Synthesis of Hybrid Silica-Carbon Tubular Structures by Chemical Vapor Deposition with Methane or Ethene
Abstract
:1. Introduction
2. Results and Discussion
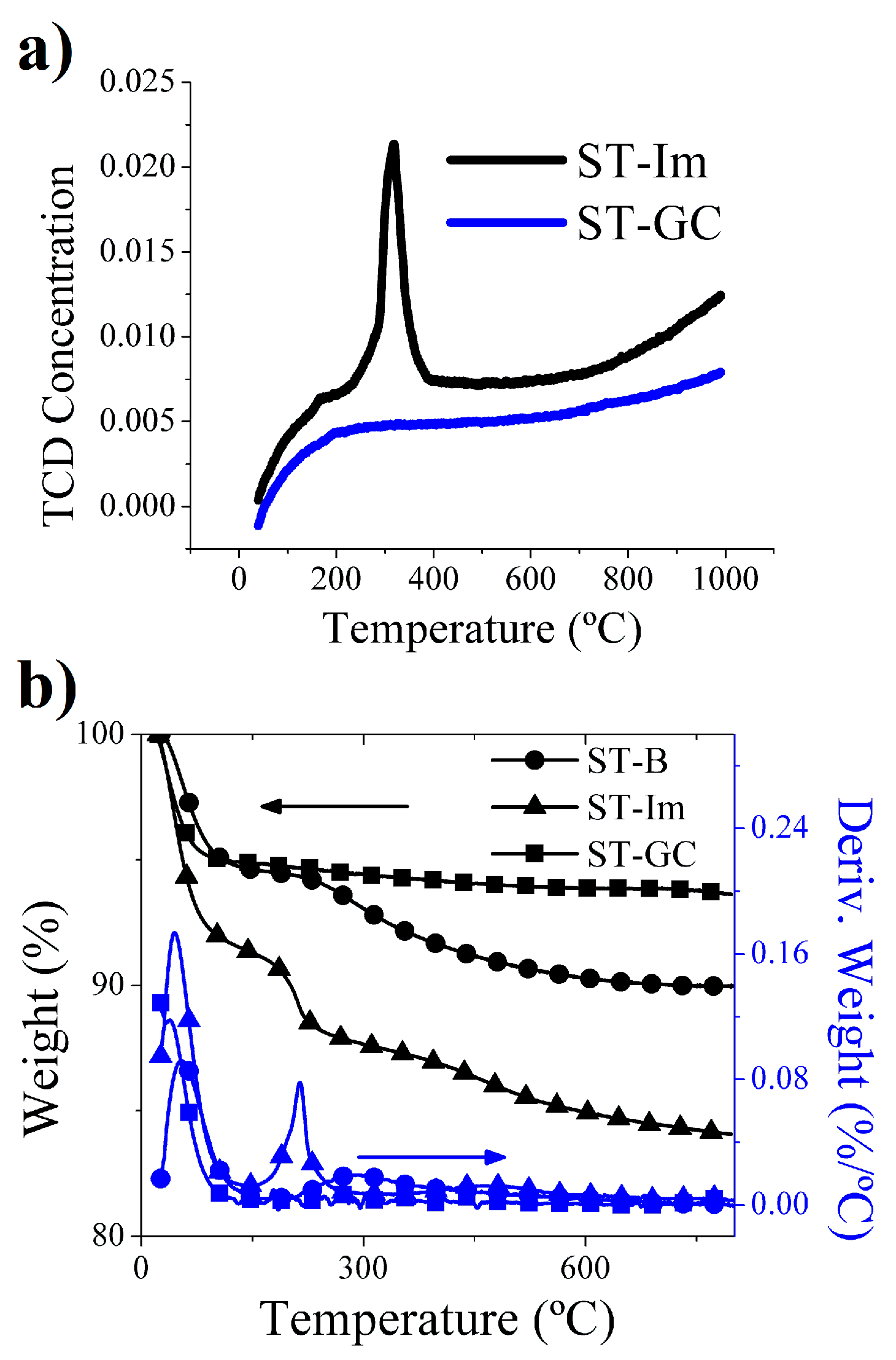
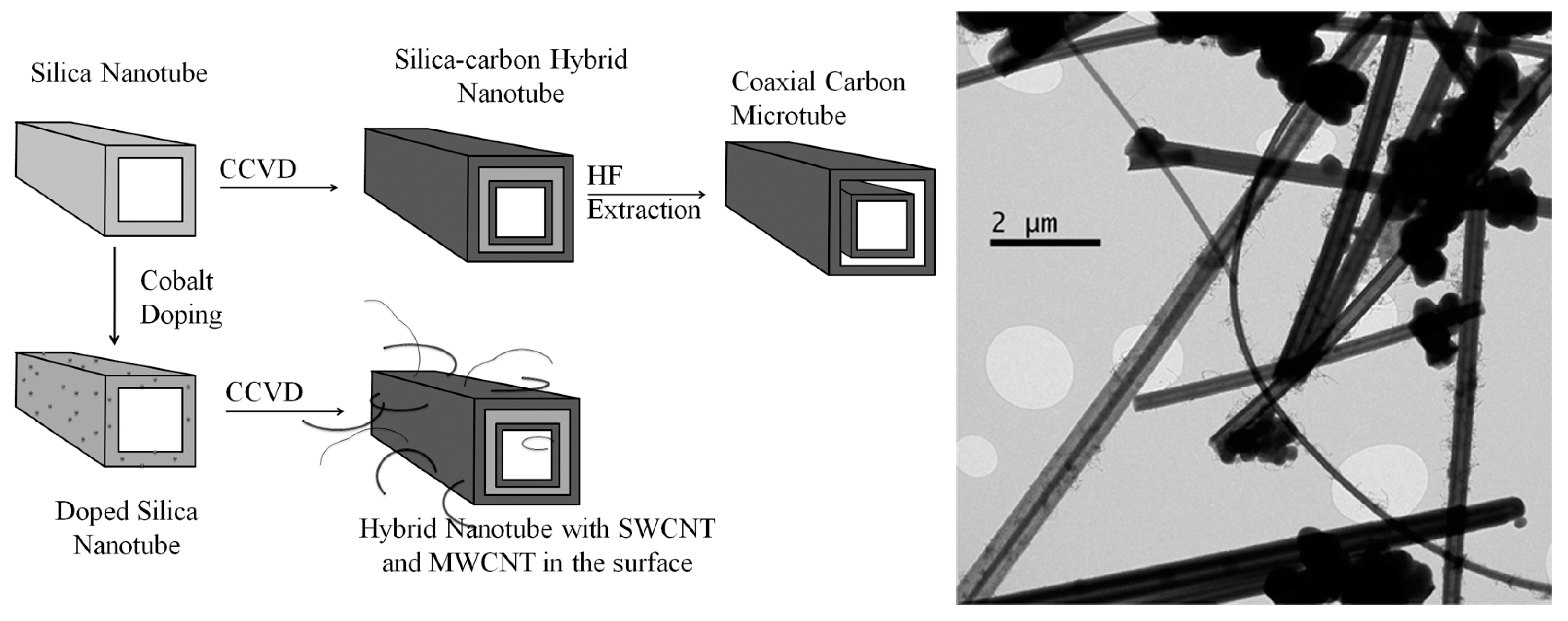
2.1. Silica Tubes
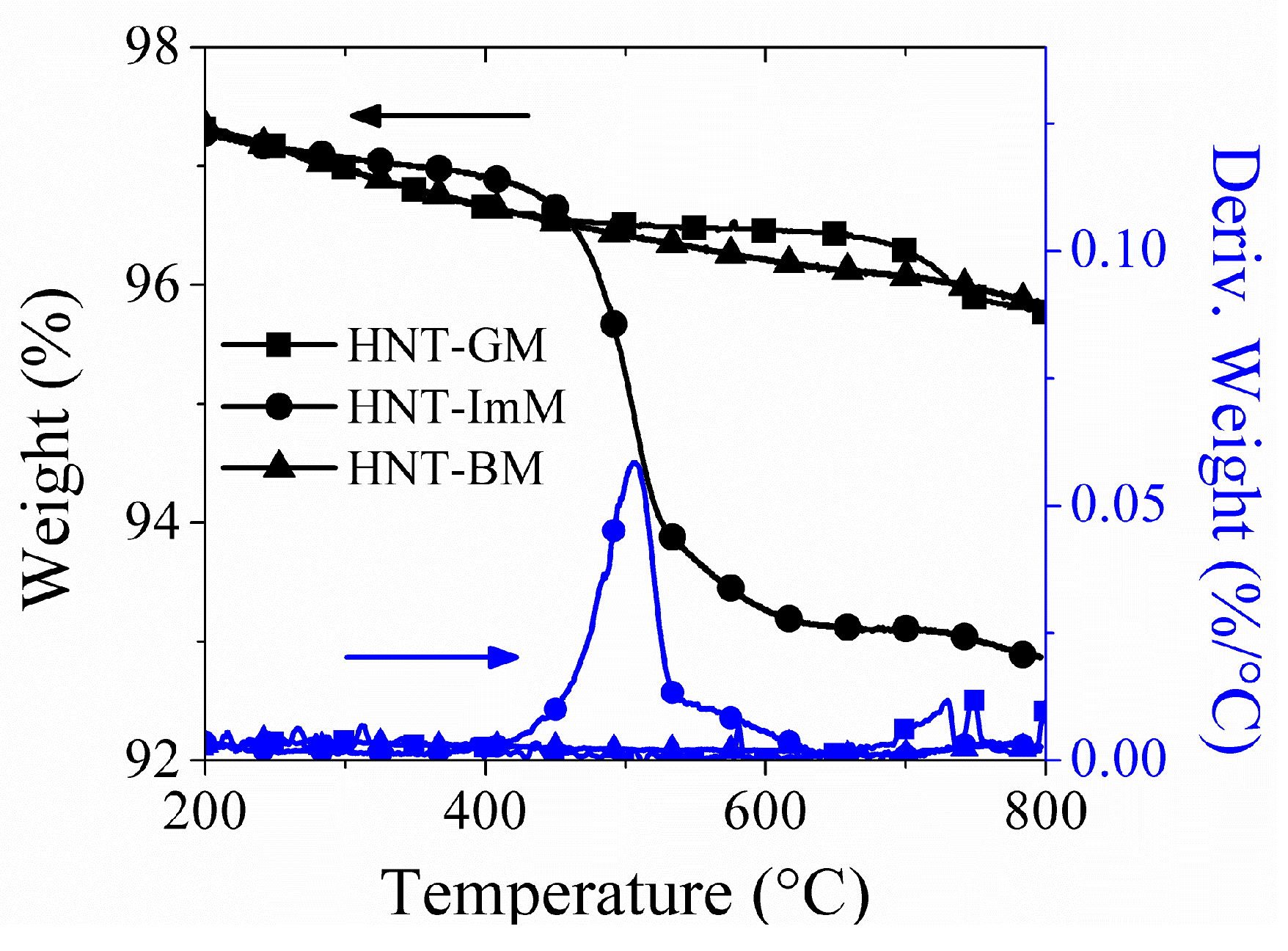
2.2. Cobalt-Silica Tube Catalysts
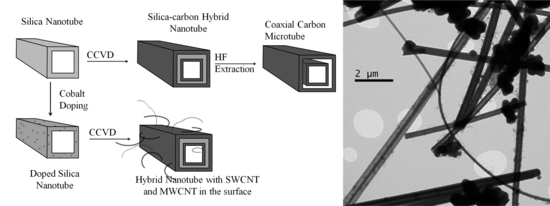
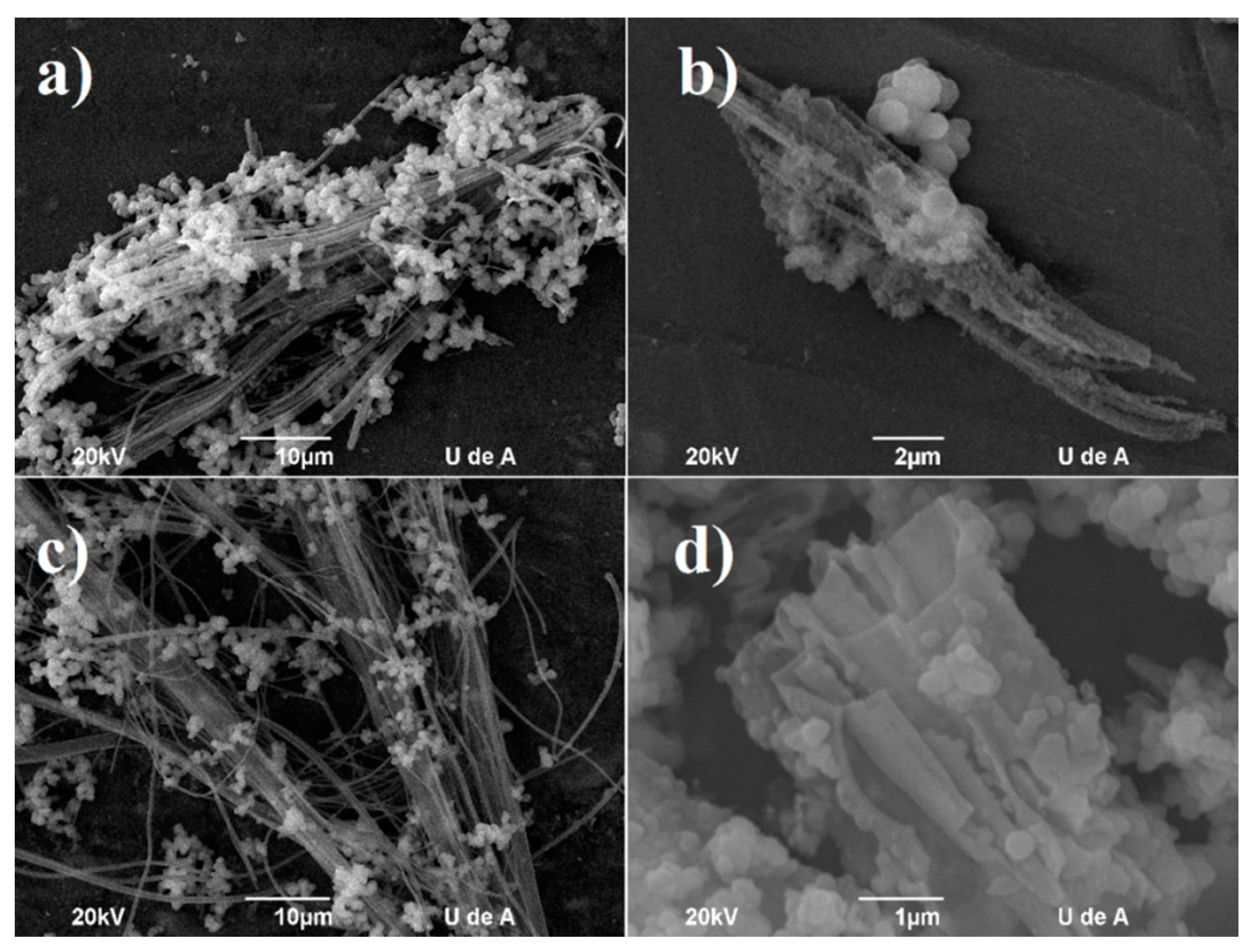
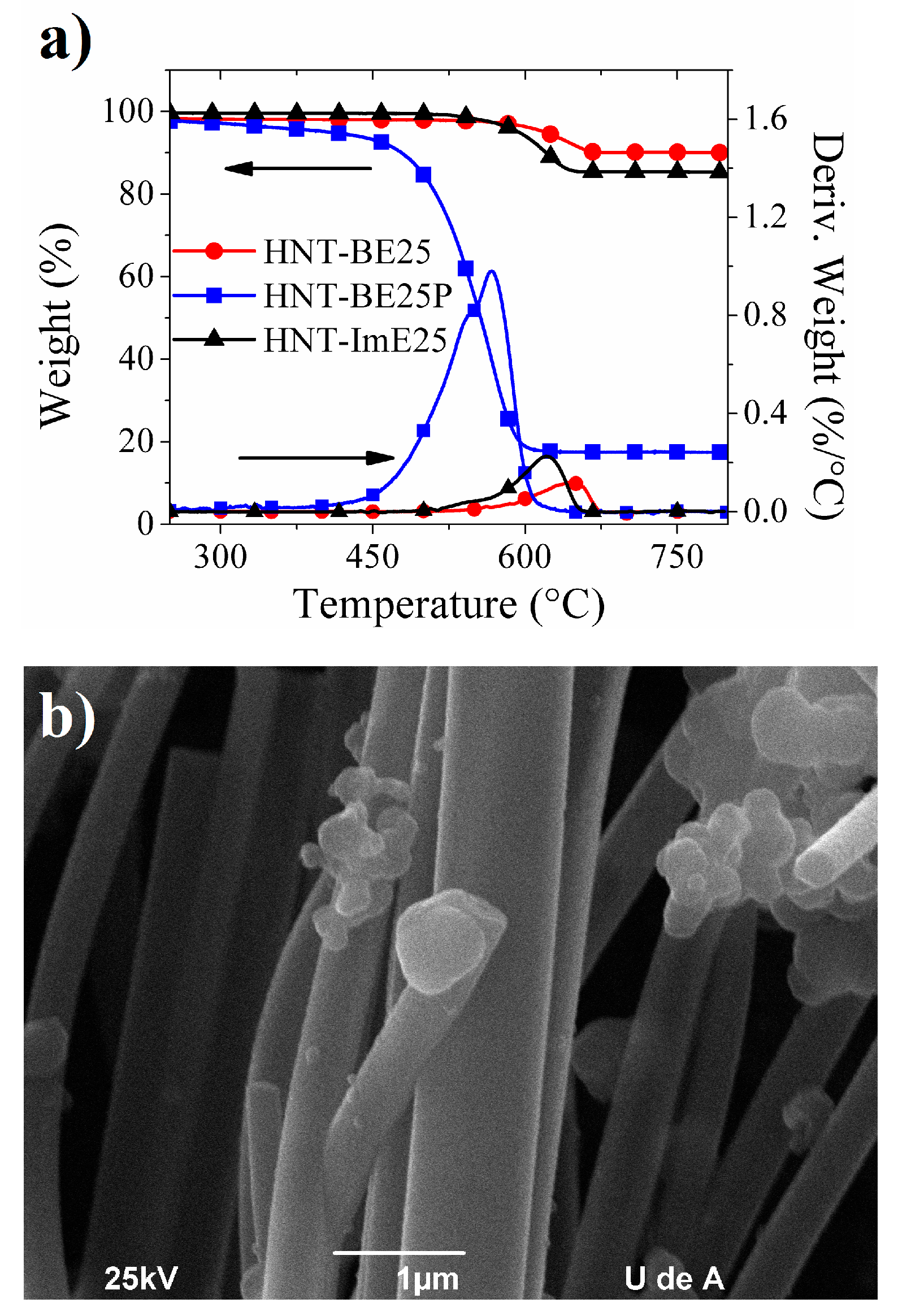
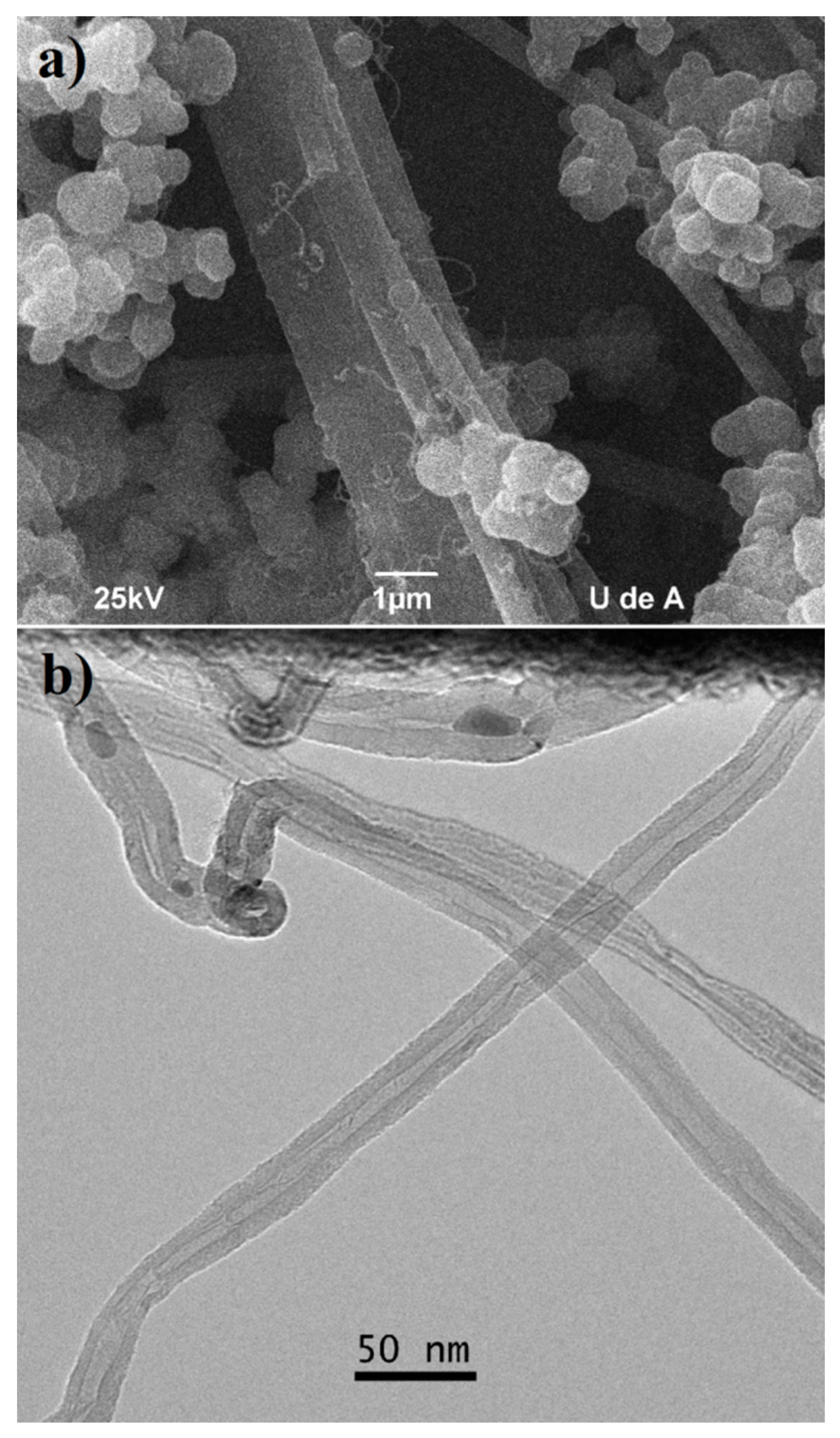
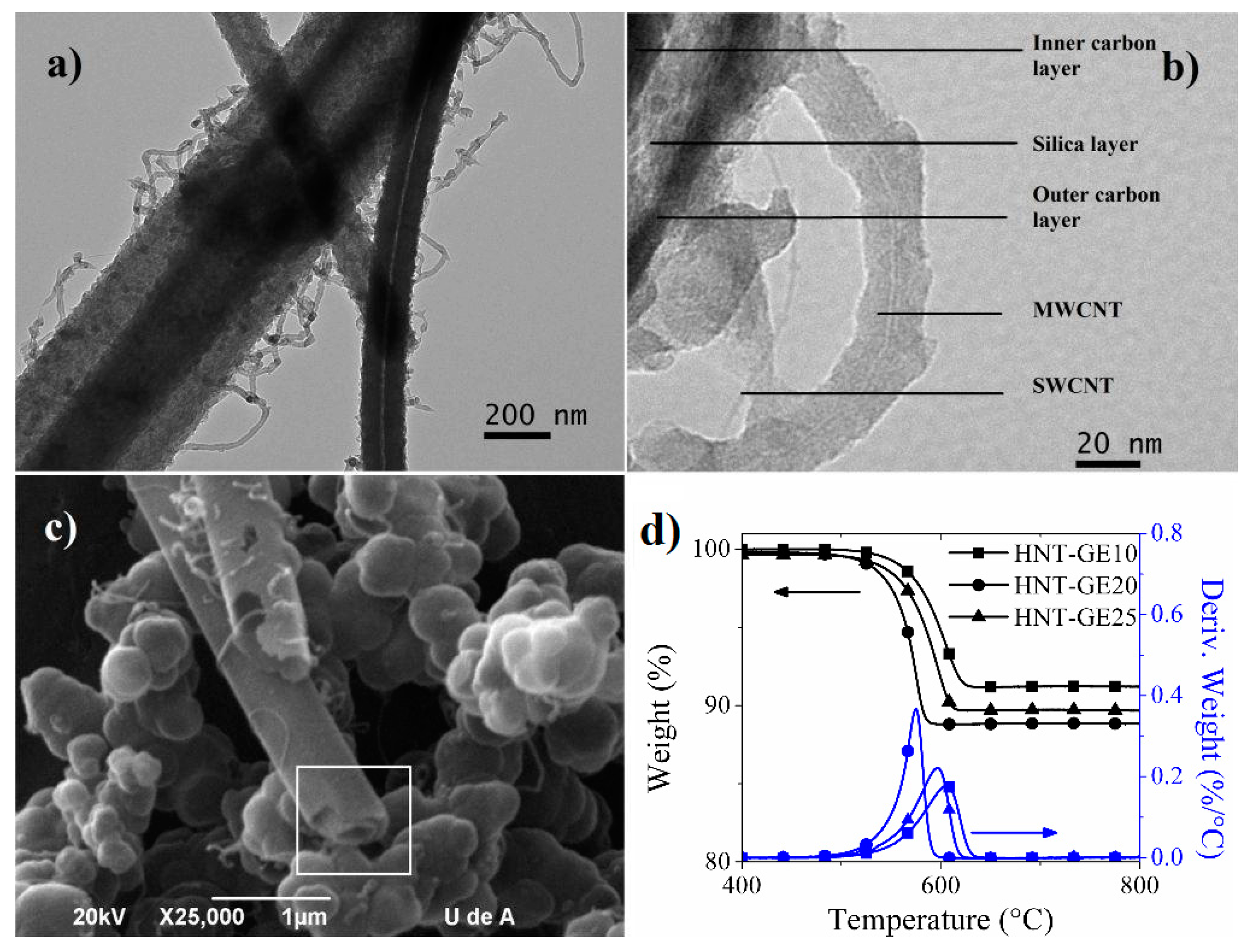
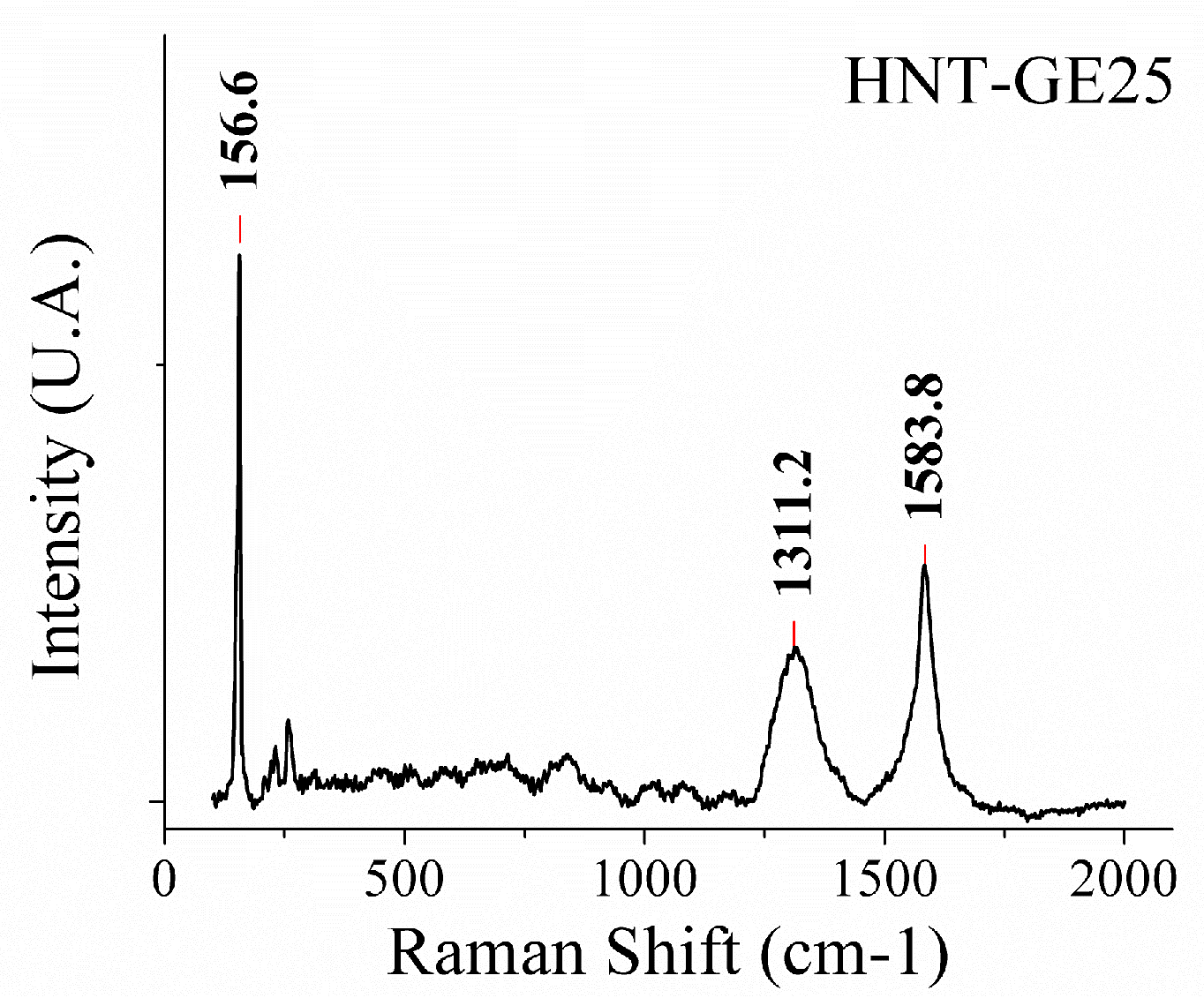
2.3. Hybrid Nanotubes
3. Materials and Methods
3.1. Materials
3.2. Silica Tubes
3.3. Catalyst Preparation
3.4. Hybrid Nanotubes
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, J.; Martel, R.; Derycke, V.; Radosavljevic, M.; Wind, S.; Neumayer, D.; Avouris, P. Carbon nanotubes as potential building blocks for future nanoelectronics. Microelectron. Eng. 2002, 64, 391–397. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.; Kim, S.; Rinzler, A.; et al. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical Microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Giraldo, L.F.; López, B.L.; Haller, G.L. Synthesis of Single Walled Carbon Nanotubes Using Cobalt Grafted in MCM-41 as Catalyst. In Nanotech: Conference & Expo 2009; CRC Press: Boca Raton, FL, US, 2009; pp. 429–432. [Google Scholar]
- Ameli, A.; Arjmand, M.; Pötschke, P.; Krause, B.; Sundararaj, U. Effects of Synthesis catalyst and temperature on broadband dielectric properties of nitrogen-doped carbon nanotube/polyvinylidene fluoride nanocomposites. Carbon 2016, 106, 260–278. [Google Scholar] [CrossRef]
- Lim, S.; Ciuparu, D.; Pak, C.; Dobek, F.; Chen, Y.; Harding, D.; Pfefferle, L.; Haller, G.L. Synthesis and characterization of highly ordered Co-MCM-41 for production of aligned single walled carbon nanotubes (SWNT). J. Phys. Chem. B 2003, 107, 11048–11056. [Google Scholar] [CrossRef]
- Somanathan, T.; Pandurangan, A. Effective Synthesis of Single-Walled Carbon Nanotubes Using Ni–MCM-41 Catalytic Template through Chemical Vapor Deposition Method. Ind. Eng. Chem. Res. 2006, 45, 8926–8931. [Google Scholar] [CrossRef]
- Balamurugan, J.; Pandurangan, A.; Thangamuthu, R.; Senthilkumar, S.M. Effective synthesis of well-graphitized carbon nanotubes on bimetallic SBA-15 template for use as counter electrode in dye- sensitized solar cells. Ind. Eng. Chem. Res. 2013, 52, 384–393. [Google Scholar] [CrossRef]
- Mirkhani, S.A.; Arjmand, M.; Sadeghi, S.; Krause, B.; Pötschke, P.; Sundararaj, U. Impact of synthesis temperature on morphology, rheology and electromagnetic interference shielding of CVD-grown carbon nanotube/polyvinylidene fluoride nanocomposites. Synth. Metal 2017, 230, 39–50. [Google Scholar] [CrossRef]
- Hu, K.-W.; Hsu, K.-C.; Yeh, C.-S. pH-Dependent biodegradable silica nanotubes derived from Gd(OH)3 nanorods and their potential for oral drug delivery and MR imaging. Biomaterials 2010, 31, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Bai, X.; Nan, A.; Ghandehari, H.; Lee, S.B. Template synthesis of multifunctional nanotubes for controlled release. J. Control Release 2006, 114, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.T.; Lee, S.B.; Trofin, L.; Li, N.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Smart nanotubes for bioseparations and biocatalysis. J. Am. Chem. Soc. 2002, 124, 11864–11865. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Lee, S.C.; Lee, S.M.; Kim, H.J. Hydrogen adsorption characteristics of Li-dispersed silica nanotubes. Chem. Phys. Lett. 2007, 436, 162–166. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Q.; Lu, Z.; Yin, Y. Templated synthesis of metal nanorods in silica nanotubes. J. Am. Chem. Soc. 2011, 133, 19706–19709. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 296, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Okamoto, K.; Son, S.J.; Luckett, C.; Gopalani, A.H.; Lee, S.B.; English, D.S. Observing capillarity in hydrophobic silica nanotubes. J. Am. Chem. Soc. 2005, 127, 17385–17392. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Wen, L.-X.; Wang, Z.-H.; Wang, M.; Shao, L.; Chen, J.-F. Facile synthesis of hollow silica nanotubes and their application as supports for immobilization of silver nanoparticles. Scr. Mater. 2004, 51, 1035–1039. [Google Scholar] [CrossRef]
- Zygmunt, J.; Krumeich, F.; Nesper, R. Novel silica nanotubes with a high aspect ratio—Synthesis and structural characterization. Adv. Mater. 2003, 15, 1538–1541. [Google Scholar] [CrossRef]
- Nakamura, H.; Matsui, Y. Silica gel nanotubes obtained by the sol-gel method. J. Am. Chem. Soc. 1995, 117, 2651–2652. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wen, L.-X.; Liu, R.-J.; Chen, J.-F. Needle-like calcium carbonate assisted self-assembly of mesostructured hollow silica nanotubes. J. Solid State Chem. 2005, 178, 2383–2389. [Google Scholar] [CrossRef]
- Rüscher, C.H.; Bannat, I.; Feldhoff, A.; Ren, L.; Wark, M. SiO2 nanotubes with nanodispersed Pt in the walls. Microporous Mesoporous Mater. 2007, 99, 30–36. [Google Scholar] [CrossRef]
- Miyaji, F.; Davis, S.A.; Charmant, J.P.H.; Mann, S. Organic crystal templating of hollow silica fibers. Chem. Mater. 1999, 11, 3021–3024. [Google Scholar] [CrossRef]
- Miyaji, F.; Watanabe, Y.; Suyama, Y. Morphology of silica derived from various ammonium carboxylate templates. Mater. Res. Bull. 2003, 38, 1669–1680. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liu, Y.-C.; Wu, C.-H.; Yeh, C.-C.; Su, M.-T.; Wu, Y.-C. Preparation of fluorescent silica nanotubes and their application in gene delivery. Adv. Mater. 2005, 17, 404–407. [Google Scholar] [CrossRef]
- Xie, C.; Liu, B.; Wang, Z.; Gao, D.; Guan, G.; Zhang, Z. Molecular imprinting at walls of silica nanotubes for TNT recognition. Anal. Chem. 2008, 80, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, S.; Cui, W.; Zhao, Q.; Cheng, X.; Li, R.; Xie, X.; Mai, Y.-W. A facile method to fabricate silica-coated carbon nanotubes and silica nanotubes from carbon nanotubes templates. J. Mater. Sci. 2009, 44, 4539–4545. [Google Scholar] [CrossRef]
- Colorado, R.; Barron, A.R. Silica-coated single-walled nanotubes: nanostructure formation. Chem. Mater. 2004, 16, 2691–2693. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Liu, T. Polymer nanocomposites using urchin-shaped carbon nanotube-silica hybrids as reinforcing fillers. Macromol. Rapid Commun. 2004, 25, 1860–1864. [Google Scholar] [CrossRef]
- Loy, D.A. Sol-Gel Processing. In Encyclopedia of Physical Science and Technolog; Academic Press: Pittsburgh, PA, USA, 2001; p. 276. [Google Scholar]
- Herrera, J.E.; Balzano, L.; Borgna, A.; Alvarez, W.E.; Resasco, D.E. Relationship between the Structure/Composition of Co–Mo Catalysts and Their Ability to Produce Single-Walled Carbon Nanotubes by CO Disproportionation. J. Catal. 2001, 204, 129–145. [Google Scholar] [CrossRef]
- Latorre, N.; Romeo, E.; Villacampa, J.I.; Cazaña, F.; Royo, C.; Monzón, A. Kinetics of carbon nanotubes growth on a Ni–Mg–Al catalyst by CCVD of methane: influence of catalyst deactivation. Catal. Today 2010, 154, 217–223. [Google Scholar] [CrossRef]
- Latorre, N.; Cazaña, F.; Martínez-Hansen, V.; Royo, C.; Romeo, E.; Monzón, A. Ni-Co-Mg-Al catalysts for hydrogen and carbonaceous nanomaterials production by CCVD of methane. Catal. Today 2011, 172, 143–151. [Google Scholar] [CrossRef]

| Sample | Molar Ratio W/TEOS/TA/E * |
|---|---|
| ST_ref | 1:1:0.04:26 |
| STw+ | 2:1:0.04:26 |
| STw- | 0.5:1:0.04:26 |
| STe- | 1:1:0.04:13 |
| STe+ | 1:1:0.04:38 |
| STta+ | 1:1:0.2:26 |
| STta- | 1:1:0.02:26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepulveda, V.R.; López, B.L. Synthesis of Hybrid Silica-Carbon Tubular Structures by Chemical Vapor Deposition with Methane or Ethene. C 2018, 4, 1. https://doi.org/10.3390/c4010001
Sepulveda VR, López BL. Synthesis of Hybrid Silica-Carbon Tubular Structures by Chemical Vapor Deposition with Methane or Ethene. C. 2018; 4(1):1. https://doi.org/10.3390/c4010001
Chicago/Turabian StyleSepulveda, Victor R., and Betty L. López. 2018. "Synthesis of Hybrid Silica-Carbon Tubular Structures by Chemical Vapor Deposition with Methane or Ethene" C 4, no. 1: 1. https://doi.org/10.3390/c4010001