhanimova, LR.; Mustafaev, M.S.; De Luca, C.; Mayer, W.; Thai, J.C.S.; Tuan, R.T.S.; Korkina, L.G. Effects of Standardised Fermented Papaya Gel on Clinical Symptoms, Inflammatory Cytokines, and Nitric Oxide Metabolites in Patients with Chronic Periodontitis: An Open Randomized Clinical Study. Mediators Inflamm. 2016, 2016, 9379840. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Bhardwaj, S.V. Papacarie® containing papain: A natural chemomechanical caries removal agent. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 660–665. [Google Scholar]
- Aruoma, O.I.; Neergheen, V.S.; Bahorun, T.; Jen, L.-S. Free Radicals, Antioxidants and Diabetes: Embryopathy, Retinopathy, Neuropathy, Nephropathy and Cardiovascular Complications. Neuroembryol. Aging 2006, 4, 117–137. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, Y.; Shen, W.-T.; Zhou, P. Content determination of benzyl glucosinolate and anti-cancer activity of its hydrolysis product in Carica papaya L. Asian Pac. J. Trop. Dis. 2012, 5, 231–233. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Shaw, P.N.; Parat, M.O.; Hewavitharana, A.K. Anticancer activity of Carica Papaya: A Review. Mol. Nutr. Food Res. 2013, 57, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ng, S.-C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 1999, 284, 696–704. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Pazhanisamy, S.K.; Li, H.; Meng, A.; Zhou, D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Rad. Biol. Med. 2010, 48, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkindel, L.; Osato, J.A.; Chivilyema, I.; Samocravyova, E.; Cheremisina, Z.; Afanas’ev, I. Radioproetctive and antioxidant effects of zinc aspartate and fpp in aspartate and fpp in Children with acute myelo-iympholeukemia. Nutrition 1995, 11, 555–558. [Google Scholar]
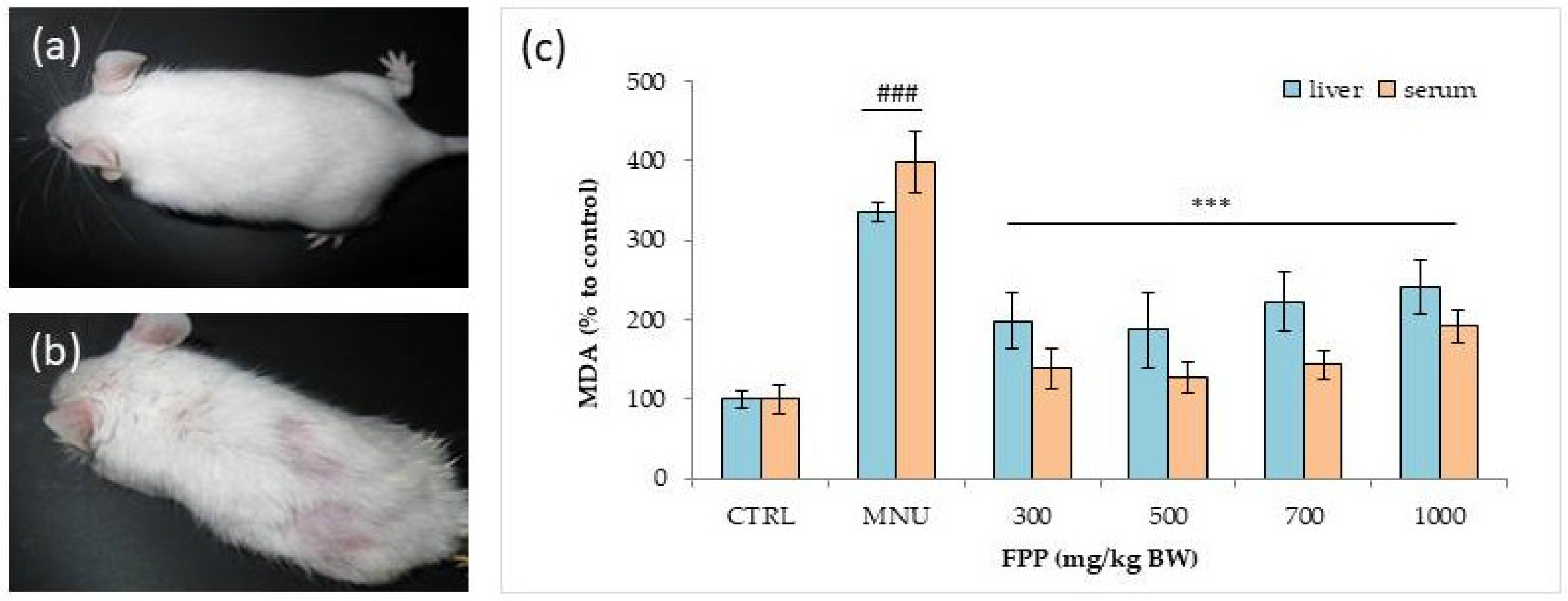
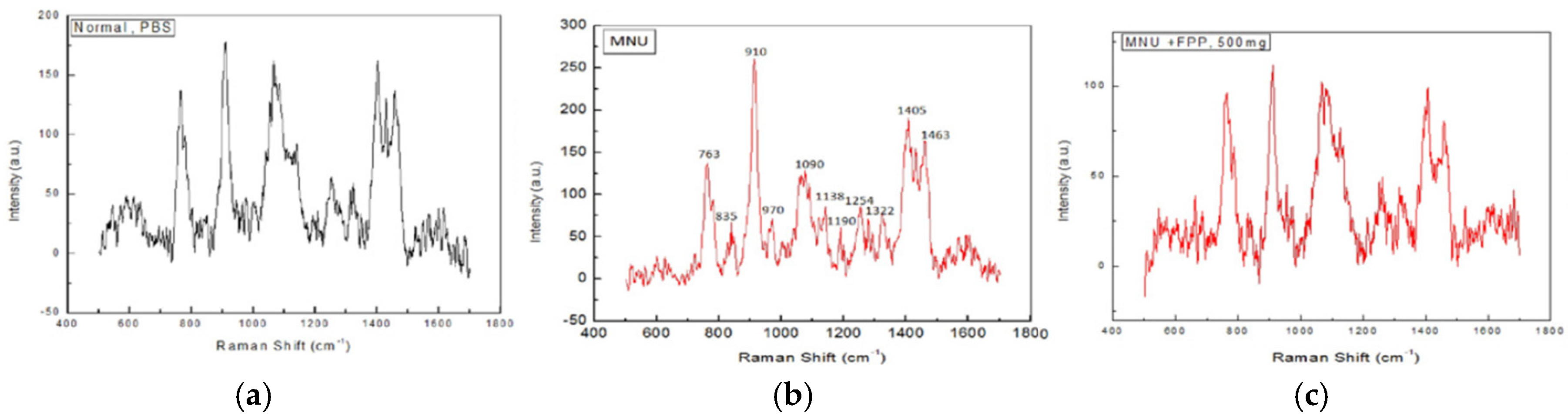
- Somanah, J.; Ramsaha, S.; Verma, S.; Kumar, A.; Sharma, P.; Singh, R.K.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Fermented papaya preparation modulates the progression of n-methyl-n-nitrosourea induced hepatocellular carcinoma in Balb/C mice. Life Sci. 2016, 151, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Imao, K.; Wang, H.; Komatsu, M.; Hiramatsu, M. Free radical scavenging activity of fermented papaya preparation and its effect on lipid peroxide level and superoxide dismutase activity in iron-induced epileptic foci of rats. Biochem. Mol. Biol. Int. 1998, 45, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.; Bahorun, T.; Singh, R.K.; Aruoma, O.I.; Kumar, A. Effect of aegle marmelos leaf extract on n-methyl n-nitrosourea-induced hepatocarcinogensis in Balb/C Mice. Pharm. Biol. 2013, 51, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.D.; Thornton, T.M.; Sabio, G.; Davis, R.A.; Rincon, M. Nuclear localization of p38 MAPK in response to DNA damage. Int. J. Biol. Sci. 2007, 5, 428–437. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somanah, J.; Putteeraj, M.; Aruoma, O.I.; Bahorun, T. Discovering the Health Promoting Potential of Fermented Papaya Preparation—Its Future Perspectives for the Dietary Management of Oxidative Stress During Diabetes. Fermentation 2018, 4, 83. https://doi.org/10.3390/fermentation4040083
Somanah J, Putteeraj M, Aruoma OI, Bahorun T. Discovering the Health Promoting Potential of Fermented Papaya Preparation—Its Future Perspectives for the Dietary Management of Oxidative Stress During Diabetes. Fermentation. 2018; 4(4):83. https://doi.org/10.3390/fermentation4040083
Chicago/Turabian StyleSomanah, Jhoti, Manish Putteeraj, Okezie I. Aruoma, and Theeshan Bahorun. 2018. "Discovering the Health Promoting Potential of Fermented Papaya Preparation—Its Future Perspectives for the Dietary Management of Oxidative Stress During Diabetes" Fermentation 4, no. 4: 83. https://doi.org/10.3390/fermentation4040083