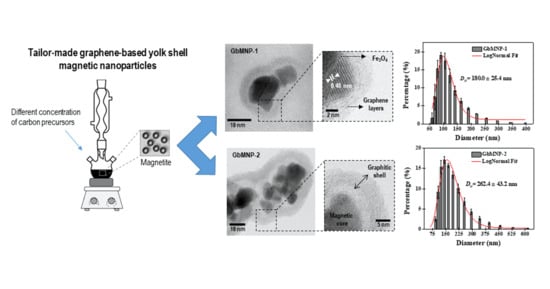
A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of GbMNPs
2.3. Colloidal Stabilization of GbMNPs
2.4. Characterization of GbMNPs
2.5. Drug Loading Studies
2.6. In Vitro pH-Dependent Drug Release and Kinetics Studies
2.7. In Vitro Biostudies
2.7.1. Cell Culture
2.7.2. Biocompatibility and Cellular Drug-Delivery Assay
3. Results and Discussion
3.1. Synthesis and Characterization of the GbMNPs
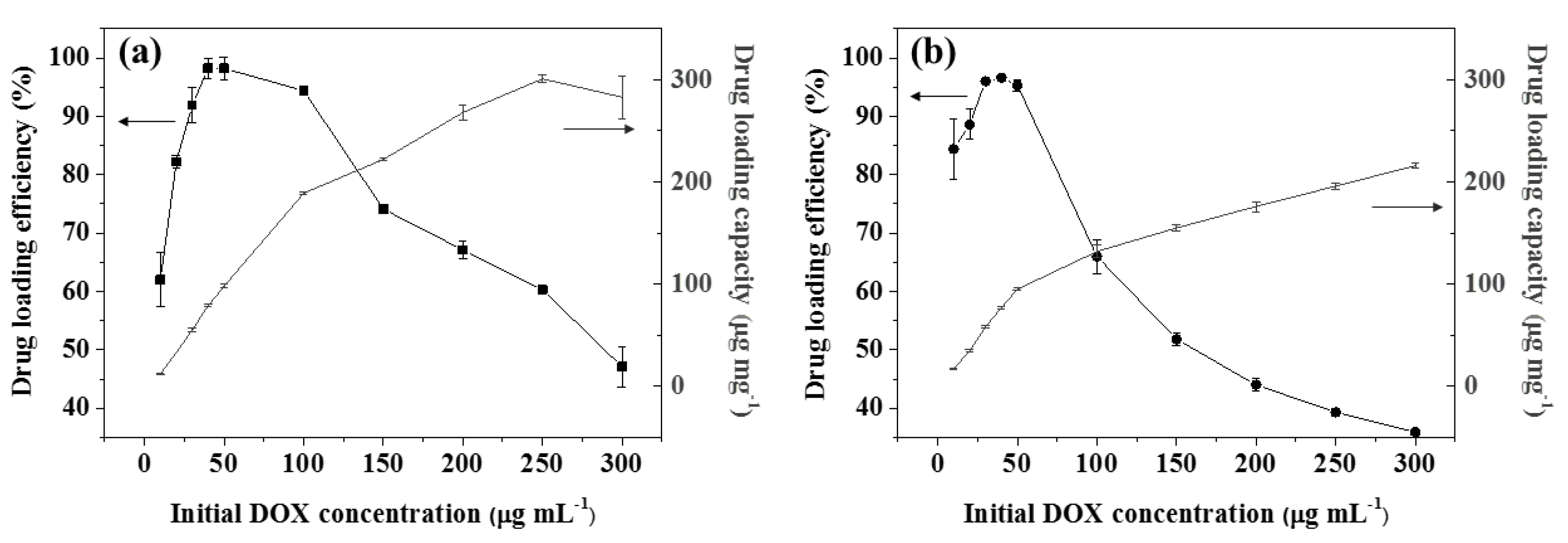
3.2. DOX Loading Studies
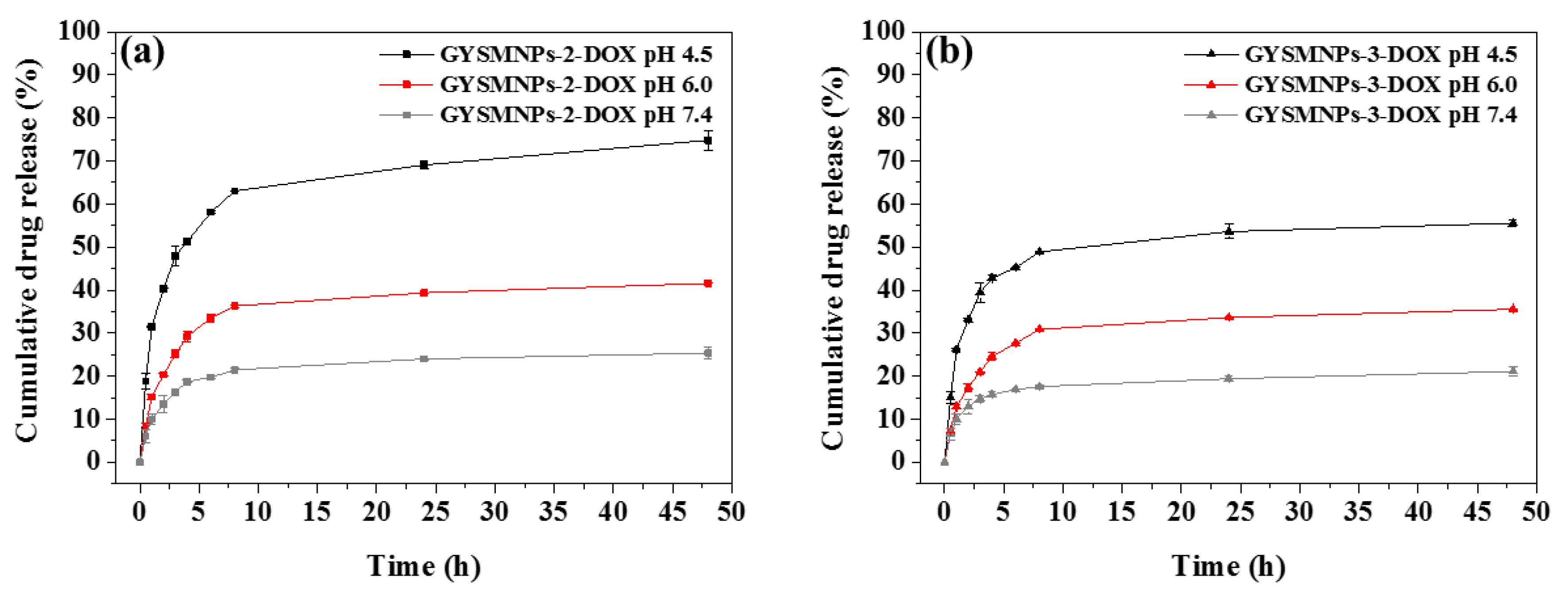
3.3. In Vitro pH-Dependent Drug Release and Kinetics Studies
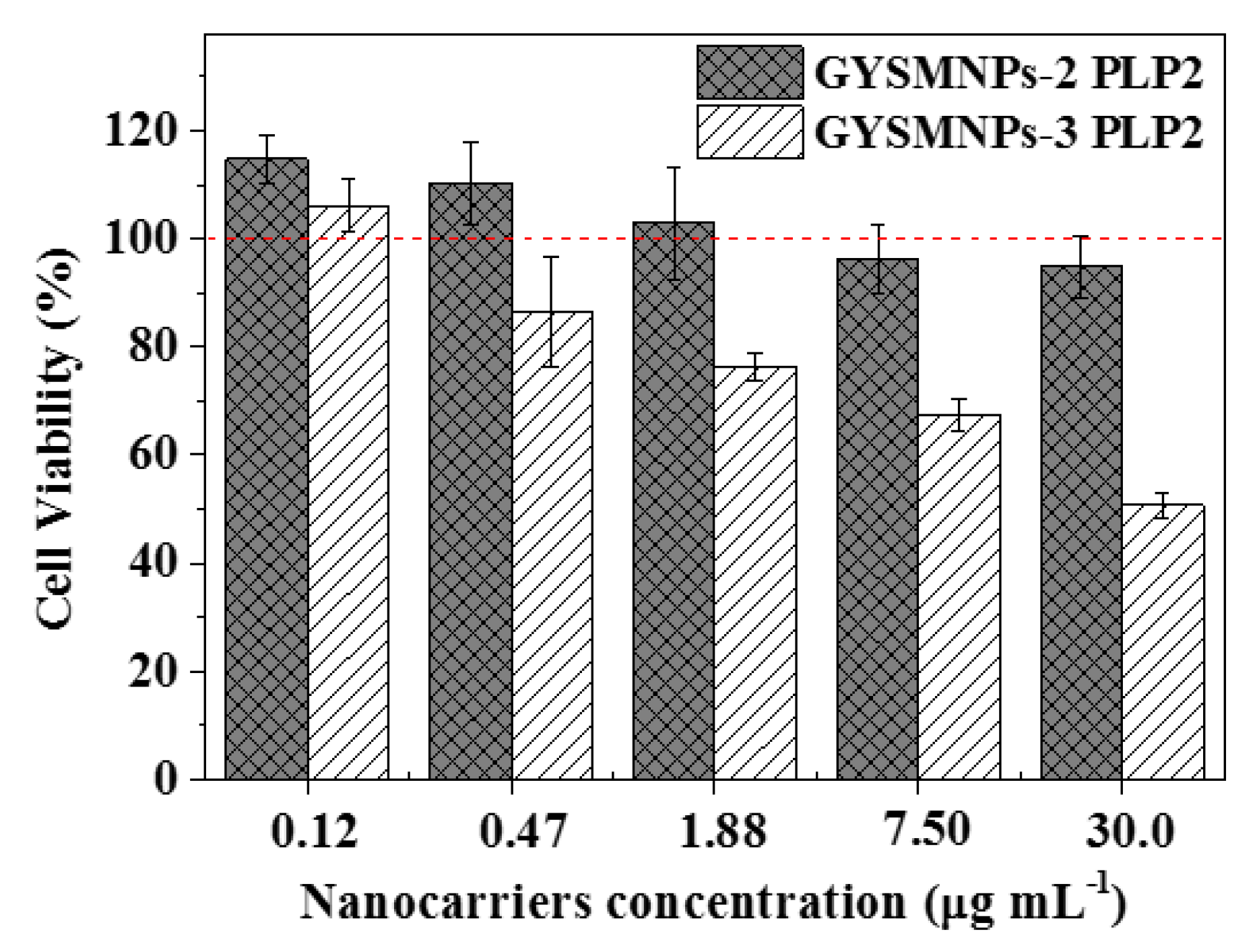
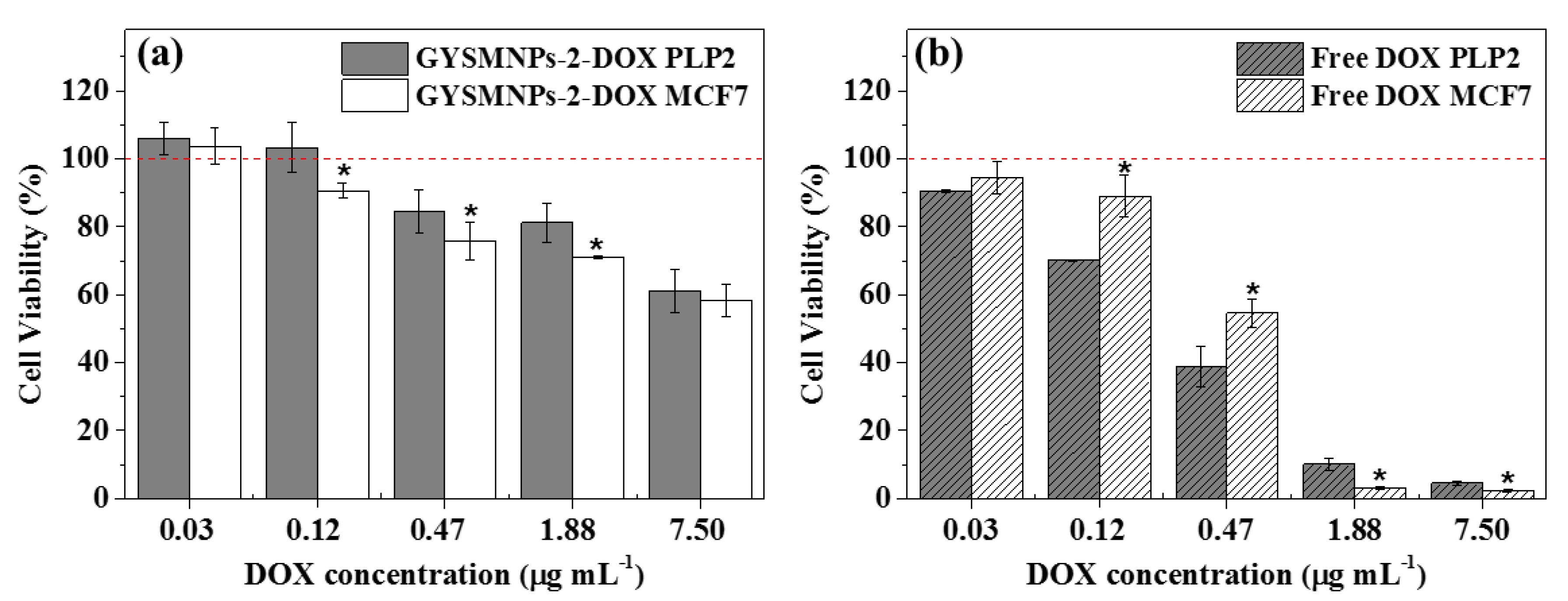
3.4. In Vitro Biocompatibility and Cellular Drug-Delivery Assay
4. Conclusions
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Schadt, M.J.; Lim, I.I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.-Y.; Luo, J.; Zhong, C.-J. Fabrication of Magnetic Core@Shell Fe Oxide@Au Nanoparticles for Interfacial Bioactivity and Bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung, L.D.; Maenosono, S.; Wälti, C.; Thanh, N.T.K. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Chao, J.; Song, C.; Liu, B.; Zhu, D.; Sun, Y.; Yang, B.; Zhang, Q.; Chen, Y.; et al. Synthesis of magnetic core-branched Au shell nanostructures and their application in cancer-related miRNA detection via SERS. Sci. China Mater. 2017, 60, 1129–1144. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, A.V.; Flint, J.A.; Varshney, M.; Morey, T.E.; Dennis, D.M.; Duran, R.S. Surface Modification of Silica Core-Shell Nanocapsules: Biomedical Implications. Biomacromolecules 2006, 7, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, C.; Wang, F.; Xil, Z.; Wang, Z.; Deng, Y.; Hel, N. Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles Coated with PEG/PEI for Biomedical Applications: A Facile and Scalable Preparation Route Based on the Cathodic Electrochemical Deposition Method. Adv. Phys. Chem. 2017, 2017, 9437487. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, S.; Du, X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon 2016, 101, 135–142. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly Hydrophilic Luminescent Magnetic Mesoporous Carbon Nanospheres for Controlled Release of Anticancer Drug and Multimodal Imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhang, Y.N.; Zhang, W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug Discov. Today 2017, 22, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Zhang, Y.S.; Khademhosseini, A. Boosting clinical translation of nanomedicine. Nanomedicine 2016, 11, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, J.; Chen, D.; Wu, Y.; Zhang, W.; Zheng, F.; Cao, J.; Ma, H.; Liu, Y. Yolk-shell hybrid nanoparticles with magnetic and pH-sensitive properties for controlled anticancer drug delivery. Nanoscale 2013, 5, 11718–11724. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Scudieri, C.; Polichetti, M.; Ciambelli, P. Electrochemical Applications of Magnetic Core-Shell Graphene-Coated FeCo Nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 3157–3166. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanopart. Res. 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Liu, Y.-X.; Yan, X.-Y.; Yong, G.-P.; Xu, Y.-P.; Liu, S.-M. One-pot synthesis of yolk-shell mesoporous carbon spheres with high magnetisation. J. Mater. Chem. A 2014, 2, 9600–9606. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svobodova, B.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Bittova, M.; Kuban, V.; Ferreira, I.C.F.R. Bioactive properties and phenolic profile of Momordica charantia L. medicinal plant growing wild in Trinidad and Tobago. Ind. Crops Prod. 2017, 95, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Bharath, G.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Mangalaraj, D.; Ponpandian, N. Solvent-free mechanochemical synthesis of graphene oxide and Fe3O4-reduced graphene oxide nanocomposites for sensitive detection of nitrite. J. Mater. Chem. A 2015, 3, 15529–15539. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Wei, Y.; Zhang, G. An in situ gelatin-assisted hydrothermal synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic performance under ultraviolet and visible light. RSC Adv. 2014, 4, 7933–7943. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Z.; Xu, F.; Thummavichai, K.; Chen, H.; Xia, Y.; Zhu, Y. A generic method to synthesise graphitic carbon coated nanoparticles in large scale and their derivative polymer nanocomposites. Sci. Rep. 2017, 7, 11829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Ryu, S.Y.; Kim, J.; Jun, Y. A study of TiO2/carbon black composition as counter electrode materials for dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.S.; Silva, A.M.T.; Tavares, P.B.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites for catalytic wet peroxide oxidation applications. Catal. Today 2017, 280, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Chen, Y.; Li, W.; Li, J.; Yu, H.; Liu, L.; Wu, G.; Yang, T.; Luo, L. Preparation of boron nitride nanosheet-coated carbon fibres and their enhanced antioxidant and microwave-absorbing properties. RSC Adv. 2018, 8, 17944–17949. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P.; Rapoport, N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, M.; Rahmani, F.; Nouranian, S. Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems. J. Mater. Chem. B 2016, 4, 7441–7451. [Google Scholar] [CrossRef]
- Al-Nahain, A.; Lee, S.Y.; In, I.; Lee, K.D.; Park, S.Y. Triggered pH/redox responsive release of doxorubicin from prepared highly stable graphene with thiol grafted Pluronic. Int. J. Pharm. 2013, 450, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of oxygen functionalities on the synthesis of photocatalytically active graphene-TiO2 composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Adnan, A.; Lam, R.; Chen, H.; Lee, J.; Schaffer, D.J.; Barnard, A.S.; Schatz, G.C.; Ho, D.; Liu, W.K. Atomistic Simulation and Measurement of pH Dependent Cancer Therapeutic Interactions with Nanodiamond Carrier. Mol. Pharm. 2011, 8, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials—An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Zhai, G. Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C 2016, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Maher, S.; Wang, Y.; Kaur, G.; Wang, L.; Erkelens, M.; Forward, P.; Lambert, M.F.; Evdokiou, A.; Losic, D. Naturally Derived Iron Oxide Nanowires from Bacteria for Magnetically Triggered Drug Release and Cancer Hyperthermia in 2D and 3D Culture Environments: Bacteria Biofilm to Potent Cancer Therapeutic. Biomacromolecules 2016, 17, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
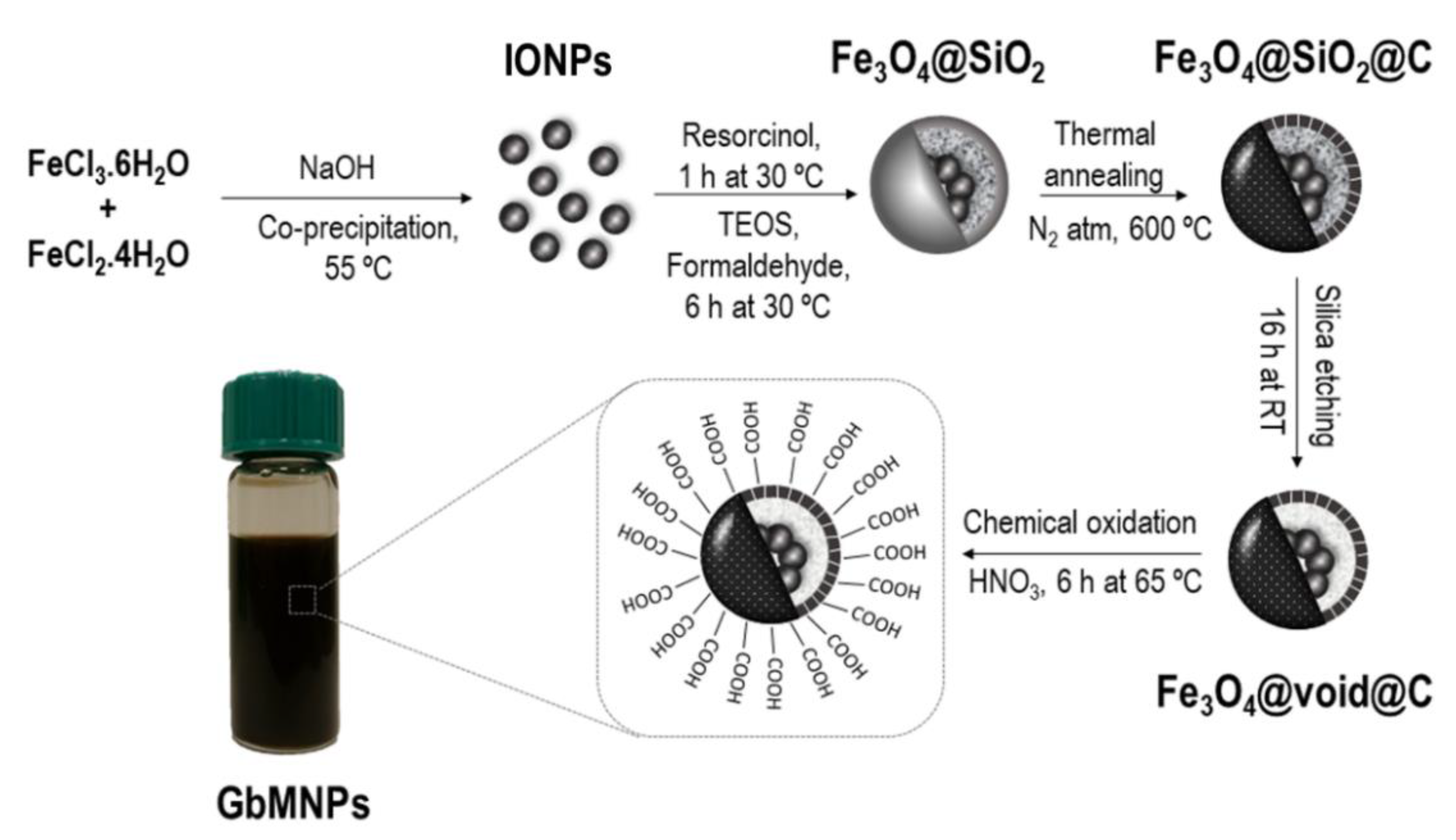
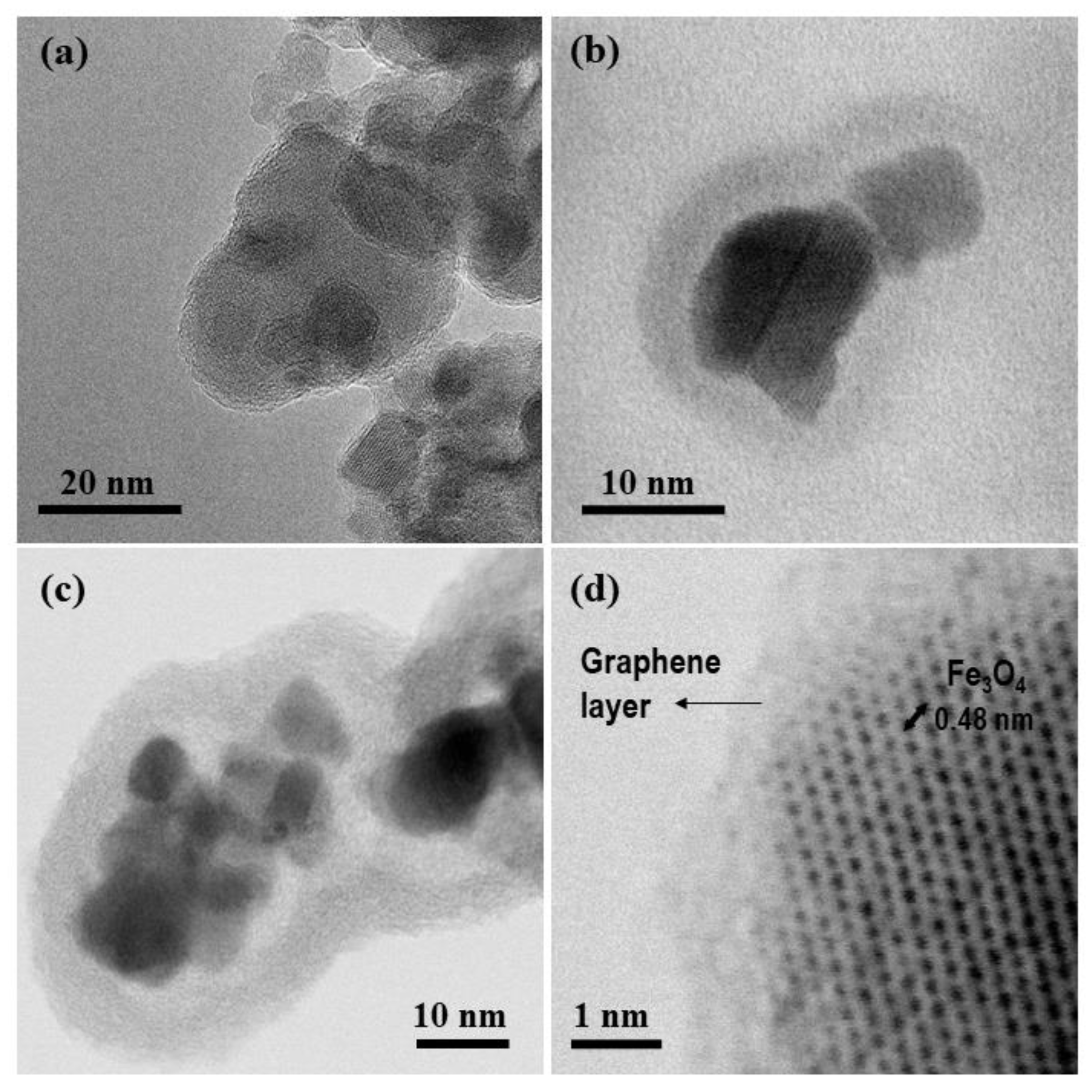
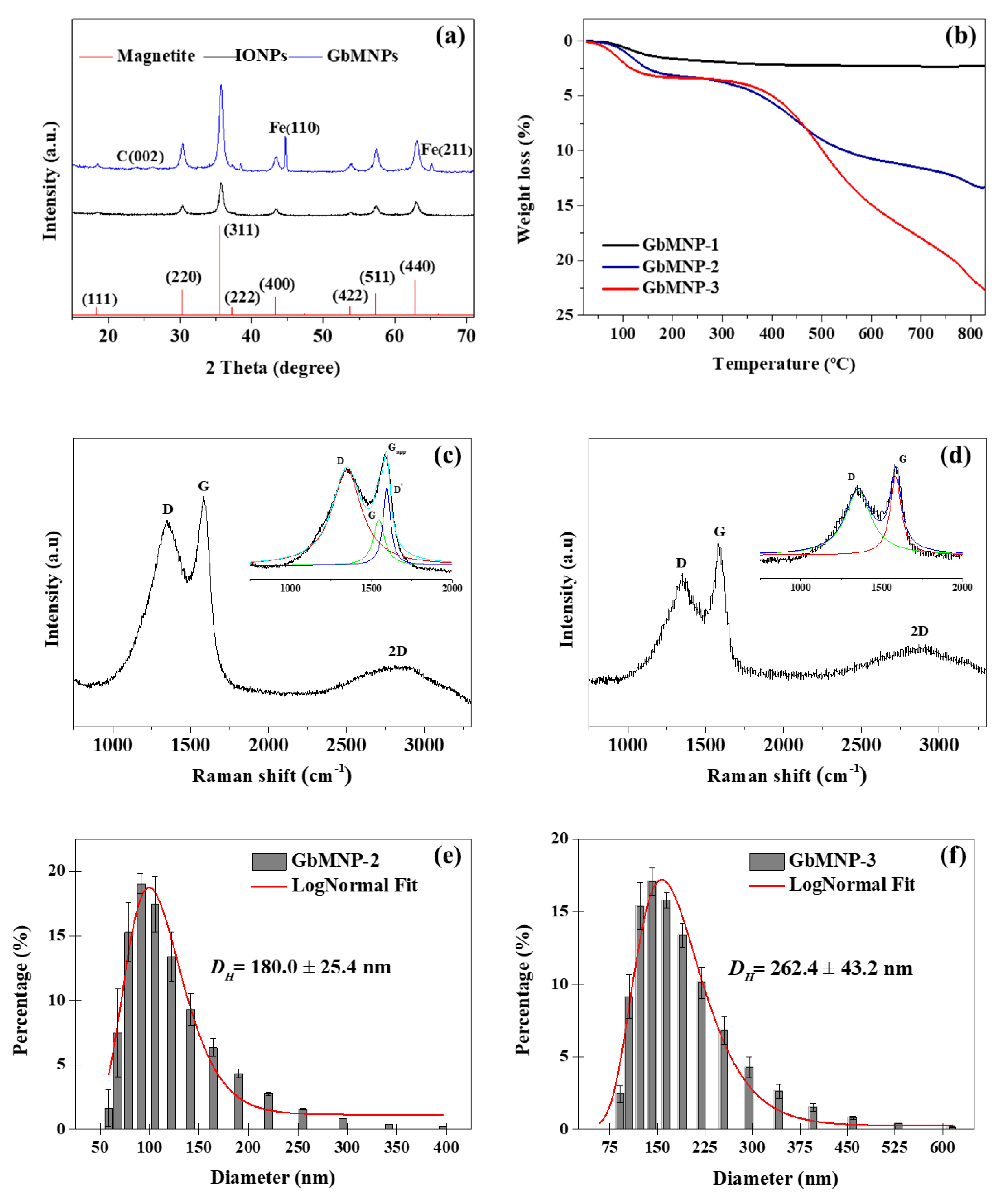
| Material | Magnetic Core (g) | Resorcinol (g) | Formaldehyde (mL) | TEOS (mL) | Hollow Thickness (nm) a | Carbon-Shell Thickness (nm) a |
|---|---|---|---|---|---|---|
| GbMNP-1 | 0.25 | 0.05 | 0.075 | 0.10 | Not detected | 1.41 ± 0.44 |
| GbMNP-2 | 0.25 | 0.10 | 0.150 | 0.21 | 0.70 ± 0.30 | 3.55 ± 1.27 |
| GbMNP-3 | 0.25 | 0.20 | 0.300 | 0.41 | 2.07 ± 0.92 | 7.07 ± 1.88 |
| Material | SBET (m2·g−1) | Smeso (m2·g−1) | Vmicro (cm3·g−1) | Vtotal (cm3·g−1) | Vmicro/Vtotal | daverage (nm) |
|---|---|---|---|---|---|---|
| GbMNP-2 | 156 | 123 | 0.013 | 0.318 | 0.041 | 8.2 |
| GbMNP-3 | 245 | 160 | 0.035 | 0.333 | 0.105 | 5.4 |
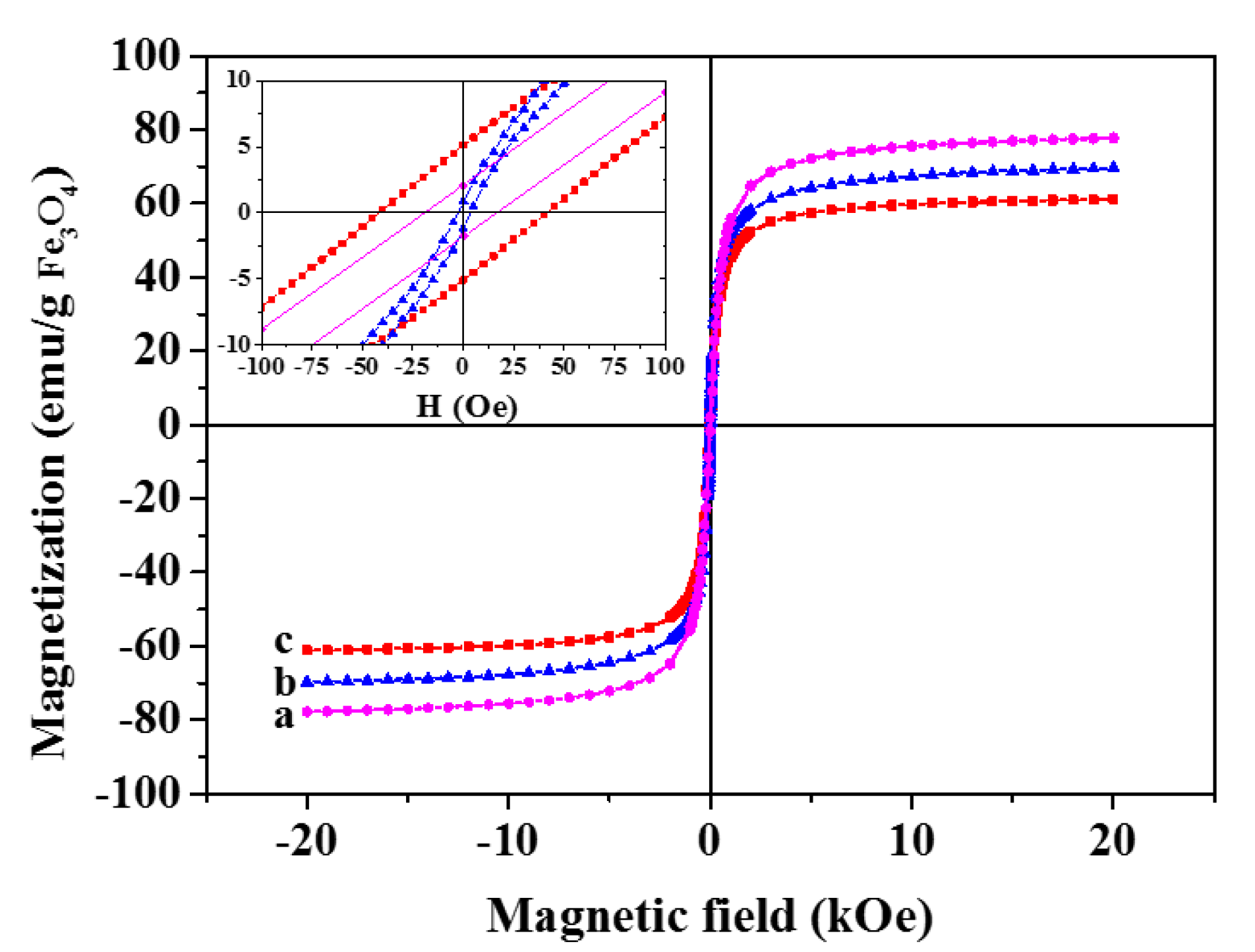
| Sample | Ms (emu·g−1 IONPs) | Hc (Oe) | Mr (emu·g−1 IONPs) |
|---|---|---|---|
| IONPs | 77.7 | 18.33 | 1.94 |
| GbMNP-2 | 69.8 | 3.54 | 1.16 |
| GbMNP-3 | 61.2 | 41.33 | 5.08 |
| Sample | pH | Zero-Order | First-Order | Hixson-Crowell | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | ||
| GbMNP-2 | 7.4 | 0.45 | 0.37 | 0.47 | 0.72 | 0.85 | 0.30 |
| 6.0 | 0.46 | 0.35 | 0.50 | 0.73 | 0.84 | 0.33 | |
| 4.5 | 0.47 | 0.39 | 0.58 | 0.73 | 0.96 | 0.44 | |
| GbMNP-3 | 7.4 | 0.41 | 0.39 | 0.43 | 0.67 | 0.85 | 0.23 |
| 6.0 | 0.48 | 0.37 | 0.51 | 0.74 | 0.85 | 0.33 | |
| 4.5 | 0.40 | 0.34 | 0.46 | 0.66 | 0.81 | 0.26 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.O.; Baldi, G.; Doumett, S.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; Silva, A.M.T.; et al. A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C 2018, 4, 55. https://doi.org/10.3390/c4040055
Rodrigues RO, Baldi G, Doumett S, Gallo J, Bañobre-López M, Dražić G, Calhelha RC, Ferreira ICFR, Lima R, Silva AMT, et al. A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C. 2018; 4(4):55. https://doi.org/10.3390/c4040055
Chicago/Turabian StyleRodrigues, Raquel O., Giovanni Baldi, Saer Doumett, Juan Gallo, Manuel Bañobre-López, Goran Dražić, Ricardo C. Calhelha, Isabel C. F. R. Ferreira, Rui Lima, Adrián M. T. Silva, and et al. 2018. "A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine" C 4, no. 4: 55. https://doi.org/10.3390/c4040055