Hydrogels as Porogens for Nanoporous Inorganic Materials
Abstract
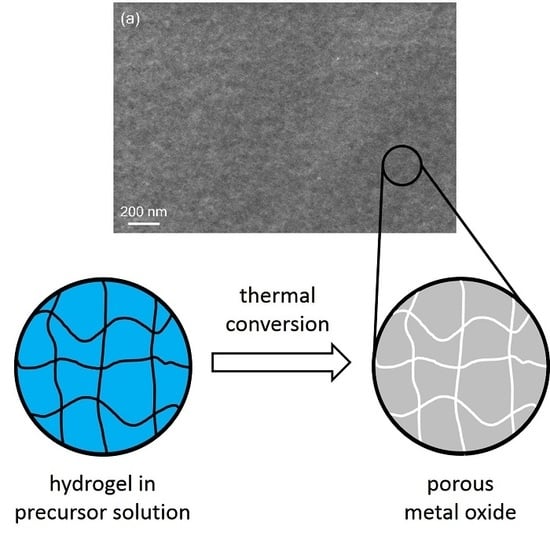
:1. Introduction
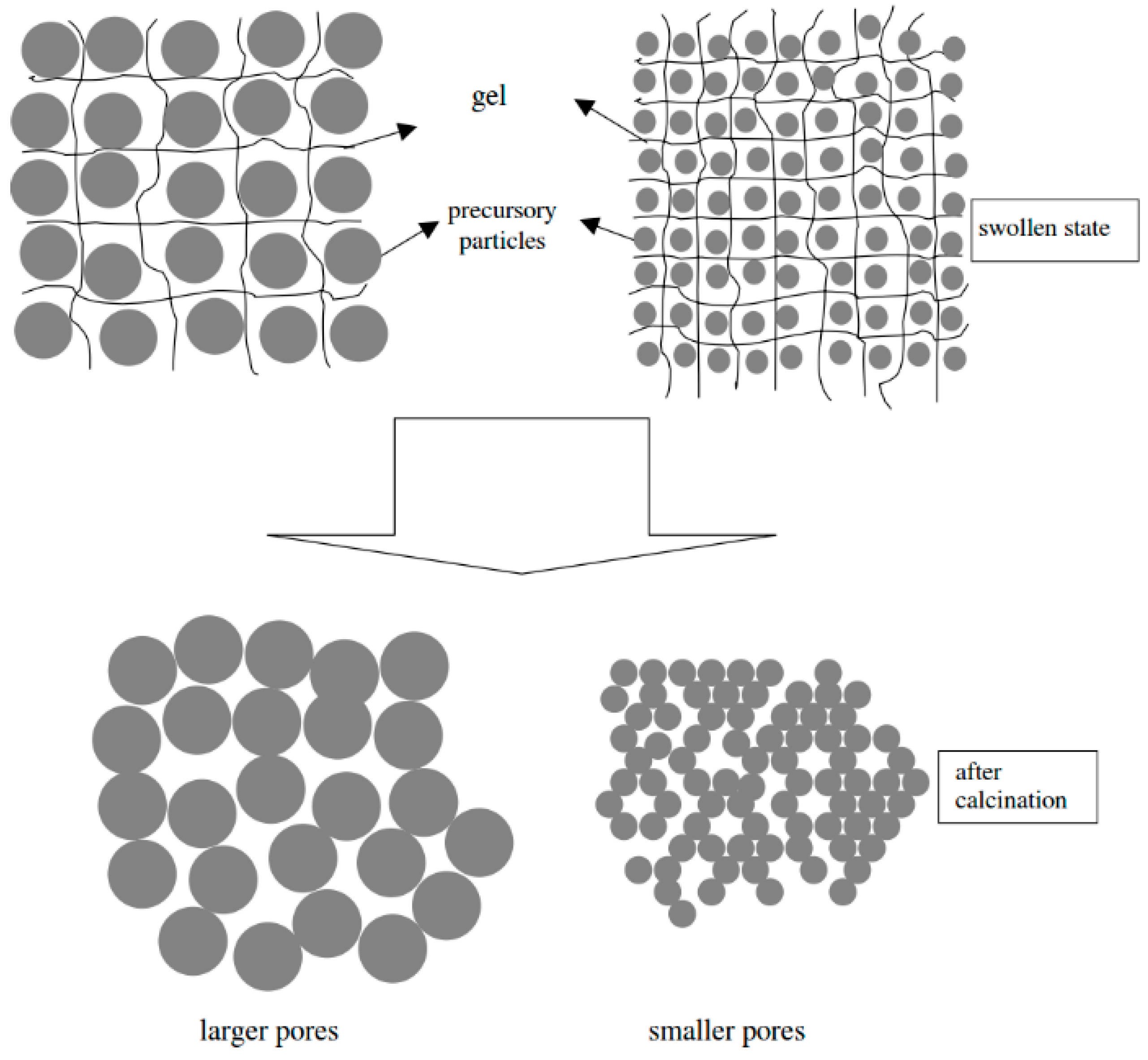
2. Physically Cross-Linked Porogenic Hydrogels
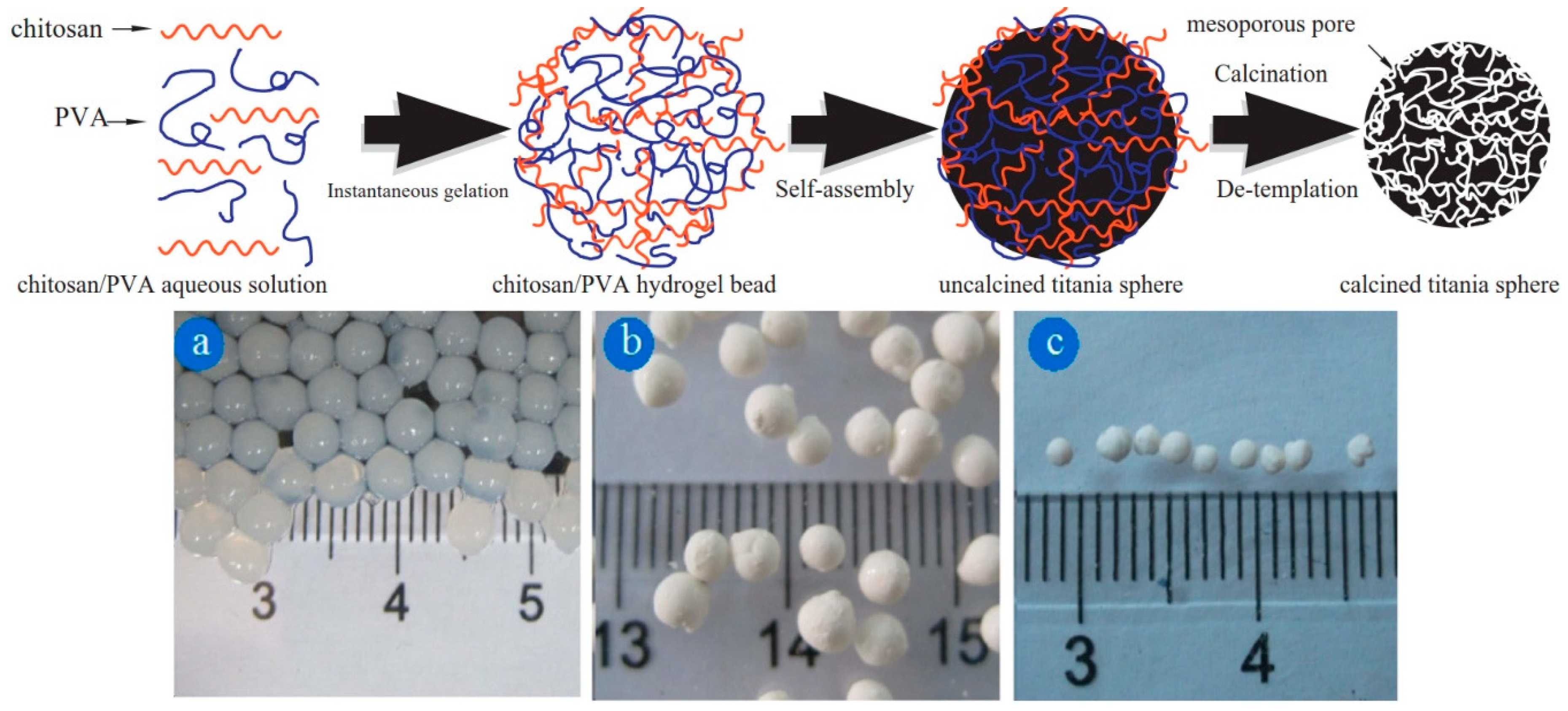
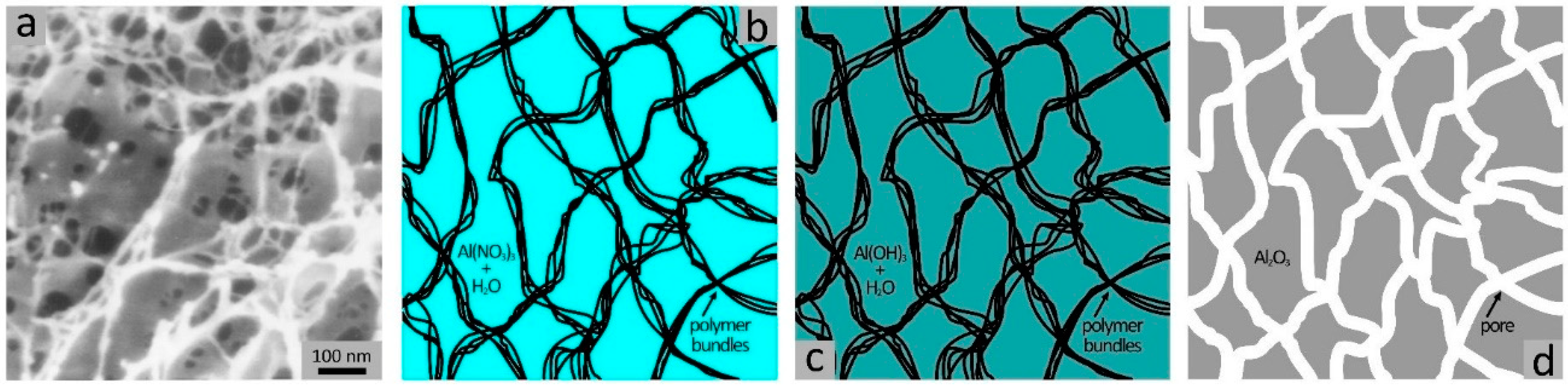
3. Chemically Cross-linked Porogenic Hydrogels
4. Further Examples of Hydrogel Matrices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Schmidt, W. Solid Catalysts on the Nanoscale: Design of Complex Morphologies and Pore Structures. ChemCatChem 2009, 1, 53–67. [Google Scholar] [CrossRef]
- Tüysüz, H.; Schüth, F. Ordered Mesoporous Materials as Catalysts. Adv. Catal. 2012, 55, 127–239. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered mesoporous metal oxides: Synthesis and applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, M. Porous metal oxides as gas sensors. Chem. Eur. J. 2007, 13, 8376–8388. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous materials as gas sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, N.; Mueller, P.P.; Stieve, M.; Lenarzd, T.; Behrens, P. Mesoporous silica films as a novel biomaterial: Applications in the middle ear. Chem. Soc. Rev. 2013, 42, 3847–3861. [Google Scholar] [CrossRef] [PubMed]
- Sierra, I.; Pérez-Quintanilla, D. Heavy metal complexation on hybrid mesoporous silicas: An approach to analytical applications. Chem. Soc. Rev. 2013, 42, 3792–3807. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, C.; Elsaesser, M.S.; Hüsing, N. Chemical phase separation strategies towards silica monoliths with hierarchical porosity. Chem. Soc. Rev. 2013, 42, 3833–3846. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990; ISBN 0-12-134970-5. [Google Scholar]
- Corma, A.; Davis, M.E. Issues in the Synthesis of Crystalline Molecular Sieves: Towards the Crystallization of Low Framework-Density Structures. ChemPhysChem 2004, 5, 304–313. [Google Scholar] [CrossRef]
- Masoumifard, N.; Guillet-Nicolas, R.; Kleitz, F. Synthesis of Engineered Zeolitic Materials: From Classical Zeolites to Hierarchical Core-Shell Materials. Adv. Mater. 2018, 30, 1704439. [Google Scholar] [CrossRef] [PubMed]
- Meynen, V.; Cool, P.; Vansant, E.F. Synthesis of siliceous materials with micro- and mesoporosity. Microporous Mesoporous Mater. 2007, 104, 26–38. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of non-siliceous mesoporous oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, Z.; Luo, W.; Li, Y.; Elzatahry, A.A.; Al-Enizi, A.M.; Deng, Y.; Zhao, D. New Insight into the Synthesis of Large-Pore Ordered Mesoporous Materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of mesostructuring through evaporation-induced self-assembly. Adv. Funct. Mater. 2004, 4, 309–322. [Google Scholar] [CrossRef]
- Tiemann, M. Repeated templating. Chem. Mater. 2007, 20, 961–971. [Google Scholar] [CrossRef]
- Deng, X.; Chen, K.; Tüysüz, H. Protocol for the Nanocasting Method: Preparation of Ordered Mesoporous Metal Oxides. Chem. Mater. 2017, 29, 40–51. [Google Scholar] [CrossRef]
- Döring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels—Structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 40, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Gkioni, K.; Leeuwenburgh, S.C.G.; Douglas, T.E.L.; Mikos, A.G.; Jansen, J.A. Mineralization of Hydrogels for Bone Regeneration. Tissue Eng. B 2010, 16, 577–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Ono, Y.; Shinkai, S. Novel preparation method for multi-layered, tubular silica using an azacrown-appended cholesterol as template and metal-deposition into the interlayer space. J. Chem. Soc. Perkin Trans. 1999, 2, 1289–1291. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Hanabusa, K.; Shinkai, S. Creation of Both Right-Handed and Left-Handed Silica Structures by Sol-Gel Transcription of Organogel Fibers Comprised of Chiral Diaminocyclohexane Derivatives. J. Am. Chem. Soc. 2000, 122, 5008–5009. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Shinkai, S. Sol-Gel Polycondensation of Tetraethoxysilane in a Cholesterol-Based Organogel System Results in Chiral Spiral Silica. Angew. Chem. Int. Ed. 2000, 39, 1862–1865. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Shinkai, S. Novel Silica Structures Which Are Prepared by Transcription of Various Superstructures Formed in Organogels. Langmuir 2000, 16, 1643–1649. [Google Scholar] [CrossRef]
- Kubo, W.; Murakoshi, K.; Kitamura, T.; Wada, Y.; Hanabusa, K.; Shirai, H.; Yanagida, S. Fabrication of Quasi-Solid-State Dye-Sensitized TiO2 Solar Cells Using Low Molecular Weight Gelators. Chem. Lett. 1998, 27, 1241–1242. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hanabusa, K.; Hamasaki, N.; Kimura, M.; Shirai, H. Preparation of TiO2 Hollow-Fibers Using Supramolecular Assemblies. Chem. Mater. 2000, 12, 1523–1525. [Google Scholar] [CrossRef]
- Llusar, M.; Pidol, L.; Roux, C.; Pozzo, J.L.; Sanchez, C. Templated Growth of Alumina-Based Fibers through the Use of Anthracenic Organogelators. Chem. Mater. 2002, 14, 5124–5133. [Google Scholar] [CrossRef]
- Cui, X.; Tang, S.; Zhou, H. Mesoporous alumina materials synthesized in different gel templates. Mater. Lett. 2013, 98, 116–119. [Google Scholar] [CrossRef]
- Bao, C.; Lu, R.; Jin, M.; Xue, P.; Tan, C.; Zhao, Y.; Liu, G. Synthesis and Characterization of Nanostructural Hydrogel and Template for CdS Nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Lu, R.; Xue, P.; Bao, C.; Zhao, Y. Synthesis of CuS nanoribbons templated by hydrogel. Mater. Chem. Phys. 2008, 112, 500–503. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Chen, H.H.; Yao, J.; Fu, Y.Q.; Zhang, Z.Y.; Xu, Y.M. Effect of calcination temperature on physical parameters and photocatalytic activity of mesoporous titania spheres using chitosan/poly(vinyl alcohol) hydrogel beads as a template. Appl. Surf. Sci. 2014, 319, 189–196. [Google Scholar] [CrossRef]
- Chen, Z.; Weinberger, C.; Tiemann, M.; Kuckling, D. Organic Polymers as Porogenic Structure Matrices for Mesoporous Alumina and Magnesia. Processes 2017, 5, 70. [Google Scholar] [CrossRef]
- Jiu, J.; Kurumada, K.I.; Tanigaki, M. Preparation of nanoporous silica using copolymer template. Mater. Chem. Phys. 2002, 78, 177–183. [Google Scholar] [CrossRef]
- Jiu, J.; Kurumada, K.I.; Tanigaki, M. Preparation of nanoporous ZnO using copolymer gel template. Mater. Chem. Phys. 2003, 81, 93–98. [Google Scholar] [CrossRef]
- Jiu, J.; Kurumada, K.I.; Tanigaki, M.; Adachi, M.; Yoshikawa, S. Preparation of nanoporous MgO using gel as structure-direct template. Mater. Lett. 2003, 58, 44–47. [Google Scholar] [CrossRef]
- Jiu, J.; Kurumada, K.; Tanigaki, M. Preparation of oxide with nano-scaled pore diameters using gel template. J. Non-Cryst. Solids 2003, 325, 124–132. [Google Scholar] [CrossRef]
- Kurumada, K.; Nakamura, T.; Suzuki, A.; Umeda, N.; Kishimoto, N.; Hiro, M. Nanoscopic replication of cross-linked hydrogel in high-porosity nanoporous silica. J. Non-Cryst. Solids 2007, 353, 4839–4844. [Google Scholar] [CrossRef]
- Kurumada, K.I.; Nakamura, T.; Suzuki, A.; Umeda, N. Replication of a nano-scale mesh of hydrogel by assembled nanoparticles. Adv. Powder Technol. 2007, 18, 763–773. [Google Scholar] [CrossRef]
- Kurumada, K.; Suzuki, A.; Baba, S.; Otsuka, E. Relationship between polarity of template hydrogel and nanoporous structure replicated in sol–gel-derived silica matrix. J. Appl. Polym. Sci. 2009, 114, 4085–4090. [Google Scholar] [CrossRef]
- Ford, J.; Yang, S. Directed Synthesis of Silica Nanoparticles on Micropatterned Hydrogel Templates Tethered with Poly(ethyleneimine). Chem. Mater. 2007, 19, 5570–5575. [Google Scholar] [CrossRef]
- Song, S.; Saiz, E.; Bertozzi, C.R. A New Approach to Mineralization of Biocompatible Hydrogel Scaffolds: An Efficient Process toward 3-Dimensional Bonelike Composites. J. Am. Chem. Soc. 2003, 125, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tong, H.; Jiang, T.; Zhu, Z.; Wan, P.; Hu, J. Homogeneous chitosan/carbonate apatite/citric acid nanocomposites prepared through a novel in situ precipitation method. Compos. Sci. Technol. 2007, 67, 2238–2245. [Google Scholar] [CrossRef]
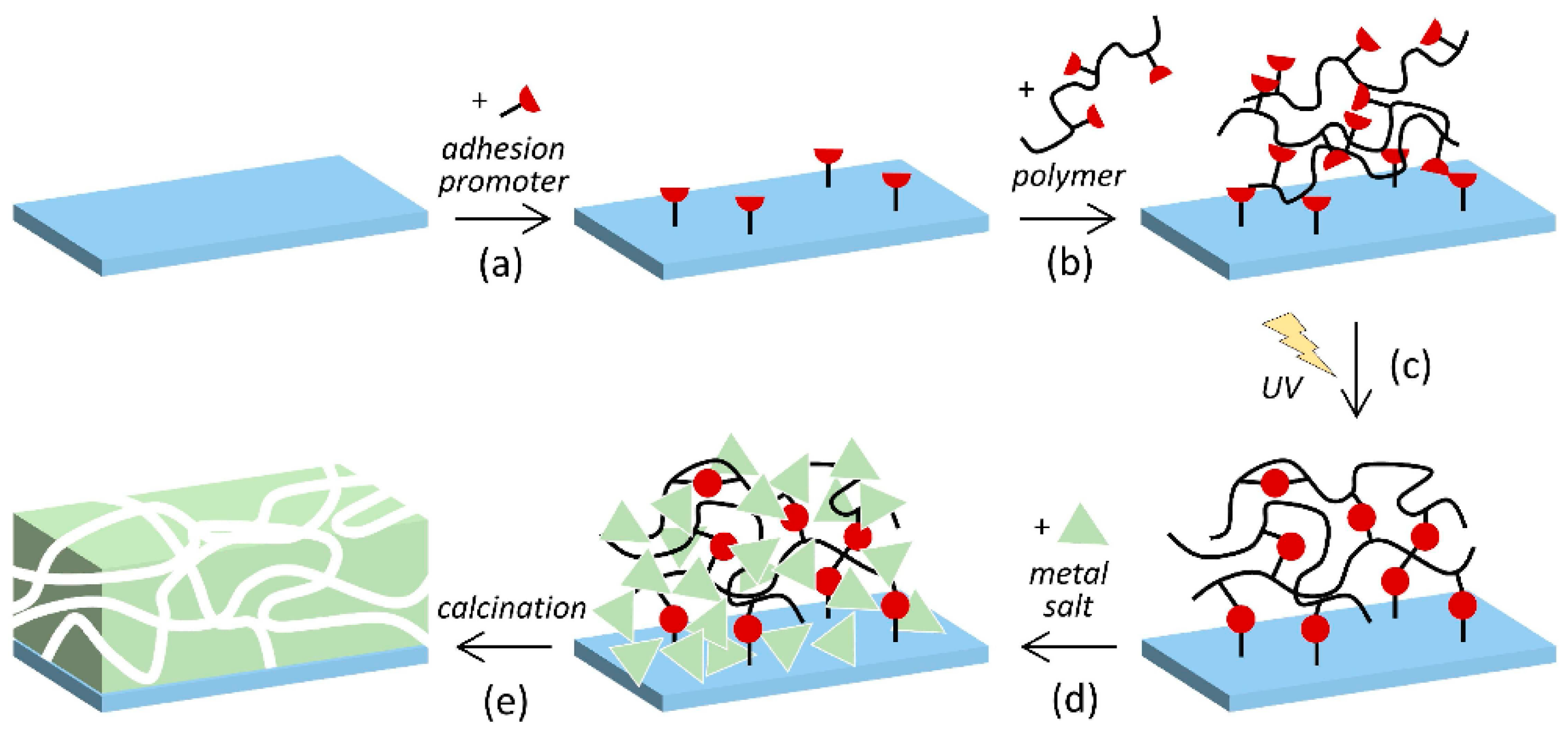
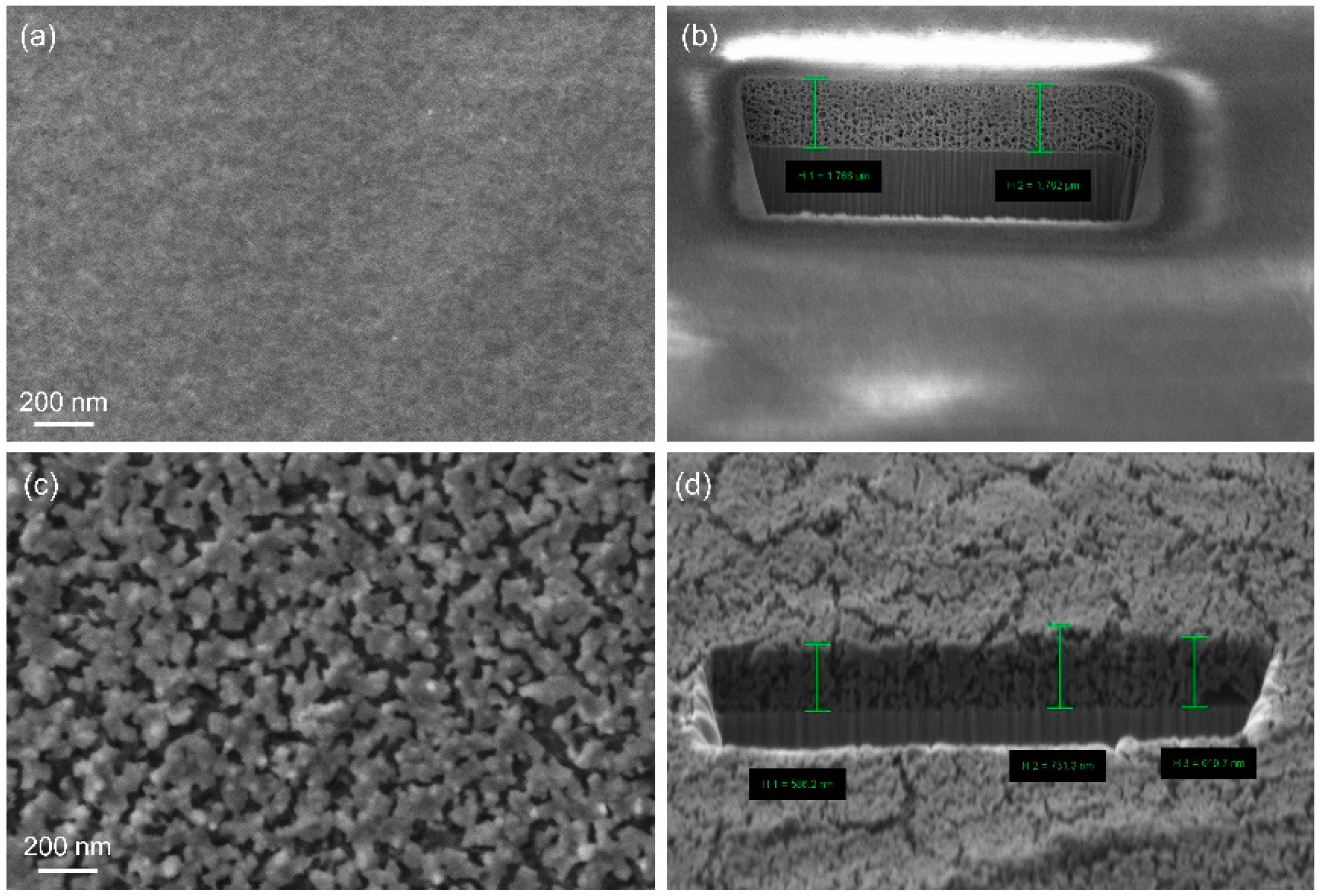
- Birnbaum, W.; Weinberger, C.; Schill, V.; Haffer, S.; Tiemann, M.; Kuckling, D. Synthesis of mesoporous alumina through photo cross-linked poly(dimethylacrylamide) hydrogels. Colloid Polym. Sci. 2014, 292, 3055–3060. [Google Scholar] [CrossRef]
- Weinberger, C.; Chen, Z.; Birnbaum, W.; Kuckling, D.; Tiemann, M. Photo-cross-linked polydimethylacrylamide hydrogels as porogens for mesoporous alumina. Eur. J. Inorg. Chem. 2017, 1026–1031. [Google Scholar] [CrossRef]
- Chen, Z.; Kuckling, D.; Tiemann, M. Porous Aluminum Oxide and Magnesium Oxide Films by Using Organic Hydrogels as Structure Matrices. Nanomaterials 2018, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N. In situ metal particle preparation in cross-linked poly(2-acrylamido-2-methyl-1-propansulfonic acid) hydrogel networks. Colloid Polym. Sci. 2006, 285, 283–292. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Chen, W.; Ning, J. Preparation of hollow CdSe nanospheres. Mater. Lett. 2004, 58, 2911–2913. [Google Scholar] [CrossRef]
- Wang, H.; Holmberg, B.A.; Yan, Y. Synthesis of Template-Free Zeolite Nanocrystals by Using in Situ Thermoreversible Polymer Hydrogels. J. Am. Chem. Soc. 2003, 125, 9928–9929. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinberger, C.; Kuckling, D.; Tiemann, M. Hydrogels as Porogens for Nanoporous Inorganic Materials. Gels 2018, 4, 83. https://doi.org/10.3390/gels4040083
Weinberger C, Kuckling D, Tiemann M. Hydrogels as Porogens for Nanoporous Inorganic Materials. Gels. 2018; 4(4):83. https://doi.org/10.3390/gels4040083
Chicago/Turabian StyleWeinberger, Christian, Dirk Kuckling, and Michael Tiemann. 2018. "Hydrogels as Porogens for Nanoporous Inorganic Materials" Gels 4, no. 4: 83. https://doi.org/10.3390/gels4040083