3.1. Emulsion Appearance and Storage Stability
The Petri dish test showed visual observation of emulsion flow behavior during storage on day 1 and day 30 (
Figure 1A,B). Almost all of the W/O emulsions were highly viscous liquids without any flow, except for the control emulsion (without LMP) with 20 wt.% water, which visually flowed on day 1 (
Figure 1A). However, the control emulsion with 50 wt.% water showed no flow due to close packing of excess water droplets, although its surface looked unsmooth and without any gloss (
Figure 1A). Emulsions with aqueous phase LMP and 20, 30, 40, and 50 wt.% water self-retained their shape on day 1. On day 30, the control emulsions with 20 wt.% water spread over the Petri dish, indicating a change in the emulsions’ structure (
Figure 1B). However, the control emulsions with 50 wt.% water showed a similar emulsion shape on day 30 as on day 1. LMP emulsions with 20 wt.% water showed a certain degree of spreading compared to day 1, although lower than that of the control emulsions. LMP emulsions with 30 wt.% water demonstrated some spreading, but it was less than that of the 20 wt.% water emulsion. The LMP emulsions with 40 and 50 wt.% water retained their form without any spreading on day 30, comparable to day 1. Recently, Rafanan and Rousseau (2019) reported that W/VO emulsions with 20 wt.% water with HSO and PGPR phase separated after 14 days; however, monoacylglycerol-stabilized emulsions exhibited soft, paste-like solids retaining their shape without separation after 28 days of storage. In the present work, shape retention was observed with more water content and at lower GMO and HSO concentrations. Perhaps the presence of LMP in the aqueous phase provided improved stability to the water droplets, leading to improved structure retention.
The flipped glass tubes were also used to detect emulsion stability and gelation after 30 days of storage (
Figure 1C). Control emulsions with 20 wt.% water content showed a partial flow. A similar emulsion with no separation evidence was also found after 28 days of storage with 14.4 wt.% HSO and 1.6 wt.% GMO by Rafanan and Rousseau [
13]. Though control emulsions with 50 wt.% water exhibited no flow, aqueous phase separation was observed at the bottom of the glass tube, indicating some emulsion destabilization. All LMP emulsions reported no phase separation or flow on day 30, suggesting that the emulsions were self-supporting, and a strong gel structure was developed. Similarly, Rafanan and Rousseau [
14] described considerable textural variations with water increment in GMO emulsions. From 5 to 10 wt.% water, the emulsions were liquid, and from 15 to 20 wt.% water, self-supporting soft solid emulsions were observed for 7 days in storage.
3.2. Emulsion Viscosity
The viscosity graphs exhibited a decreasing trend with the increase in shear rate (
Figure 2A). The solution of 1.5 wt.% LMP began with 4.3 ± 0.7 Pa·s at 0.01 s
−1, and after that, the viscosity decreased due to the LMP molecules’ detangling, and more flow was dominant in the system. The mixture of 7 wt.% HSO in CO showed an apparent viscosity of 8.8 ± 1.1 Pa·s at 0.01 s
−1, which also decreased with an increase in shear rate due to the rupture of the fat crystal network, and nearly reached a plateau beyond 20 s
−1 (evidence of liquid-like Newtonian flow behavior).
All emulsions showed a shear-thinning behavior where viscosity decreased with an increase in shear rate on day 1 and day 30 (
Figure 2B,C). Control emulsions with 20 wt.% water showed the lowest viscosity trend with shear, which began with a low-shear plateau at 13.2 ± 1.7 Pa·s at 0.01 s
−1 and decreased to at 0.1 ± 0.01 Pa·s at the end of the run at 100 s
−1 (
Figure 2B). However, control emulsions with 50 wt.% water showed an initial viscosity higher than all emulsions at 0.01 s
−1, which showed a slightly irregular viscosity curve as a function of shear than all other emulsions. Such behavior could be related to their uneven texture observed in the Petri dish (
Figure 1A). LMP emulsions with 20 wt.% water exhibited an initial viscosity significantly higher (137.05 ± 25.02 Pa·s) compared to control 20 wt.% water emulsions. There was not much difference in the viscosity trend of other LMP emulsions. In contrast to control emulsions with 50 wt.% water, all of the LMP emulsions showed a brief low-shear viscosity plateau, which indicates their Newtonian fluid-like behavior at a very low shear rate.
After aging, control emulsions with 20 wt.% water exhibited a significant viscosity drop (
Figure 2C), which was expected based on the visual observation of its flow behavior (
Figure 1B). Control emulsions with 50 wt.% water had a similar irregular viscosity curve, but in lesser magnitude than on day 1. For all LMP emulsions, the viscosity curves shifted to higher values after 30 days of storage. The viscosity profiles of LMP emulsions with 50 and 40 wt.% water were even higher than the 50 wt.% water control emulsion after 30 days of storage. Similarly, emulsions with 50 wt.% water content reported substantially higher viscosity than 40, 30, and 20 wt.% water for W/MO emulsions stabilized with wax and GMO after 28 days of storage [
8].
3.2.1. Apparent Viscosity at a Low Shear Rate
For direct comparison, the emulsions’ apparent viscosities at two different shear rates were plotted in
Figure 2D,E. Apparent viscosity at a 0.1 s
−1 shear rate (
Figure 2D) would represent emulsion viscosity when the structure was not completely broken down due to shear, while apparent viscosity at a 100 s
−1 shear rate (
Figure 2E) would be an indication of free-flowing emulsion where the original structures were largely broken down. On day 1, at a 0.1 s
−1 shear rate, the control emulsions with 20 wt.% water showed the lowest viscosity (6.4 ± 1.3 Pa·s), which increased to 42.9 ± 8.0 Pa·s for the control emulsions with 50 wt.% water. Such an increase in viscosity is related to the rise in the amount of dispersed water droplets in the emulsion (
Figure 2D). LMP emulsions with 20 wt.% water reported a significant rise in viscosity (27 ± 6.7 Pa·s) compared to the control emulsions with 20 wt.% water (
p < 0.05), which must be related to the improved stability of the water droplets in the presence of LMP in the aqueous phase. Upon increasing the water content, a significant increase in LMP emulsion viscosity at 0.1 s
−1 was observed at 30 wt.% water, which did not change with further increase in water content to 50 wt.% (on average, 48.5 Pa·s) (
p > 0.05). In contrast, Haj-shafiei et al. [
8] showed that the apparent viscosity of fresh W/MO emulsion stabilized with paraffin wax and GMO at a 10 s
−1 shear rate increased proportionally to the increment of the water content (20–50 wt.%). In the present case, perhaps the emulsions’ structure was broken even at a 0.1 s
−1 shear rate, and a proper indication of their gelation behavior could be obtained from small-deformation oscillatory rheological measurement (discussed below). The low-shear viscosity of 50 wt.% water control emulsions was not significantly different compared to 50 wt.% LMP emulsions (
p > 0.05), which indicates that under fresh conditions the predominant interaction influencing the viscosity of concentrated emulsions was the active filler property of the water droplets.
On day 30, control emulsions with 20 wt.% water demonstrated a decrease in viscosity (1.4 ± 0.3 Pa·s) from day 1 (
Figure 2D), which could be associated with the visual observation of their flow behavior under the quiescent conditions shown in
Figure 1B. Nevertheless, control emulsions with 50 wt.% water content revealed a viscosity increase after storage (57.5 ± 4.2 Pa·s.), related to further HSO crystallization in the continuous emulsion phase. A slight increase in viscosity was also observed after 30 days for 20 wt.% water emulsions with LMP, which did not change for 30 wt.% water (
p > 0.05). Nevertheless, a significant viscosity rise (~2x) was observed for aged LMP emulsions with 40 and 50 wt.% water (106 ± 9.8 and 142 ± 14.2 Pa·s, respectively), suggesting the synergistic effect of HSO crystallization along with water droplet packing during storage. On day 30, the viscosity of the LMP emulsions was significantly higher than the control emulsions, which could be due to the improved stability of the aged droplet network in the presence of LMP in the aqueous phase.
3.2.2. Apparent Viscosity at a High Shear Rate
The apparent viscosity decreased for all emulsions at a high shear rate (100 s
−1) due to the breakdown of fat crystal networks and water droplet packing. On day 1, control emulsions reported a viscosity range of 0.1–0.15 Pa·s, while the LMP emulsions showed a range from 0.2 to 0.26 Pa·s (
Figure 2E). This indicates that, even at a higher shear rate, the LMP’s presence in the water droplets was better against the shear force than its absence. Interestingly, on day 1, high shear viscosity decreased for 40 and 50 wt.% water emulsions, compared to 20 and 30 wt.% water, which could be associated with the water droplets’ destabilization from tight packing under high shear force.
After aging, all emulsions reported an increase in apparent viscosity even at a higher shear rate, which could be due to HSO crystallization, as discussed above. Control emulsions with only water showed a viscosity of 0.18 ± 0.04 and 0.21 ± 0.03 for 20 and 50 wt.% water, respectively. LMP emulsions presented higher viscosity compared to control emulsions. Emulsions with 20 and 30 wt.% water exhibited an average viscosity of 0.27 Pa·s (
p > 0.05). However, the highest viscosity was reported by 40 wt.% water content followed by 50 wt.% water content. The decrease in high-shear viscosity of 50 wt.% water LMP emulsions after storage could be due to higher droplet destabilization under high shear force when they were closely packed. An increase in viscosity from 10 to 40 wt.% water followed the trends observed by Iqbal et al. [
9] for PGPR-stabilized water-in-soybean-oil emulsions containing whey protein isolate (WPI) and NaCl in the aqueous phase. Heating the emulsions to denature the WPI in the aqueous phase, and subsequent cooling, led to a steep increase in apparent viscosity at 10 s
−1 for 30 and 40 wt.% water (~38 and 54 Pa·s, respectively) compared to 10 and 20 wt.% water [
9]. The authors reported that the viscosity increase was due to the 3D network of the highly aggregated water droplets containing protein networks after heating—leading to an increase in effective dispersed phase volume fraction—and due to the shift in their shape from spherical to non-spherical under shear.
3.2.3. Modeling Emulsion Viscosity
Pal [
15] proposed a new generalized viscosity model for concentrated suspensions and emulsions, which considered shear-induced droplet aggregation as an advancement over the Mooney and Krieger–Dougherty models. Pal proposed that the effective volume fraction of aggregated particles (ϕ
eff) could be calculated by multiplying droplet actual volume fraction (ϕ) with an aggregation coefficient,
k. The aggregation coefficient (
k) was, in turn, calculated from the boundary condition of maximum packing volume fraction (ϕ
m). Using these two parameters, the relative viscosity (η
r, ratio of suspension to continuous phase viscosity) of a concentrated hard-sphere suspension was expressed as [
16]:
In order to use this model for emulsions, a correction factor for η
r must be considered for the viscosity ratio (λ) of droplets to the continuous phase. When emulsions are sheared, the fluid inside the droplets circulates, which reduces the alteration of the flow behavior of the continuous phase, leading to a lowering of viscosity compared to a hard-sphere suspension. Pal proposed that the relationship between emulsion relative viscosity with its droplet volume fraction can be calculated by replacing η
r with η
r ((2η
r + 5λ)/(2 + 5λ))
3/2 [
17]. By applying this correction to η
r, the new generalized viscosity model for concentration emulsions became [
15]:
In the present case, Equation (2) was unable to predict our emulsion viscosity using ϕ
m as a fitting parameter. The calculated relative viscosities with the correction factor became several orders of magnitude higher than the model-predicted values. This could be due to several factors. First, in the present emulsions, the continuous phase was structured due to the presence of HSO fat crystal networks. Second, it has been proposed that when HSO crystallizes in the continuous phase containing GMO-stabilized water droplets, crystallization initiates at the water droplet surface, where GMO could act as a favorable site for nucleation [
3]. This could lead to a combination of crystal network and Pickering stabilization of water droplets [
10]. The crystal-coated Pickering water droplets could essentially act as hard spheres. Therefore, the water droplets are not only aggregated in the present case, but they are also connected to the continuous phase structure, acting as active fillers [
2]. Third, the water droplets could additionally take various irregular shapes due to fat crystallization at the surface, which was not considered in the model. Fourth, LMP can also interact at the interface not only with GMO, but also with LMP molecules at the surface of other water droplets. Therefore, the Pal equation (Equation (2)) was not able to model the complex structure of our emulsion.
We found that a weighted viscosity ratio (λ
w) of dispersed (η
d) to continuous phase (η
c) could be more successfully used as a correction to the hard-sphere model proposed in Equation (1). The λ
w can be defined as the ratio η
d ϕ/η
c (1–ϕ). The proposed equation becomes:
Figure 3 shows the model fitting of Equation (3) to the corrected relative viscosity values of the W/CO emulsions from the present work, using ϕ
m as a fitting parameter. For λ
w, a viscosity of 1.5 wt.% LMP and 7 wt.% HSO in CO at a 0.1 s
−1 shear rate were used for the dispersed and continuous phase values, respectively. For day 1 emulsion viscosities, the model fitting was poor (R
2 = 0.50), which could be associated with the high viscosity for 30 wt.% W/CO emulsions. The fitting parameter—maximum packing volume fraction—was 0.85, which is unusually high and indicates that crystal-coated droplet surfaces would no longer be spherical, and the droplets could be severely compressed at this stage. Clearly, more research is needed in order to further understand the highly compressed state of such Pickering and network-stabilized emulsions. Interestingly, on day 30, W/CO emulsions reported a decrease in ϕ
m to 0.62 with an R
2 = 0.92 (
Figure 3). Even then, the model was not able to predict higher viscosities of the emulsions at lower water volume fractions. Lowering of the ϕ
m after storage could be associated with fat crystals’ growth over time, which filled up the space leaving less space for the droplets to pack.
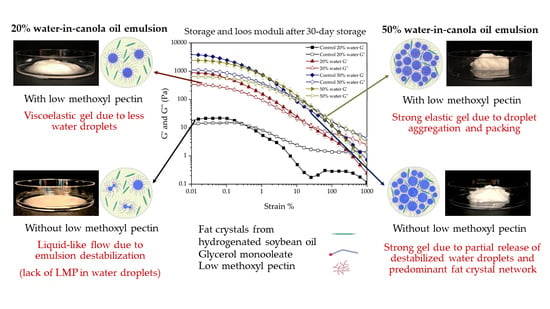
3.5. Emulsion Stability Analysis from Their Thermal Behaviour
All emulsions were subjected to freeze/thaw cycles in DSC to understand their dispersed phase stability better. Thermal cycles included a cooling from 25 °C to −70 °C, heating to 70 °C, and a second cooling to −70 °C at 5 °C/min (
Figure 6). On day 1, control emulsions with 20 wt.% water (
Figure 7A) showed small peaks at around −18 °C due to partially destabilized large water droplets, and again between −27 and −37 °C (peak 1), attributed to the crystallization of smaller water droplets, indicating stable emulsion. After the dispersed phase crystallization, the continuous phase CO crystallized at −50 °C (peak 2). During heating, CO melted between −35 and −8 °C (peak 3), the ice melted between −2 and 5 °C (peak 4), GMO melted between 10 and 16 °C (peak 5), and HSO melted between 50 and 60 °C (peak 6). The second cooling scan exhibited HSO crystallization at 30 °C (peak 7). A large water bulk crystallization peak and several small, dispersed water droplet crystallization peaks between −16 and −36 °C (peak 8) were related to a partial emulsion destabilization. The dispersed droplets’ crystallization peaks appeared at a higher temperature in the second cooling cycle than the first cooling cycle due to the increase in water droplet size due to the melting of stabilizing HSO crystals. The second cooling cycle also showed CO crystallization at −50 °C (peak 9). Control emulsions with 50 wt.% water (
Figure 7A) demonstrated a large water crystallization peak from −15 to −20 °C in the first cooling cycle (peak 10), responsible for destabilized bulk water crystallization, followed by CO crystallization (peak 11). During the heating cycle, CO melted at peak 12, followed by ice melting (peak 13), GMO melting (peak 14), and HSO melting (peak 15). The ice melting peak for the control 50 wt.% water emulsions (peak 13) was more significant than that for the control 20 wt.% water emulsions (peak 4), due to the presence of more water in the former. The second cooling scan showed HSO crystallization at 30 °C (peak 16), followed by a large bulk water crystallization peak ranging from −13 to −20 °C (peak 17), which was even larger than the first cooling water crystallization peak (peak 10), indicating extensive water droplet instability, followed by CO crystallization in peak 18.
From the above discussion of DSC freeze/thaw cycles, emulsion stability can be predicted from water crystallization peak analysis. The appearance of multiple emulsified-water droplet crystallization peaks in the first cooling cycle could be ascribed to homogeneous nucleation of water where the impurities are divided among the numerous water droplets, indicating emulsion stability [
22]. In contrast, the appearance of a bulk water crystallization peak in the first cooling cycle around −20 °C is a sign of extensive water droplet coalescence and emulsion destabilization. The effect of water droplet size on water crystallization temperature has been studied by Clausse et al. [
23,
24] via thermogranulometry, where the authors showed that the crystallization temperature increased with an increase in droplet size. Therefore, a water droplet crystallization peak at a higher temperature indicates larger coalesced water droplets. Using a similar DSC methodology, Ghosh, Tran and Rousseau [
10] reported a full water droplet destabilization upon heating a 20 wt.% W/O emulsion (4 wt.% GMO and 10 wt.% hydrogenated canola oil), showing a large bulk water crystallization peak during the second cooling cycle at −19 °C. Suppose that a stable emulsion, with droplet crystallization at between −30 and −40 °C, shows a bulk water crystallization peak (at around −20 °C) in the second cooling cycle. In that case, it indicates that the melting of fat crystals during the heating cycle was the emulsion’s main stabilization mechanism. On the other hand, if an emulsion shows stable water droplet crystallization in the second cooling cycle, its stabilization mechanism must be dependent not on fat crystals, but on the emulsifier’s interfacial stability. Such insights from using DSC freeze/thaw cycles make them a powerful technique for predicting the emulsion stabilization mechanism.
For the rest of the DSC thermograms, only the water droplet crystallization peak was identified as being key to understanding emulsion stability. Aged control emulsions (
Figure 7B) with 20 wt.% water showed similar water crystallization peaks (peak 19) in the first cooling cycle as in the fresh emulsions, indicating unchanged stability. The second cooling also exhibited a large bulk water peak surrounded by a few smaller peaks (peak 20), indicating partial destabilization related to the day 1 emulsions. Aged control emulsions with 50 wt.% water showed similar large bulk water crystallization peaks in the first and second cooling cycles (peaks 21 and 22, respectively) to the day 1 emulsions, which illustrated extensive emulsion destabilization. Therefore, between the two controls, 20 wt.% water emulsions showed better stability than the 50 wt.% water emulsions.
The thermograms of day 1 and day 30 LMP emulsions are shown in
Figure 7C–F. On day 1, LMP emulsions with 20 wt.% water showed water crystallization in a small peak (peak 23) at −18 °C during the first cooling due to crystallization of large water droplets, and a tiny peak at −35 °C (peak 24) due to very small water droplets, indicating stable emulsions (
Figure 7C). The second cooling scan illustrated water crystallization at −14 °C (peak 25) with multiple tiny peaks, related to water droplets, followed by another small stable water droplet crystallization peak at −35 °C (peak 26), indicating that the emulsion was able to prevent complete destabilization even by complete melting of the stabilizing HSO crystals. Khudyakov et al. [
25] found that pectins can modify the ice morphology during the transition of liquid water to ice. Even a small pectin concentration (0.2 and 0.4 wt.%) exhibited cryoprotective properties in frozen cells’ integrity. In the present case, utilizing water droplet crystallization enthalpy, we calculated that LMP emulsions with 20 wt.% water presented a 65% reduction in the crystallization enthalpy of water droplets in the second cooling cycle (
Figure 7A), indicating 65% destabilization due to freeze/thaw, which was much lower than the 93% destabilization for 20 wt.% control emulsions (
Figure 7C). This indicates a similar cryoprotective effect associated with LMP incorporation in the emulsion.
LMP emulsions with 30 wt.% water illustrated a large bulk water crystallization peak at −10 °C (peak 27), followed by two small spikes at around −19 °C during the first cooling cycle. The second cooling exhibited destabilized bulk water crystallization at −10 °C, with a large peak and subsequent small peaks until −20 °C (peak 28). This indicates that most water droplets were unstable, but the emulsions did not completely destabilize, even after melting the stabilizing HSO crystals. Aged LMP emulsions with 20 and 30 wt.% water (
Figure 7D) showed similar peaks to those observed on day 1 (peaks 29–34), which indicates that the emulsion stability remained unchanged after 30 days of storage.
LMP emulsions with 40 wt.% water (
Figure 7E) exhibited water crystallization at around −10 °C during the first cooling scan on day 1 (peak 35). In the second cooling, water crystallized with a similar large peak at around −10 °C, followed by a few tiny peaks related to some very large water droplets (peak 36). LMP emulsions with 50 wt.% water (day 1,
Figure 7E) also illustrated a long sharp peak of water crystallization at −10 °C during the first cooling (peak 37), and a similar peak during the second cooling (peak 38), which suggested entirely unstable water droplets. Aged emulsions with 40 wt.% water (
Figure 7F) reported similar water crystallization peaks (peak 39) as on day 1, showing that some droplets were still present in the second cooling (peak 40). Moreover, LMP emulsions with 50 wt.% water reported similar water crystallization peaks as on day 1, but with few spikes and peaks that followed the large peaks (peaks 41 and 42), indicating only a few stable water droplets. From the DSC freeze/thaw cycles of emulsions, it is clear that as the water content increased, more water crystallized at a higher temperature, similar to bulk water instead of emulsified water droplets. In highly concentrated emulsions, impurities would be distributed in many droplets, leading to faster heterogeneous nucleation in many droplets. Moreover, as the water droplets were closely packed, crystallization in one droplet could lead to ice crystals rupturing other surrounding droplets’ membranes, leading to interdroplet heterogeneous nucleation and crystallization of all droplets at a higher temperature [
22]. Upon melting, the ice crystal bridge between the droplets would lead to collision-mediated coalescence and destabilization of emulsions. Therefore, the ability of the DSC to predict emulsion stability is not quite applicable to concentrated emulsions.
Similar freeze/thaw-induced emulsion destabilization via ice-crystal-induced water droplet collision was also observed by Lin et al. [
26]. Aronson and Petko [
27] also reported freeze/thaw experiments of 92 wt.% water-in-paraffin-oil emulsion (10 wt.% GMO in the oil phase and magnesium sulfate in the aqueous phase). The emulsion without the salt in the aqueous phase consisted of 90% ice when frozen and could not resist destabilization via the freeze/thaw cycle. During freezing, the water droplets progressively distorted to create ice blocks, breaking the interdroplet oil film and puncturing other water droplets. During thawing, the ice melted, leading to the coalescence of water droplets and emulsion destabilization. In the emulsion with magnesium sulfate in the aqueous phase, 83% ice was formed, while 9% water remained liquid due to salt-induced depression of the freezing point. This emulsion was stable to the freeze/thaw cycle. The authors proposed that the presence of liquid water wetted the ice crystals and provided protection against fusion among the ice crystals from adjacent water droplets.
3.6. Solid Fat Content of Emulsions
The viscoelastic properties of the W/O emulsions originate from the presence of water droplets and the fat crystal network in the continuous phase and around the water droplets. To investigate the effect of water content on emulsion viscoelasticity, we kept the amount of HSO constant in all of the emulsions (7 wt.%). However, depending on the water content, the amount of GMO varied. The amount of total solid fat in the emulsions at 4 °C also varied based on the solubility of HSO in CO [
10]. Therefore, the emulsions’ solid fat content at 4 °C was determined by measuring the solid fat enthalpies using a DSC (
Figure 8). On day 1, all emulsions showed solid fat content ranging from 6.4 ± 0.5 wt.% to 7.4 ± 0.4 wt.% (
p > 0.05). After 30 days, solid fat content increased in all emulsions (6.8 ± 0.3 wt.% to 7.8 ± 0.4 wt.%), although it was not significant (
p > 0.05). Such an increase over time could be due to crystal growth. Similar results have been reported by Ghosh and Rousseau [
28] using hydrogenated canola oil with an initial solid fat content of ~ 6 wt.% in fresh emulsions. After 4 weeks, the solid fat content increased to 7 wt.%. The authors implied that in fresh emulsions, GMO’s presence might hinder the packing of saturated fatty acids into crystal lattices [
28]. In this study, the increase in solid fat content of more than 7 wt.% could be due to partial GMO crystallization at the water droplet surface. The DSC thermograms of all emulsions showed a GMO melting peak at around 10 °C; we also observed GMO crystallization at around 13 °C from CO in the bulk oil phase (data not shown). The minor increase in solid fat content could also be due to water evaporation during storage. Emulsions slowly destabilize over time due to water droplet coalescence, and the free water is more accessible to evaporate during storage leading to a slight increase in solid fat content. However, such a minor increase in solid fat content would not significantly influence emulsion viscoelasticity for various emulsions; therefore, the change in the water volume fraction is the most important factor for the variation in emulsions’ viscoelasticity.