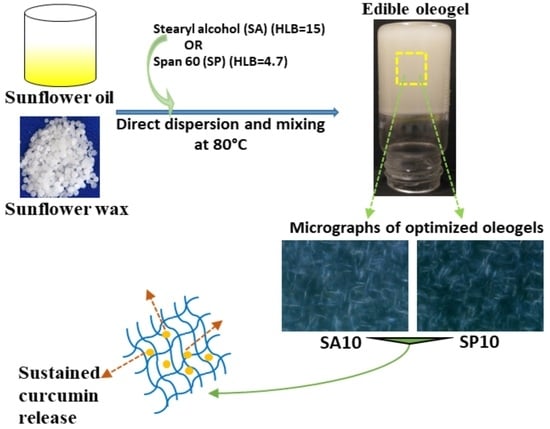
Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels
Abstract
:1. Introduction
2. Results and Discussion
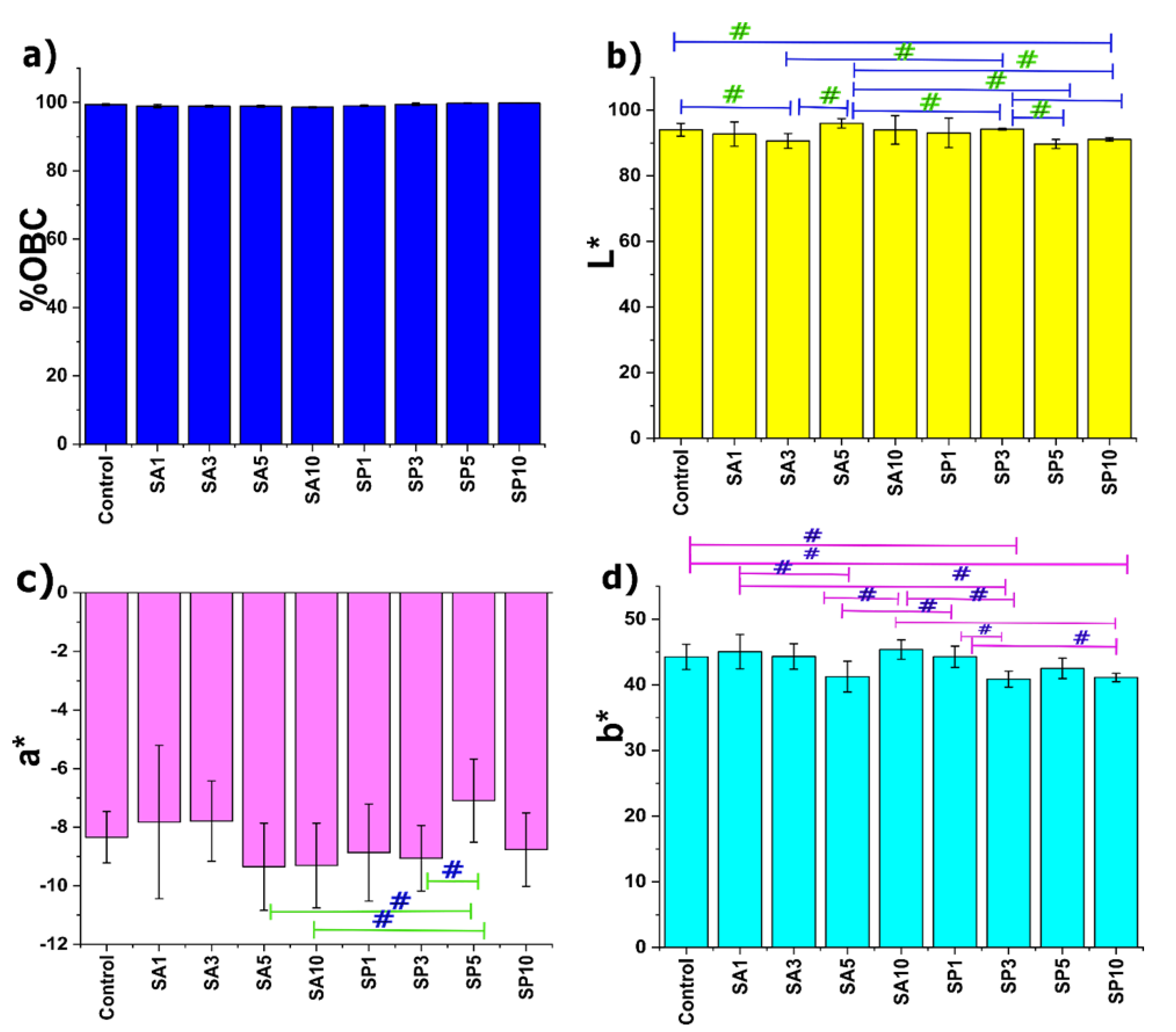
2.1. Oil-Binding Capacity
2.2. Colorimetry Study
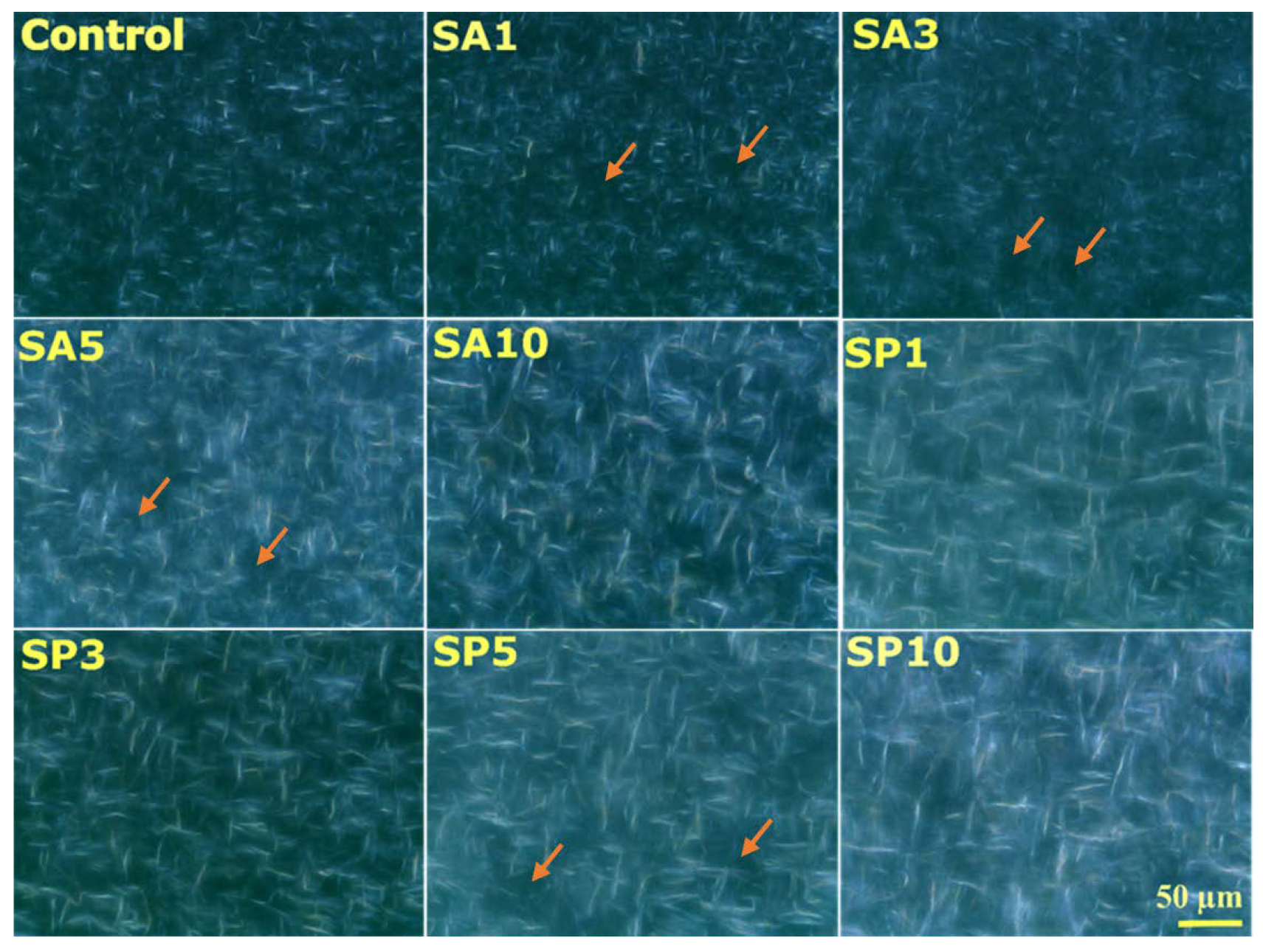
2.3. Microscopy
2.3.1. Surface Topology
2.3.2. Microstructure Analysis
2.4. Molecular Characterizations
2.4.1. XRD Analysis
2.4.2. FTIR Analysis
2.5. Thermal Analysis
2.5.1. Crystallization Kinetics
2.5.2. Differential Scanning Calorimetry
2.6. Mechanical Studies
2.7. Nutrient Release
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oleogel Synthesis
4.3. Quantification of OBC
4.4. Colorimetric Study
4.5. Microscopic Studies
4.5.1. Surface Features
4.5.2. Microstructural Observation
4.6. Molecular Studies
4.6.1. Fourier-Transform Infrared (FTIR) Spectroscopy
4.6.2. X-ray Diffraction Analysis (XRD)
4.7. Thermal Analysis
4.7.1. Crystallization Studies
4.7.2. Differential Scanning Calorimeter (DSC)
4.8. Mechanical Characterization
4.9. Nutrient Release
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcus, J.B. Flavor Enhancement Techniques. In Aging, Nutrition and Taste; Academic Press: Cambridge, MA, USA, 2019; pp. 207–247. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible oil structuring: An overview and recent updates. Food Funct. 2015, 7, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Formation, characterization, and potential food application of rice bran wax oleogels: Expeller-pressed corn germ oil versus refined corn oil. Food Chem. 2019, 309, 125704. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, A.A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Borriello, A.; Miele, N.A.; Masi, P.; Aiello, A.; Cavella, S. Effect of fatty acid composition of vegetable oils on crystallization and gelation kinetics of oleogels based on natural wax. Food Chem. 2022, 375, 131805. [Google Scholar] [CrossRef]
- Botega, D.C.Z.; Marangoni, A.G.; Smith, A.K.; Goff, H.D. The Potential Application of Rice Bran Wax Oleogel to Replace Solid Fat and Enhance Unsaturated Fat Content in Ice Cream. J. Food Sci. 2013, 78, C1334–C1339. [Google Scholar] [CrossRef]
- Kinyanjui, T.; Artz, W.; Mahungu, S. EMULSIFIERS|Uses in Processed Foods. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 2080–2086. [Google Scholar] [CrossRef]
- Uvanesh, K.; Sagiri, S.S.; Banerjee, I.; Shaikh, H.; Pramanik, K.; Anis, A.; Pal, K. Effect of Tween 20 on the Properties of Stearate Oleogels: An in-Depth Analysis. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 711–719. [Google Scholar] [CrossRef]
- Sarig, J.; Garti, S. Emulsifiers as Additives in Fats: Effect on Polymorphic Transformations and Crystal Properties of Fatty Acids and Triglycerides. Food Struct. 1990, 9, 4. Available online: https://digitalcommons.usu.edu/foodmicrostructureAvailableat:https://digitalcommons.usu.edu/foodmicrostructure/vol9/iss4/1 (accessed on 9 November 2021).
- Golodnizky, D.; Davidovich-Pinhas, M. The Effect of the HLB Value of Sucrose Ester on Physiochemical Properties of Bigel Systems. Foods 2020, 9, 1857. [Google Scholar] [CrossRef]
- Öğütcü, M.; Yılmaz, E. Characterization of Hazelnut Oil Oleogels Prepared with Sunflower and Carnauba Waxes. Int. J. Food Prop. 2014, 18, 1741–1755. [Google Scholar] [CrossRef]
- Omonov, T.S.; Bouzidi, L.; Narine, S.S. Quantification of oil binding capacity of structuring fats: A novel method and its application. Chem. Phys. Lipids 2010, 163, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Barbut, S. Fat reduction in comminuted meat products-effects of beef fat, regular and pre-emulsified canola oil. Meat Sci. 2011, 87, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S.; Kim, S.; Evans, K.O.; Koga, C.; Lee, Y. Morphology and networks of sunflower wax crystals in soybean oil organogel. Food Struct. 2015, 5, 10–20. [Google Scholar] [CrossRef]
- Pinto, T.; Martins, A.; Pastrana, L.; Pereira, M.; Cerqueira, M. Oleogel-Based Systems for the Delivery of Bioactive Compounds in Foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- de Man, J.M. Microscopy in the Study of Fats and Emulsions. Food Struct. 1982, 1, 2. Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1038&context=foodmicrostructure (accessed on 23 February 2022).
- Rizzo, G.; Norton, J.; Norton, I. Emulsifier effects on fat crystallisation. Food Struct. 2015, 4, 27–33. [Google Scholar] [CrossRef]
- Purohit, S.R.; Jayachandran, L.E.; Raj, A.S.; Nayak, D.; Rao, P.S. X-ray diffraction for food quality evaluation. In Evaluation Technologies for Food Quality; Woodhead Publishing: Sawston, UK, 2019; pp. 579–594. [Google Scholar] [CrossRef]
- Rincón-Cardona, J.A.; Martini, S.; Candal, R.J.; Herrera, M.L. Polymorphic behavior during isothermal crystallization of high stearic high oleic sunflower oil stearins. Food Res. Int. 2013, 51, 86–97. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, C.; Ji, X.; Hu, D.; Chen, Y.; Liu, M. Surfactants assisted synthesis and electrochemical properties of nano-LiFePO4/C cathode materials for low temperature applications. J. Power Sources 2015, 288, 337–344. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of biodegradable hydrophilic and hydrophobic emulsifiers on the oleogels containing sunflower wax and sunflower oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Garcés, R.; Martínez-Force, E.; Salas, J.J.; Venegas-Calerón, M. Current advances in sunflower oil and its applications. Lipid Technol. 2009, 21, 79–82. [Google Scholar] [CrossRef]
- Ongpipattanakul, B.; Francoeur, M.L.; Potts, R.O. Polymorphism in stratum corneum lipids. Biochim. Biophys. Acta Biomembr. 1994, 1190, 115–122. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Liu, Y.; Xu, Y.; Tan, C.-P. Crystal network structure and stability of beeswax-based oleogels with different polyunsaturated fatty acid oils. Food Chem. 2021, 381, 131745. [Google Scholar] [CrossRef]
- Douaire, M.; di Bari, V.; Norton, J.; Sullo, A.; Lillford, P.; Norton, I. Fat crystallisation at oil–water interfaces. Adv. Colloid Interface Sci. 2014, 203, 1–10. [Google Scholar] [CrossRef]
- Sonwai, S.; Podchong, P.; Rousseau, D. Crystallization kinetics of cocoa butter in the presence of sorbitan esters. Food Chem. 2017, 214, 497–506. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Meng, Z.; Chai, X.; Cao, C.; Liu, Y. Beeswax and carnauba wax modulate the crystallization behavior of palm kernel stearin. LWT 2019, 115, 108446. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Bin Sintang, M.D.; Danthine, S.; van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: A Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2016, 12, 97–108. [Google Scholar] [CrossRef]
- Rogers, A.M.; Wright, A.J.; Marangoni, A.G. Post-crystallization increases in the mechanical strength of self-assembled fibrillar networks is due to an increase in network supramolecular ordering. J. Phys. D Appl. Phys. 2008, 41, 5. [Google Scholar] [CrossRef]
- Hani, U. Solubility enhancement and delivery systems of curcumin a herbal medicine: A review. Curr. Drug Deliv. 2014, 11, 792–804. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Cunha, R.L.; Vicente, A.A. Fortified beeswax oleogels: Effect of β-carotene on the gel structure and oxidative stability. Food Funct. 2017, 8, 4241–4250. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Joseph, J.; Sharma, A.; Paul, J. Particle size and shape effects on the surface mechanical properties of aluminium coated with carbonaceous materials. J. Compos. Mater. 2018, 53, 261–270. [Google Scholar] [CrossRef]
- Saffarionpour, S.; Diosady, L.L. Curcumin, a potent therapeutic nutraceutical and its enhanced delivery and bioaccessibility by pickering emulsions. Drug Deliv. Transl. Res. 2021, 12, 124–157. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Qureshi, D.; Choudhary, B.; Mohanty, B.; Sarkar, P.; Anis, A.; Cerqueira, M.A.; Banerjee, I.; Maji, S.; Pal, K. Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels. Gels 2020, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Aklakur, M.; Rather, M.A.; Kumar, N. Nanodelivery: An Emerging Avenue for Nutraceuticals and Drug Delivery. Crit. Rev. Food Sci. Nutr. 2016, 56, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Jindal, R. Comparative Evaluation for Controlled Release of Amoxicillin from RSM-CCD-Optimized Nanocomposites Based on Sodium Alginate and Chitosan-Containing Inclusion Complexes. Cite This Mol. Pharm. 2021, 18, 3795–3810. [Google Scholar] [CrossRef]
- Qureshi, D.; Nadikoppula, A.; Mohanty, B.; Anis, A.; Cerqueira, M.; Varshney, M.; Pal, K. Effect of carboxylated carbon nanotubes on physicochemical and drug release properties of oleogels. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125695. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Saleh, A.S.; Yang, H.; Wang, N.; Wang, P.; Yue, X.; Xiao, Z. Functional Characteristics of Oleogel Prepared from Sunflower Oil with β-Sitosterol and Stearic Acid. J. Am. Oil Chem. Soc. 2017, 94, 1153–1164. [Google Scholar] [CrossRef]
- Jain, A.; Pradhan, B.K.; Mahapatra, P.; Ray, S.S.; Chakravarty, S.; Pal, K. Development of a low-cost food color monitoring system. Color Res. Appl. 2020, 46, 430–445. [Google Scholar] [CrossRef]
- Das, P.; Qureshi, D.; Paul, S.; Mohanty, B.; Anis, A.; Verma, S.; Wilczyński, S.; Pal, K. Effect of sorbitan monopalmitate on the polymorphic transitions and physicochemical properties of mango butter. Food Chem. 2021, 347, 128987. [Google Scholar] [CrossRef] [PubMed]


| Formulations | Peak | Peak Position (° 2θ) | FWHM (° 2θ) | d-Spacing (Å) | Crystallite Size (nm) | Lattice Strain | Dislocation Density (δ) × 10 17 lines/m2 |
|---|---|---|---|---|---|---|---|
| Control | 1 | 22.82 | 8.102 | 4.52 | 1.21 | 0.17 | 0.68 |
| 2 | 22.82 | 4.09 | 4.52 | 2.40 | 0.08 | 0.17 | |
| 3 | 25.06 | 0.42 | 4.12 | 23.35 | 0.00 | 0.00 | |
| 4 | 25.06 | 14.17 | 4.12 | 0.70 | 0.27 | 2.04 | |
| 5 | 27.85 | 0.43 | 3.71 | 23.18 | 0.00 | 0.00 | |
| Average | - | - | 4.20 | 10.16 | 0.11 | 0.58 | |
| SA1 | 1 | 21.19 | 10.07 | 4.86 | 0.97 | 0.23 | 1.06 |
| 2 | 22.23 | 4.61 | 4.63 | 2.13 | 0.10 | 0.22 | |
| 3 | 24.69 | 0.53 | 4.18 | 18.36 | 0.01 | 0.00 | |
| 4 | 24.69 | 8.88 | 4.11 | 1.11 | 0.17 | 0.81 | |
| 5 | 27.47 | 0.53 | 3.76 | 18.53 | 0.01 | 0.00 | |
| Average | - | - | 4.32 | 8.22 | 0.11 | 0.42 | |
| SA3 | 1 | 23.09 | 8.46 | 4.46 | 1.16 | 0.18 | 0.74 |
| 2 | 23.09 | 4.29 | 4.46 | 2.29 | 0.09 | 0.19 | |
| 3 | 25.33 | 0.42 | 4.07 | 23.39 | 0.00 | 0.00 | |
| 4 | 25.33 | 14.42 | 4.07 | 0.69 | 0.27 | 2.10 | |
| 5 | 28.10 | 0.44 | 3.68 | 22.41 | 0.00 | 0.00 | |
| Average | - | - | 4.15 | 9.98 | 0.11 | 0.60 | |
| SA5 | 1 | 23.01 | 9.02 | 4.48 | 1.09 | 0.19 | 0.84 |
| 2 | 23.01 | 4.47 | 4.48 | 2.20 | 0.09 | 0.20 | |
| 3 | 25.13 | 0.38 | 4.11 | 25.33 | 0.01 | 0.00 | |
| 4 | 27.84 | 0.45 | 3.71 | 21.99 | 0.01 | 0.00 | |
| 5 | 27.84 | 11.96 | 3.71 | 0.83 | 0.21 | 1.45 | |
| Average | Average | - | - | 4.10 | 10.28 | 0.10 | 0.50 |
| SA10 | 1 | 22.31 | 9.13 | 4.62 | 1.08 | 0.20 | 0.86 |
| 2 | 22.85 | 4.58 | 4.51 | 2.15 | 0.09 | 0.21 | |
| 3 | 25.14 | 0.40 | 4.10 | 24.58 | 0.00 | 0.00 | |
| 4 | 25.14 | 10.73 | 4.10 | 0.92 | 0.21 | 1.18 | |
| 5 | 27.87 | 0.43 | 3.71 | 22.96 | 0.00 | 0.00 | |
| Average | - | - | 4.21 | 10.33 | 0.10 | 0.45 | |
| SP1 | 1 | 22.62 | 10.02 | 4.56 | 0.98 | 0.22 | 1.04 |
| 2 | 22.86 | 4.78 | 4.51 | 2.05 | 0.10 | 0.23 | |
| 3 | 25.15 | 0.39 | 4.10 | 4.10 | 0.10 | 0.05 | |
| 4 | 27.66 | 0.96 | 3.74 | 10.25 | 0.01 | 0.00 | |
| 5 | 27.66 | 6.52 | 3.74 | 1.52 | 0.11 | 0.43 | |
| Average | - | - | 4.133 | 3.78 | 0.11 | 0.35 | |
| SP3 | 1 | 22.93 | 9.73 | 4.49 | 1.01 | 0.20 | 0.98 |
| 2 | 22.93 | 4.55 | 4.49 | 2.16 | 0.9 | 0.21 | |
| 3 | 25.18 | 0.41 | 4.10 | 2.16 | 0.09 | 0.21 | |
| 4 | 27.95 | 0.47 | 3.70 | 20.98 | 0.00 | 0.00 | |
| 5 | 27.95 | 11.30 | 3.70 | 0.88 | 0.19 | 1.29 | |
| Average | - | - | 4.10 | 5.44 | 0.12 | 0.54 | |
| SP5 | 1 | 23.20 | 7.93 | 4.45 | 1.24 | 0.16 | 0.65 |
| 2 | 23.20 | 4.39 | 4.45 | 2.24 | 0.09 | 0.19 | |
| 3 | 25.36 | 0.43 | 4.07 | 22.5 | 0.00 | 0.00 | |
| 4 | 25.36 | 13.58 | 4.07 | 0.73 | 0.26 | 1.87 | |
| 5 | 28.12 | 28.12 | 3.68 | 21.37 | 0.00 | 0.00 | |
| Average | - | - | 4.14 | 9.616 | 0.11 | 0.54 | |
| SP10 | 1 | 21.88 | 9.66 | 4.71 | 1.02 | 0.21 | 0.96 |
| 2 | 22.75 | 4.63 | 4.53 | 2.12 | 0.10 | 0.22 | |
| 3 | 25.10 | 0.41 | 4.11 | 23.71 | 0.00 | 0.00 | |
| 4 | 25.10 | 9.13 | 4.11 | 1.08 | 0.17 | 0.85 | |
| 5 | 27.85 | 0.45 | 3.71 | 21.97 | 0.00 | 0.00 | |
| Average | -- | -- | 4.24 | 9.98 | 0.10 | 0.10 |
| Formulations | Temperature vs. Time | Exponential Decay Model | |
|---|---|---|---|
| Onset of Secondary Crystallization (s) | Time to Reach Thermal Equilibrium (s) | Rate of Crystallization (°C/ms) | |
| Control | 999 | 2217 | 0.99 |
| SA1 | 765 | 2089 | 1.50 |
| SA3 | 633 | 2109 | 1.60 |
| SA5 | 783 | 2169 | 1.32 |
| SA10 | 688 | 2393 | 1.26 |
| SP1 | 530 | 2199 | 1.76 |
| SP3 | 991 | 2568 | 1.11 |
| SP5 | 910 | 2515 | 1.14 |
| SP10 | 720 | 2288 | 1.17 |
| Formulations | Melting | Crystallization | Degree of Supercooling (°C) | ||||
|---|---|---|---|---|---|---|---|
| T0m (°C) | Tm (°C) | ∆Hm (J/g) | T0c (°C) | Tc (°C) | ∆Hc (J/g) | ||
| Control | 49.60 | 62.00 | 09.94 | 62.40 | 60.70 | 10.46 | 1.30 |
| SA1 | 48.60 | 62.10 | 09.40 | 62.00 | 60.30 | 10.30 | 1.80 |
| SA3 | 47.20 | 61.50 | 10.42 | 62.60 | 61.00 | 11.24 | 0.50 |
| SA5 | 47.40 | 62.00 | 09.81 | 62.40 | 60.70 | 11.30 | 1.30 |
| SA10 | 46.10 | 61.30 | 10.17 | 62.50 | 60.70 | 10.45 | 0.60 |
| SP1 | 47.50 | 61.70 | 09.91 | 62.30 | 60.20 | 10.86 | 1.50 |
| SP3 | 46.70 | 62.10 | 09.48 | 62.00 | 60.40 | 09.89 | 1.70 |
| SP5 | 47.30 | 61.80 | 09.65 | 62.10 | 60.40 | 10.18 | 1.40 |
| SP10 | 47.70 | 61.60 | 09.95 | 60.40 | 60.40 | 10.77 | 1.20 |
| Samples | CPRD at 180 min | Parameters of PS Model | ||||
|---|---|---|---|---|---|---|
| Kd | Kr | Kd/Kr | m | R2 | ||
| Control | 65.95 ± 4.68 a | 3.00 ± 0.01 bc | 0.67 ± 0.17 a | 4.67 ± 1.37 bd | 0.34 ± 0.01 de | 0.99 |
| SA1 | 73.58 ± 3.91 b | 4.21 ± 0.21 abd | 0.63 ± 0.18 ab | 6.71 ± 2.04 cd | 0.37± 0.02 ce | 0.99 |
| SA3 | 85.44 ± 2.49 a | 4.06 ± 0.06 a | 0.39 ± 0.05 a | 11.26 ± 1.82 ac | 0.39 ± 0.01 bc | 0.99 |
| SA5 | 70.03 ± 3.01 b | 4.02 ± 0.20 a | 0.59 ± 0.12 a | 6.98 ± 1.09 c | 0.50 ± 0.01 a | 0.99 |
| SA10 | 49.00 ± 2.85 c | 1.02 ± 0.02 d | 0.19 ± 0.05 b | 3.00 ± 0.51 cd | 0.43 ± 0.02 b | 0.99 |
| SP1 | 72.53 ± 1.17 b | 4.17 ± 0.15 a | 0.36 ± 0.06 ac | 15.48 ± 2.92 a | 0.40 ± 0.01 c | 0.99 |
| SP3 | 91.68 ± 3.26 a | 4.57 ± 0.07 abd | 0.35 ± 0.06 ac | 10.83 ± 0.89 abc | 0.42 + 0.16 abd | 0.99 |
| SP5 | 50.31 ± 71.5 c | 3.57 ± 0.58 ac | 0.40 ± 0.07 ac | 9.13 ± 2.77 abc | 0.34 ± 0.02 de | 0.99 |
| SP10 | 40.09 ± 2.29 d | 2.59 ± 0.57 bc | 0.24 ± 0.01 bc | 9.01 ± 2.19 bc | 0.39 ± 0.09 abcd | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. https://doi.org/10.3390/gels8080520
Bharti D, Kim D, Banerjee I, Rousseau D, Pal K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels. 2022; 8(8):520. https://doi.org/10.3390/gels8080520
Chicago/Turabian StyleBharti, Deepti, Doman Kim, Indranil Banerjee, Derick Rousseau, and Kunal Pal. 2022. "Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels" Gels 8, no. 8: 520. https://doi.org/10.3390/gels8080520