The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts
Abstract
:1. Introduction
2. Results and Discussion
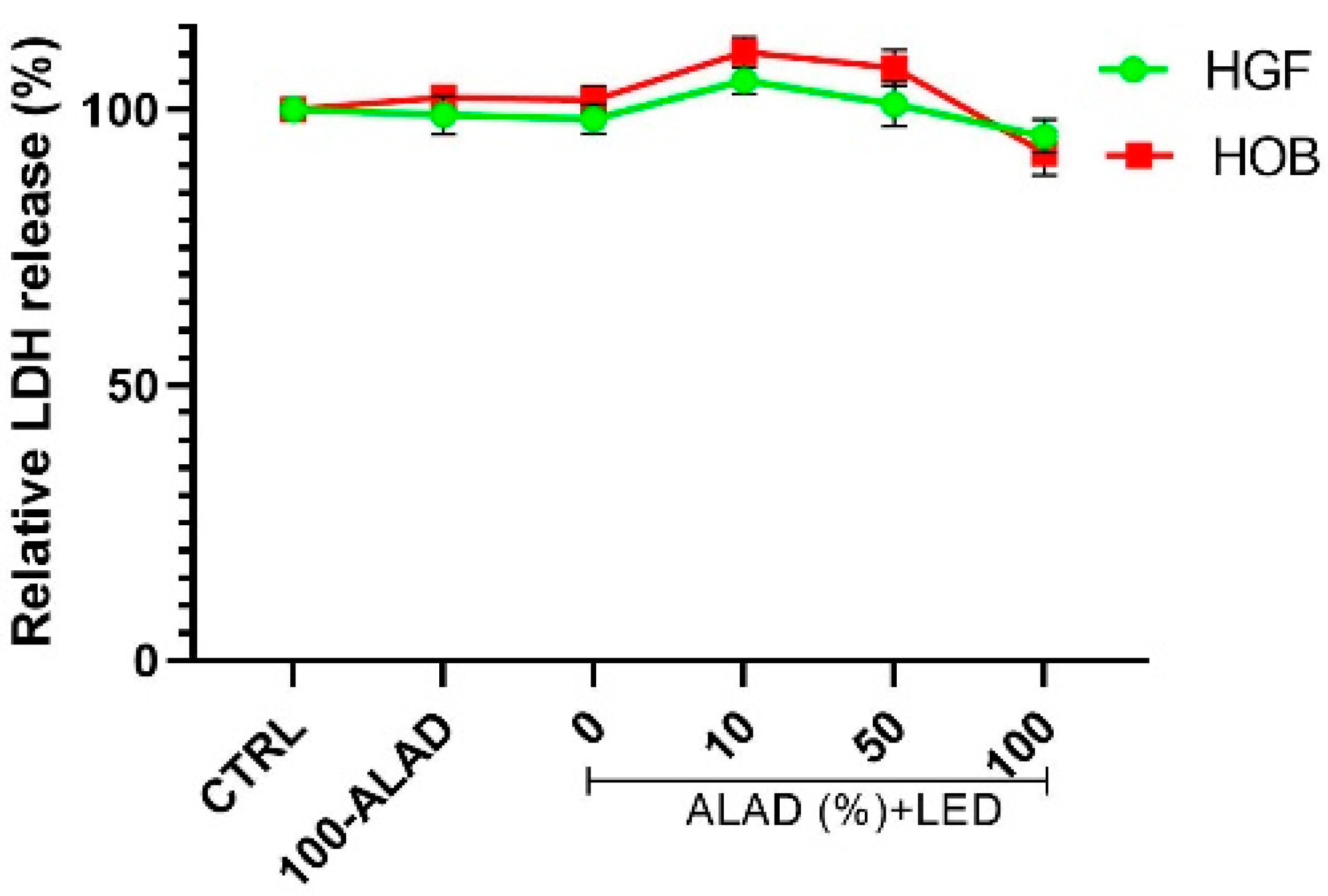
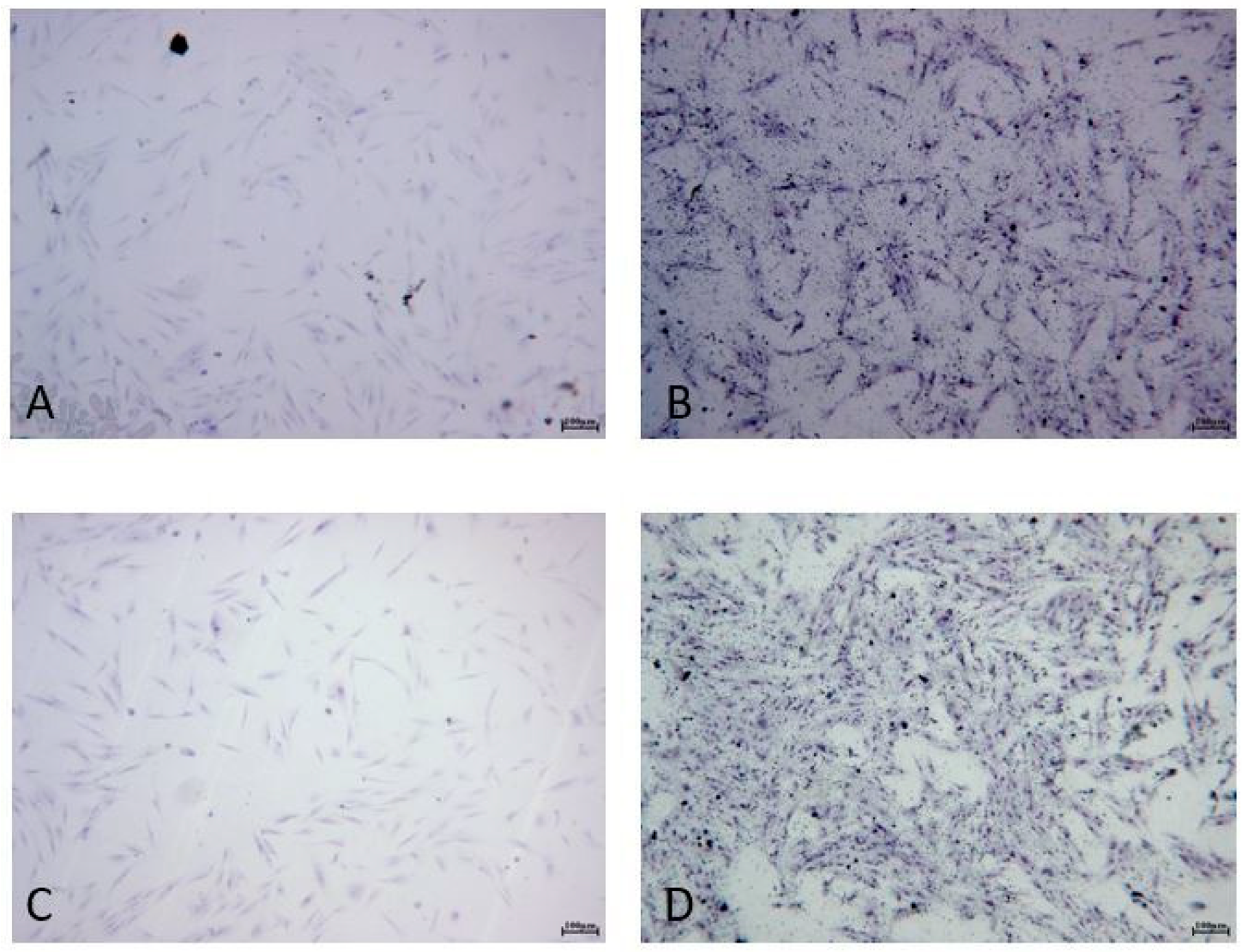
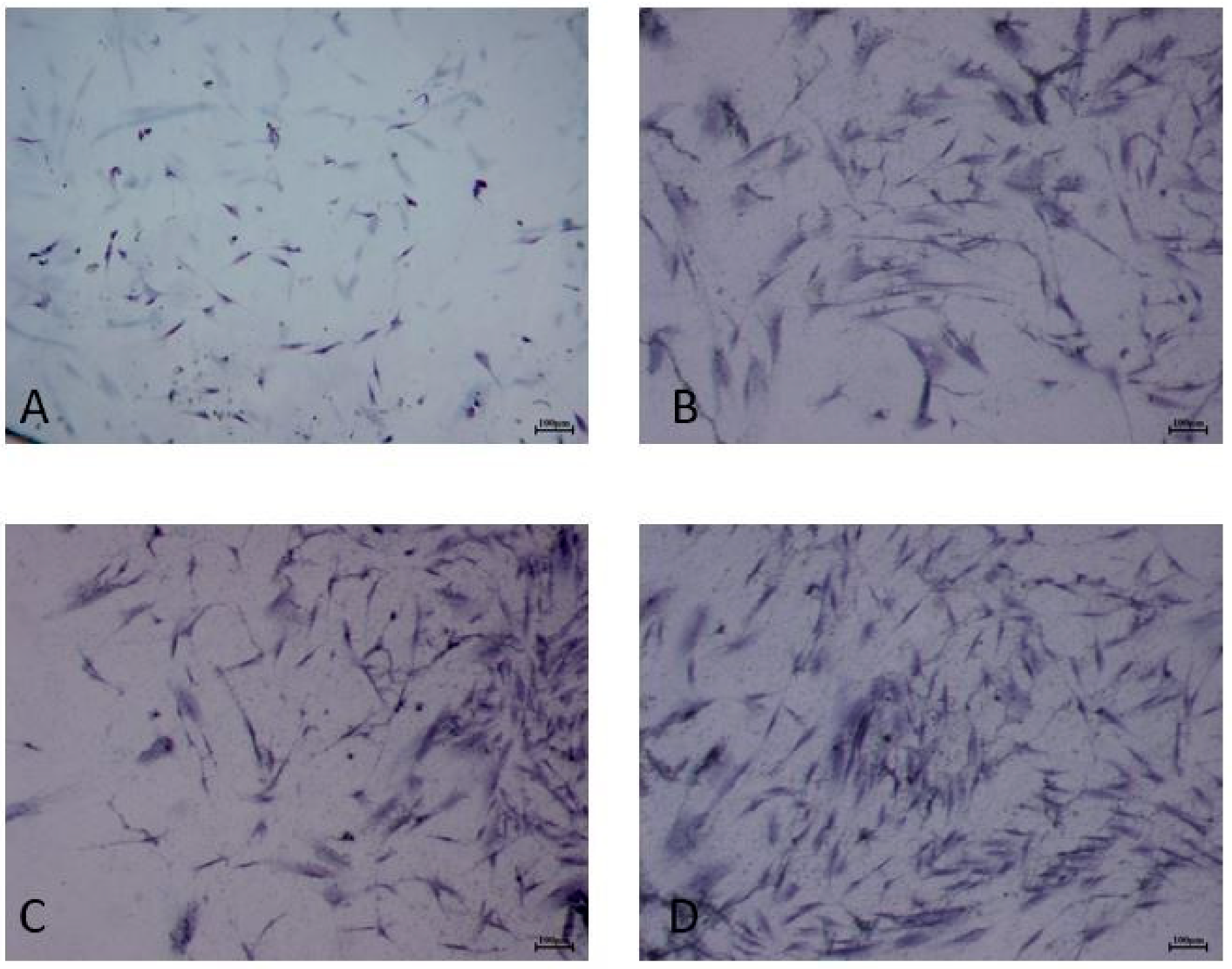
2.1. Cell Survival and Morphology after ALAD-PDT
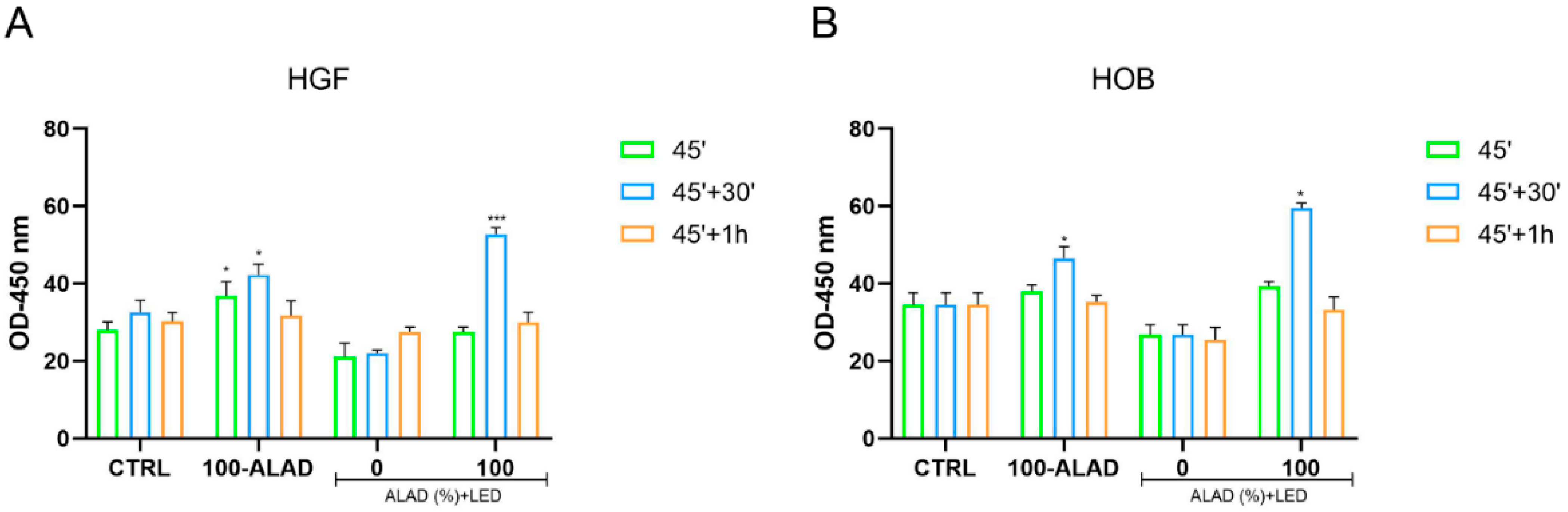
2.2. Cell Proliferation after ALAD-PDT
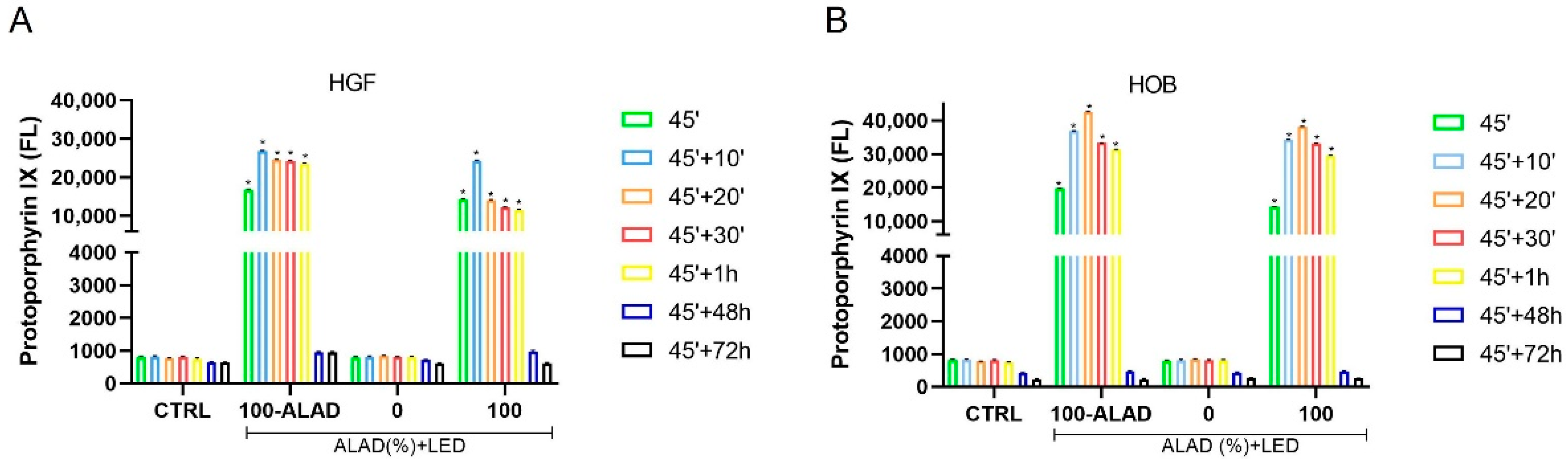
2.3. ALAD Induces Accumulation of PpIX
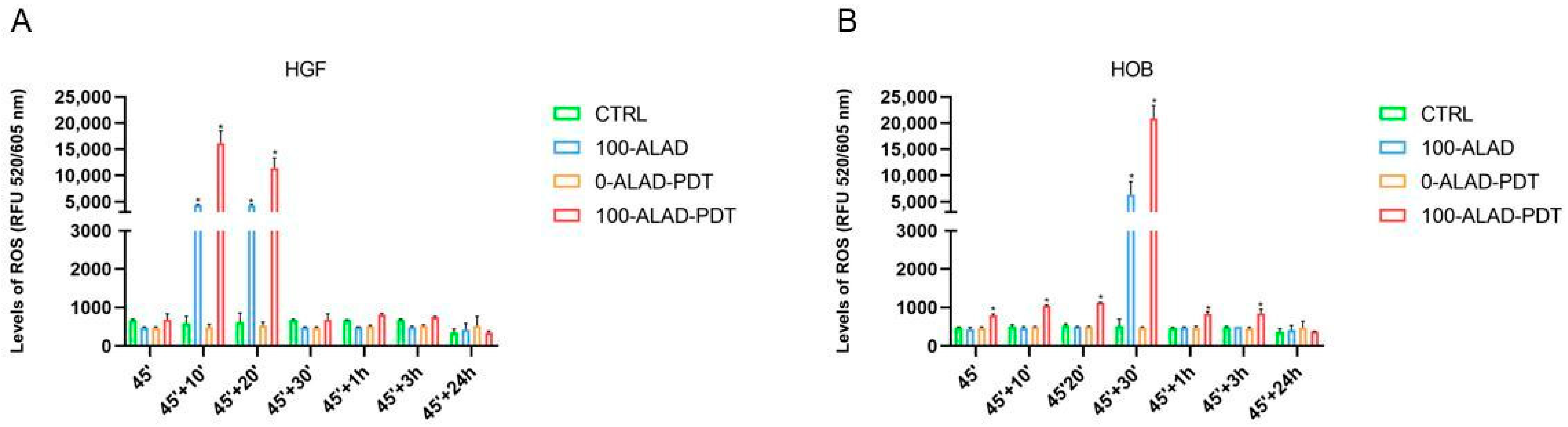
2.4. Intracellular ROS Levels after ALAD-PDT
2.5. SOD Activity after ALAD-PDT
3. Conclusions
4. Materials and Methods
4.1. Experimental Design
4.2. Cell Culture and Isolation
4.3. Treatments
4.4. Lactate Dehydrogenase (LDH) Release Assay
4.5. Toluidine Blue Staining
4.6. Cell Proliferation Assay
4.7. PpIX Fluorescence
4.8. Reactive Oxygen Species (ROS) Levels
4.9. Superoxide Dismutase (SOD) Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’ercole, S.; di Lodovico, S.; Iezzi, G.; Pierfelice, T.V.; D’amico, E.; Cipollina, A.; Piattelli, A.; Cellini, L.; Petrini, M. Complex Electromagnetic Fields Reduce Candida Albicans Planktonic Growth and Its Adhesion to Titanium Surfaces. Biomedicines 2021, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; di Lodovico, S.; Iezzi, G.; Cipollina, A.; Piattelli, A.; Cellini, L.; D’Ercole, S. Effects of Complex Electromagnetic Fields on Candida Albicans Adhesion and Proliferation on Polyacrylic Resin. Appl. Sci. 2021, 11, 6786. [Google Scholar] [CrossRef]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In Vitro Antimicrobial Activity of LED Irradiation on Pseudomonas Aeruginosa. J. Photochem. Photobiol. B 2017, 168, 25–29. [Google Scholar] [CrossRef]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In Vitro Inactivation of Enterococcus Faecalis with a Led Device. J. Photochem. Photobiol. B 2016, 160, 172–177. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. Faecalis. J. Photochem. Photobiol. B 2019, 197, 111527. [Google Scholar] [CrossRef]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of Bacteria by Endogenous Photosensitizers and Exposure to Visible Light of Different Wavelengths—A Review on Existing Data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef]
- Harris, F.; Pierpoint, L. Photodynamic Therapy Based on 5-Aminolevulinic Acid and Its Use as an Antimicrobial Agent. Med. Res. Rev. 2012, 32, 1292–1327. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The Inhibition of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Photodynamic Action for Prostate Cancer. Photodiagn. Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef]
- Petrini, M.; di Lodovico, S.; Iezzi, G.; Cellini, L.; Tripodi, D.; Piattelli, A.; D’ercole, S. Photodynamic Antibiofilm and Antibacterial Activity of a New Gel with 5-Aminolevulinic Acid on Infected Titanium Surfaces. Biomedicines 2022, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Carlesi, T.; Iezzi, G.; D’arcangelo, C.; di Lodovico, S.; Piattelli, A.; D’ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef] [PubMed]
- di Lodovico, S.; Diban, F.; di Fermo, P.; Petrini, M.; Fontana, A.; di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; di Piazza, S.; Chan, J.; Zotti, M.; Hanna, R.; Gheno, E.; Zekiy, A.O.; Pasquale, C.; de Angelis, N.; Amaroli, A. Newly Formulated 5% 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans. Photodiagn. Photodyn. 2020, 29, 101575. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Palmieri, A.; della Vella, F.; Petruzzi, M.; Botticelli, D.; Carinci, F. Photodynamic Therapy Using 5-Aminolevulinic Acid (Ala) for the Treatment of Chronic Periodontitis: A Prospective Case Series. Appl. Sci. 2022, 12, 3102. [Google Scholar] [CrossRef]
- Costin, G.-E.; Birlea, S.A.; Norris, D.A. Trends in Wound Repair: Cellular and Molecular Basis of Regenerative Therapy Using Electromagnetic Fields. Curr. Mol. Med. 2012, 12, 14–26. [Google Scholar] [CrossRef]
- Weinreb, M.; Nemcovsky, C.E. In Vitro Models for Evaluation of Periodontal Wound Healing/Regeneration. Periodontology 2000 2015, 68, 41–54. [Google Scholar] [CrossRef]
- Serini, S.M.; Cannizzaro, M.V.; Dattola, A.; Garofalo, V.; del Duca, E.; Ventura, A.; Milani, M.; Campione, E.; Bianchi, L. The Efficacy and Tolerability of 5-Aminolevulinic Acid 5% Thermosetting Gel Photodynamic Therapy (PDT) in the Treatment of Mild-to-Moderate Acne Vulgaris. A Two-Center, Prospective Assessor-Blinded, Proof-of-Concept Study. J. Cosmet. Derm. 2019, 18, 156–162. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Concepts of Osteoblast Growth and Differentiation: Basis for Modulation of Bone Cell Development and Tissue Formation. Crit. Rev. Oral Biol. Med. 1992, 3, 269–305. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Gutknecht, D.; Simon, J.C.; Schulz, J.N.; Eckes, B.; Anderegg, U.; Saalbach, A. Controlling the Balance of Fibroblast Proliferation and Differentiation: Impact of Thy-1. J. Investig. Dermatol. 2015, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and Transcriptional Control of Bone Formation. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egli, R.J.; di Criscio, A.; Hempfing, A.; Schoeniger, R.; Ganz, R.; Hofstetter, W.; Leunig, M. In Vitro Resistance of Articular Chondrocytes to 5-Aminolevulinic Acid Based Photodynamic Therapy. Lasers Surg. Med. 2008, 40, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bastian, J.D.; Egli, R.J.; Ganz, R.; Hofstetter, W.; Leunig, M. Differential Response of Porcine Osteoblasts and Chondrocytes in Cell or Tissue Culture after 5-Aminolevulinic Acid-Based Photodynamic Therapy. Osteoarthr. Cartil. 2009, 17, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushibiki, T.; Tu, Y.; Abu-Yousif, A.O.; Hasan, T. Photodynamic Activation as a Molecular Switch to Promote Osteoblast Cell Differentiation via AP-1 Activation. Sci. Rep. 2015, 5, 13114. [Google Scholar] [CrossRef] [Green Version]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem. Anticancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef]
- Moan, J.; van den Akker, J.T.H.M.; Juzenas, P.; Ma, L.W.; Angell-Petersen, E.; Gadmar, B.; Iani, V. On the Basis for Tumor Selectivity in the 5-Aminolevulinic Acid-Induced Synthesis of Protoporphyrin IX. J. Porphyr. Phthalocyanines 2001, 5, 170–176. [Google Scholar] [CrossRef]
- Egli, R.J.; Schober, M.; Hempfing, A.; Ganz, R.; Hofstetter, W.; Leunig, M. Sensitivity of Osteoblasts, Fibroblasts, Bone Marrow Cells, and Dendritic Cells to 5-Aminolevulinic Acid Based Photodynamic Therapy. J. Photochem. Photobiol. B 2007, 89, 70–77. [Google Scholar] [CrossRef]
- Moan, J.; Streckyte, G.; Bagdonas, S.; Bech, Ø.; Berg, K. Photobleaching of Protoporphyrin IX in Cells Incubated with 5-Aminolevulinic Acid. Int. J. Cancer 1997, 70, 90–97. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Milward, M.R.; Hadis, M.A.; Kuehne, S.A.; Cooper, P.R. Photobiomodulation of Mineralisation in Mesenchymal Stem Cells. Photochem. Photobiol. Sci. 2021, 20, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Chausse, B.; da Cunha, F.M.; Luévano-Martínez, L.A.; Marazzi, T.B.M.; Pessoa, P.S.; Queliconi, B.B.; Kowaltowski, A.J. Mitochondrial Compartmentalization of Redox Processes. Free Radic. Biol. Med. 2012, 52, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative Stress Modulates Osteoblastic Differentiation of Vascular and Bone Cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Lyublinskaya, O.G.; Borisov, Y.G.; Pugovkina, N.A.; Smirnova, I.S.; Obidina, J.V.; Ivanova, J.S.; Zenin, V.V.; Shatrova, A.N.; Borodkina, A.V.; Aksenov, N.D.; et al. Reactive Oxygen Species Are Required for Human Mesenchymal Stem Cells to Initiate Proliferation after the Quiescence Exit. Oxid. Med. Cell. Longev. 2015, 2015, 502105. [Google Scholar] [CrossRef] [Green Version]
- Sart, S.; Song, L.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid. Med. Cell. Longev. 2015, 2015, 105135. [Google Scholar] [CrossRef] [Green Version]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene Blue Mediated Antimicrobial Photodynamic Therapy in Clinical Human Studies: The State of the Art. Photodiagn. Photodyn. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Dascalu, L.M.; Moldovan, M.; Sarosi, C.; Sava, S.; Dreanca, A.; Repciuc, C.; Purdoiu, R.; Nagy, A.; Badea, M.E.; Paun, A.G.; et al. Photodynamic Therapy with Natural Photosensitizers in the Management of Periodontal Disease Induced in Rats. Gels 2022, 8, 134. [Google Scholar] [CrossRef]
- Liu, P.; Shi, J.; Wang, Z.A. Pattern Formation of the Attraction-Repulsion Keller-Segel System. Discret. Contin. Dyn. Syst.-B. 2013, 18, 2597. [Google Scholar] [CrossRef]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-Erythrocyte Hybrid Membrane-Coated Hollow Copper Sulfide Nanoparticles for Targeted and Photothermal/Anti-Inflammatory Therapy of Osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.; Qu, L.; Chen, S.; Wu, S. Electrospun Strong, Bioactive, and Bioabsorbable Silk Fibroin/Poly (L-Lactic-Acid) Nanoyarns for Constructing Advanced Nanotextile Tissue Scaffolds. Mater. Today Bio 2022, 14, 100243. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv. Funct. Mater. 2022, 2202470. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; D’amico, E.; Iezzi, G.; Piattelli, A.; di Pietro, N.; D’arcangelo, C.; Comuzzi, L.; Petrini, M. Nanoporous Titanium Enriched with Calcium and Phosphorus Promotes Human Oral Osteoblast Bioactivity. Int. J. Environ. Res. Public Health 2022, 19, 6212. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; di Pietro, N.; Pandolfi, A.; D’arcangelo, C.; de Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A.; et al. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. [Google Scholar] [CrossRef]
- Moan, J.; Bech, Ø.; Gaullier, J.M.; Stokke, T.; Steen, H.B.; Ma, L.W.; Berg, K. Protoporphyrin IX Accumulation in Cells Treated with 5-Aminolevulinic Acid: Dependence on Cell Density, Cell Size and Cell Cycle. Int. J. Cancer 1998, 75, 134–139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierfelice, T.V.; D’Amico, E.; Petrini, M.; Pandolfi, A.; D’Arcangelo, C.; Di Pietro, N.; Piattelli, A.; Iezzi, G. The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts. Gels 2022, 8, 491. https://doi.org/10.3390/gels8080491
Pierfelice TV, D’Amico E, Petrini M, Pandolfi A, D’Arcangelo C, Di Pietro N, Piattelli A, Iezzi G. The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts. Gels. 2022; 8(8):491. https://doi.org/10.3390/gels8080491
Chicago/Turabian StylePierfelice, Tania Vanessa, Emira D’Amico, Morena Petrini, Assunta Pandolfi, Camillo D’Arcangelo, Natalia Di Pietro, Adriano Piattelli, and Giovanna Iezzi. 2022. "The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts" Gels 8, no. 8: 491. https://doi.org/10.3390/gels8080491






