Production and New Extraction Method of Polyketide Red Pigments Produced by Ascomycetous Fungi from Terrestrial and Marine Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Fermentation and Biomass Production
2.3. Quantitative Colorimetric Analysis of Extracellular Extracts
2.4. UV-Visible Spectrophotometry and Extracellular Polyketide Metabolites Quantification
2.5. New Extraction Method of Intracellular Polyketide Pigments
2.6. Absorbance and HPLC-DAD Analyses
2.7. UHPLC-HRMS Analyses
3. Results
3.1. Biomass and Polyketide Extrolites Production Capacities under Various Growth Conditions
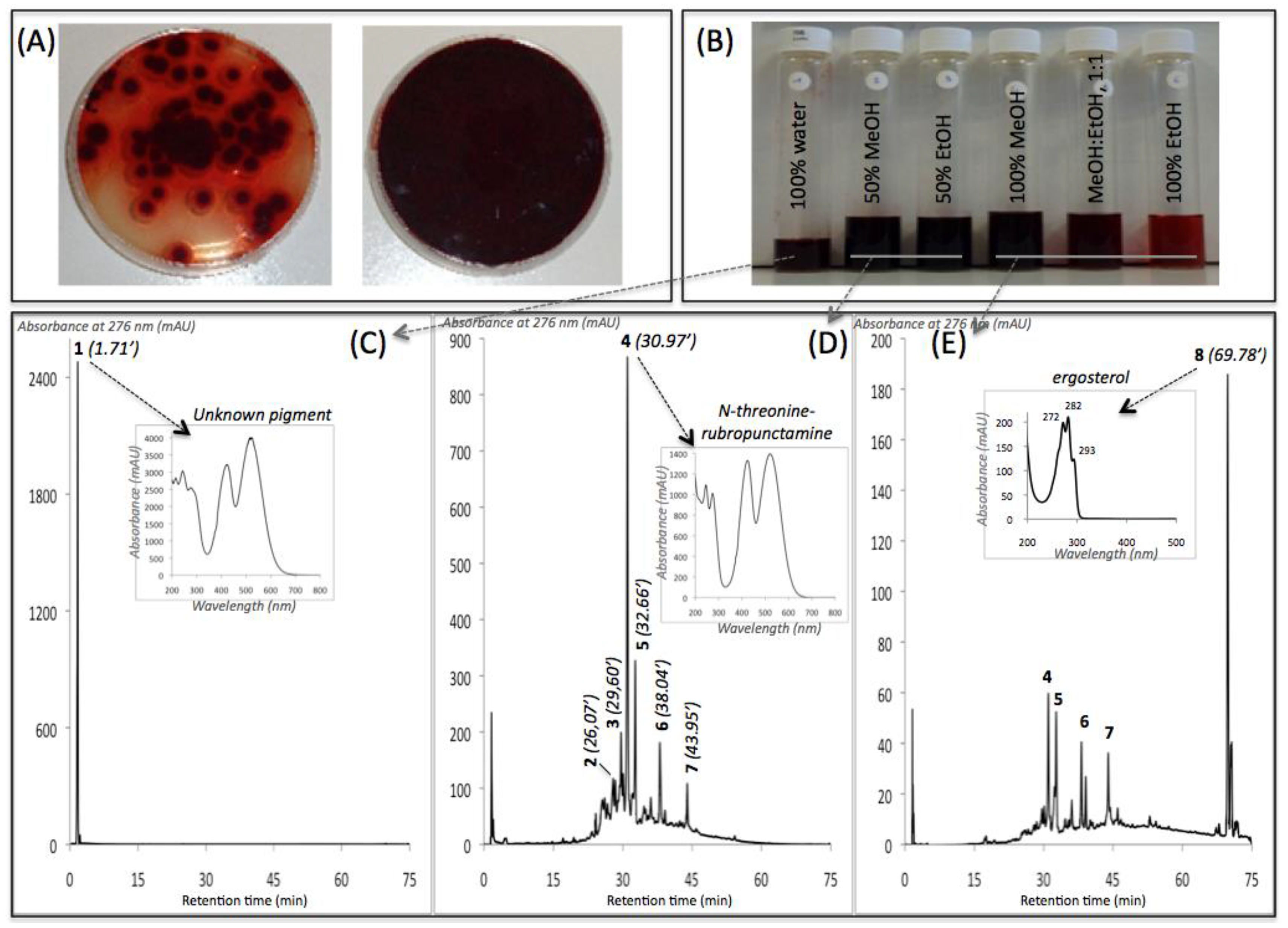
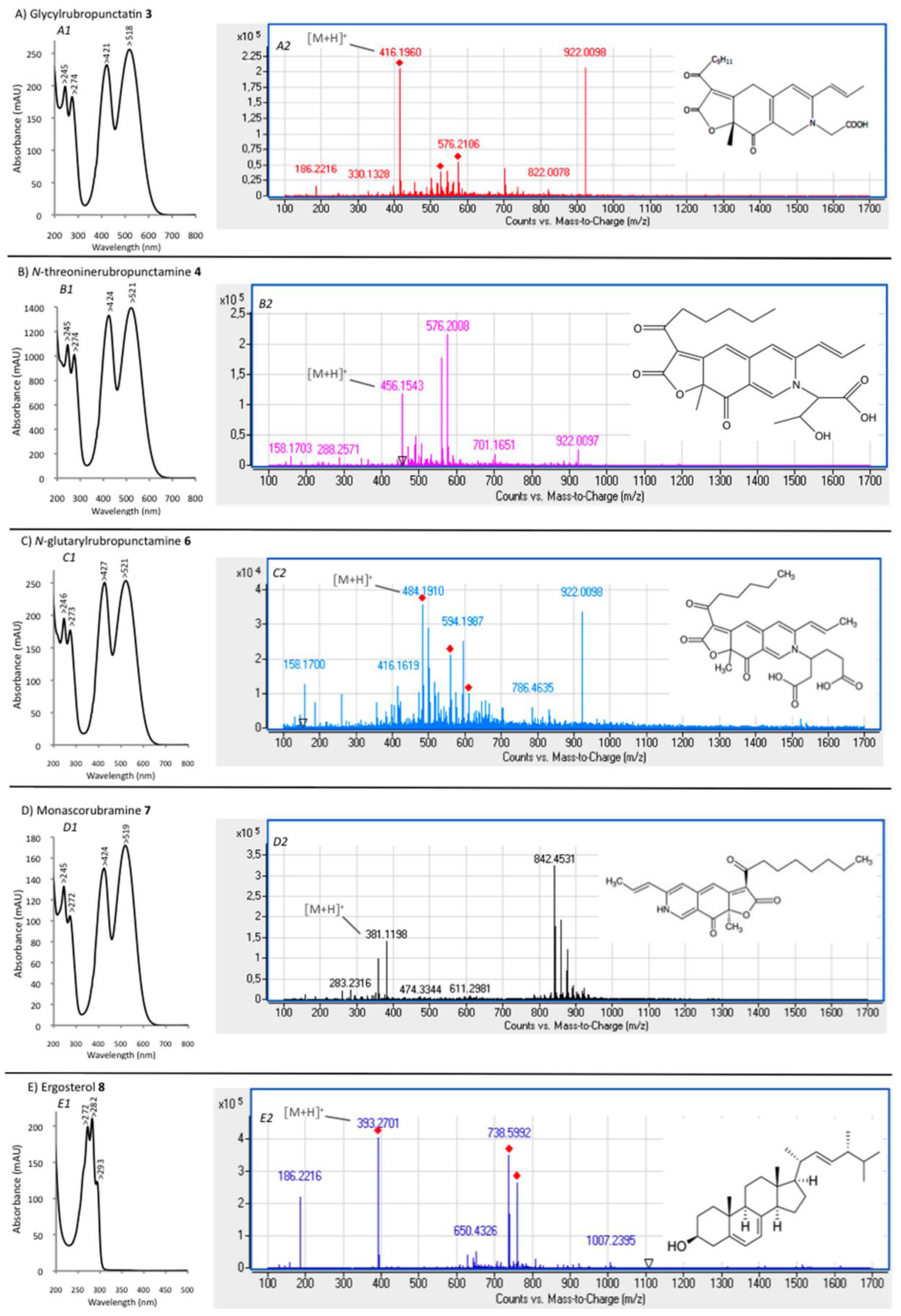
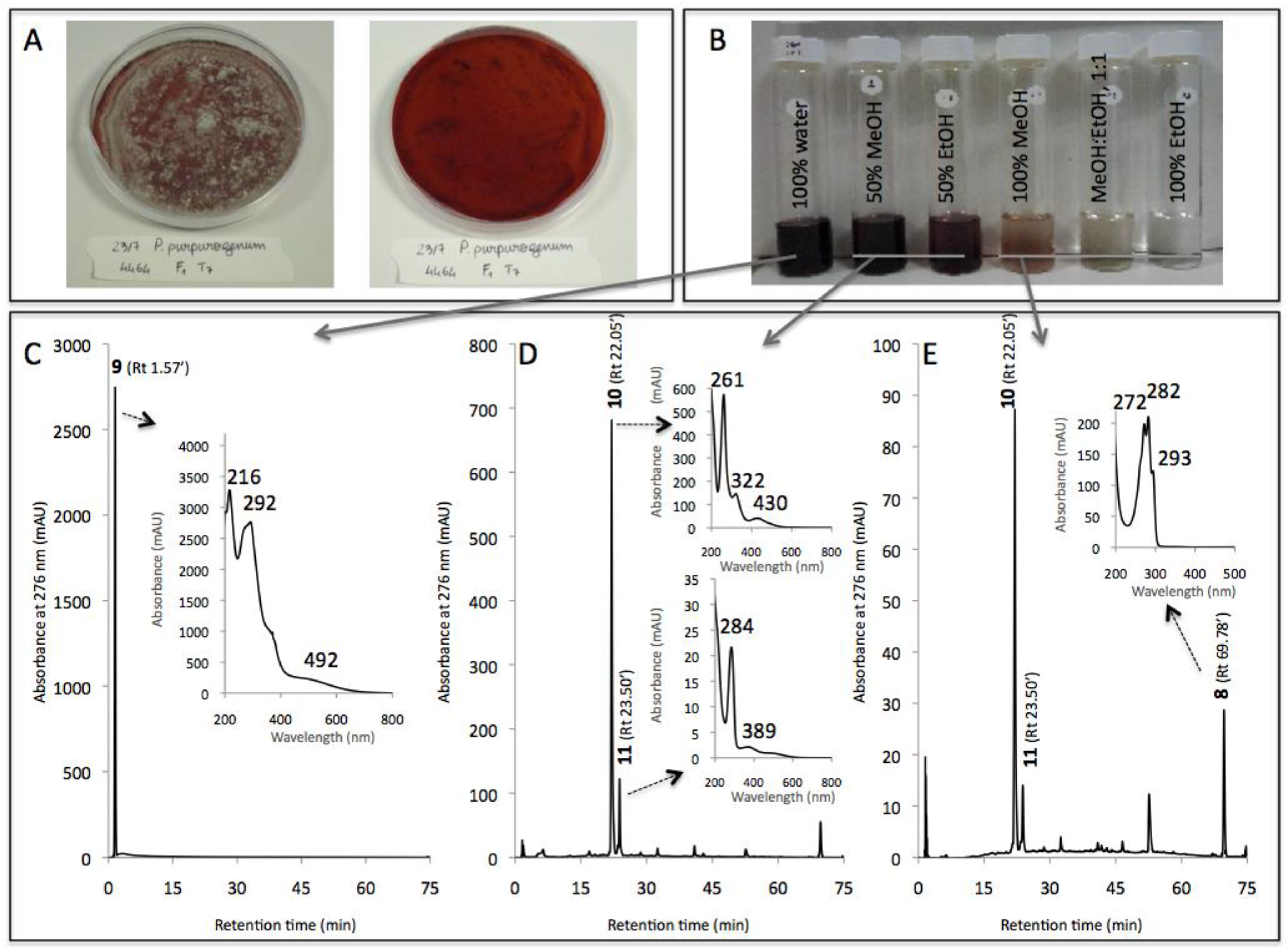
3.2. New Extraction Procedure and Nature of the Polyketide Red Pigments Produced by Talaromyces spp. Marine Isolate and P. purpurogenum rubisclerotium Terrestrial Isolate
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sutthiwong, N.; Caro, Y.; Laurent, P.; Fouillaud, M.; Valla, A.; Dufossé, L. Production of Biocolors. In Biotechnology in Agriculture and Food Processing: Opportunities and Challenges, 1st ed.; Panesar, P.S., Marwaha, S.S., Eds.; Francis & Taylor, CRC Press: Boca Raton, FL, USA, 2013; pp. 417–445. [Google Scholar]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufossé, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Dufossé, L. Anthraquinones, the Dr Jekyll and Mr Hyde of the food pigment family. Food Res. Int. 2014, 65, 132–136. [Google Scholar] [CrossRef]
- Durán, N.; Teixera, M.F.; Conti, R.D.; Esposito, E. Ecological-Friendly Pigments From Fungi. Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Dufosse, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and Colorants from Filamentous Fungi. In Fungal Metabolites, 1st ed.; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Berlin, Germany, 2015; pp. 499–568. [Google Scholar]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Houbraken, J.; Hoekstra, E.S.; Frisvad, J.C.; Visagie, C.M.; Samson, R.A. Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 2012, 29, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ebinuma, V.C.; Teixeira, M.F.; Pessoa, A., Jr. Submerged culture conditions for the production of alternative natural colorants by a new isolated Penicillium purpurogenum DPUA 1275. J. Microbiol. Biotechnol. 2013, 23, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell. Fact. 2009, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Meyer, A.S.; Frisvad, J.C.; Thrane, U. Production of Monascus-like azaphilone pigments. European patent EP2262862 B1, 28 March 2012. [Google Scholar]
- Arai, T.; Koganei, K.; Umemura, S.; Kojima, R.; Kato, J.; Kasumi, T.; Ogihara, J. Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V, production. AMB Express 2013, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, R.J.; Nielsen, N.J.; Maolanon, N.; Sorensen, J.C.; Olsson, S.; Nielsen, J.; Giese, H. The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones. Mol. Microbiol. 2006, 61, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Limon, M.C.; Rodriguez-Ortiz, R.; Avalos, J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. Trend. Anal. Chem. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Lee, Y.H.; Venil, C.K.; Lakshmanaperumalsamy, P.; Chae, J.-C.; Oh, B.-T. Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci. Bioeng. 2010, 109, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Guyomarc’h, F.; Binet, A.; Dufossé, L. Production of carotenoids by Brevibacterium linens: variation among strains, kinetic aspects and HPLC profiles. J. Ind. Microbiol. Biotechnol. 2000, 24, 64–70. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Carlet, E.; Flamin, C.; Valla, A. Spectrocolorimetry in the CIE L*a*b* color space as useful tool for monitoring the ripening process and the quality of PDO red-smear soft cheeses. Food Res. Int. 2005, 38, 919–924. [Google Scholar] [CrossRef]
- Sutthiwong, N.; Caro, Y.; Milhau, C.; Valla, A.; Fouillaud, M.; Dufossé, L. Arthrobacter arilaitensis strains isolated from ripened cheeses: Characterization of their pigmentation using spectrocolorimetry. Food Res. Int. 2014, 65, 184–192. [Google Scholar] [CrossRef]
- Klitgaard, A.; Iversen, A.; Andersen, M.R.; Larsen, T.O.; Frisvad, J.C.; Nielsen, K.F. Aggressive dereplication using UHPLC-DAD-QTOF: Screening extracts for up to 3000 fungal secondary metabolites. Anal. Bioanal. Chem. 2014, 406, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, C.; Kim, K.; Shin, C.S. Color Characteristics of Monascus Pigments Derived by Fermentation with Various Amino Acids. J. Agric. Food Chem. 2003, 51, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Hansen, M.E.; Meyer, A.S.; Thrane, U. Computerized Screening for Novel Producers of Monascus-like Food Pigments in Penicillium Species. J. Agric. Food Chem. 2008, 56, 9981–9989. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, A.; Singgih, M.; Julianti, E.; Blanc, P.J. Derivates of azaphilone Monascus pigments. Biocatal. Agric. Biotechnol. 2017, 9, 183–194. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Cjas, D.; Palfner, G.; Astuya, A.; Aballay, A.; Pérez, C.; Hernandez, V.; Becerra, J. Steroidal composition and cytotoxic activity from fruiting body of Cortinarius xiphidipus. Nat. Prod. Res. 2017, 31, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Dame, Z.T.; Silima, B.; Gryzenhout, M.; van Ree, T. Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat. Prod. Res. 2016, 30, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 4. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017. [Google Scholar] [CrossRef]
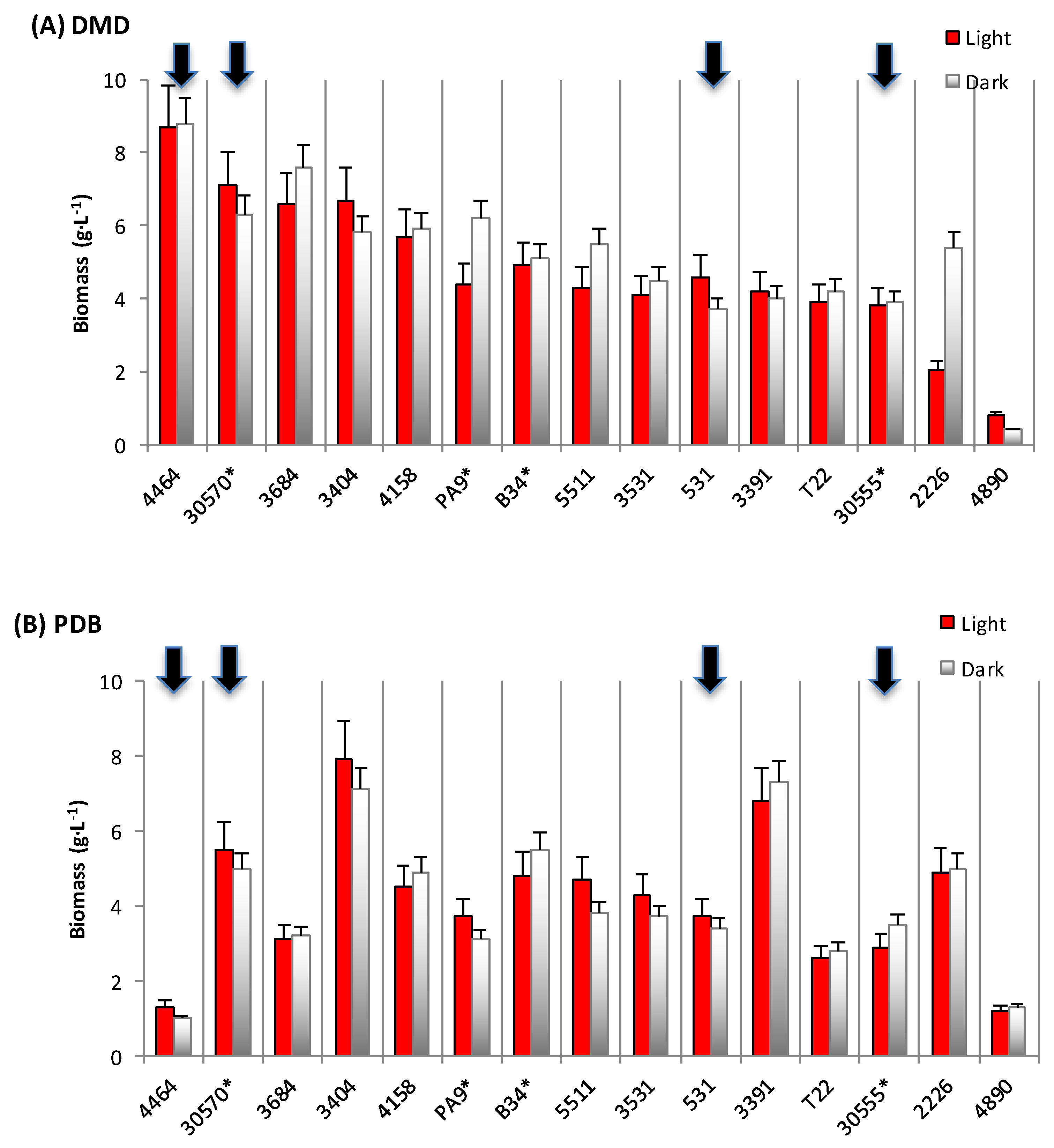
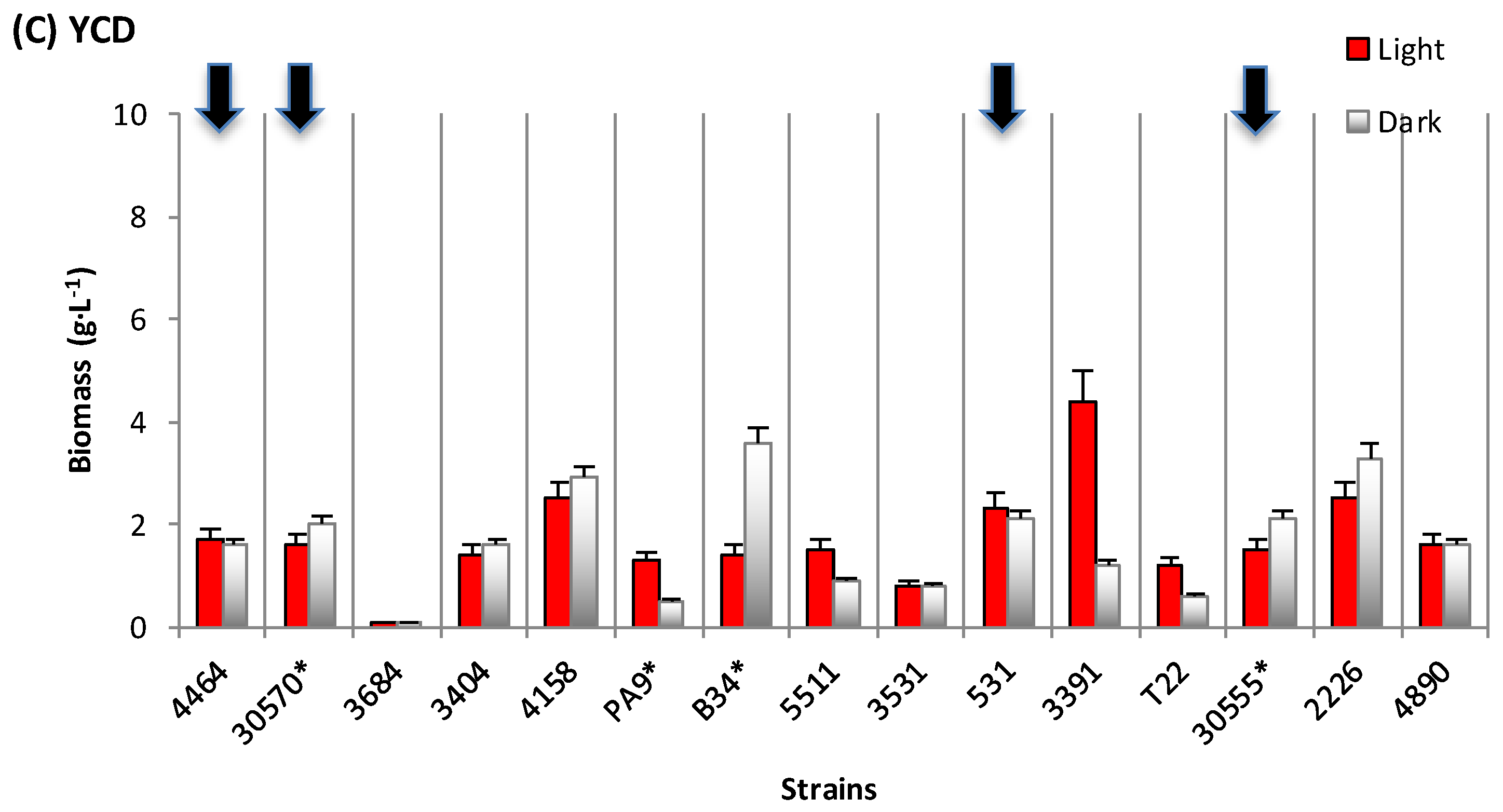
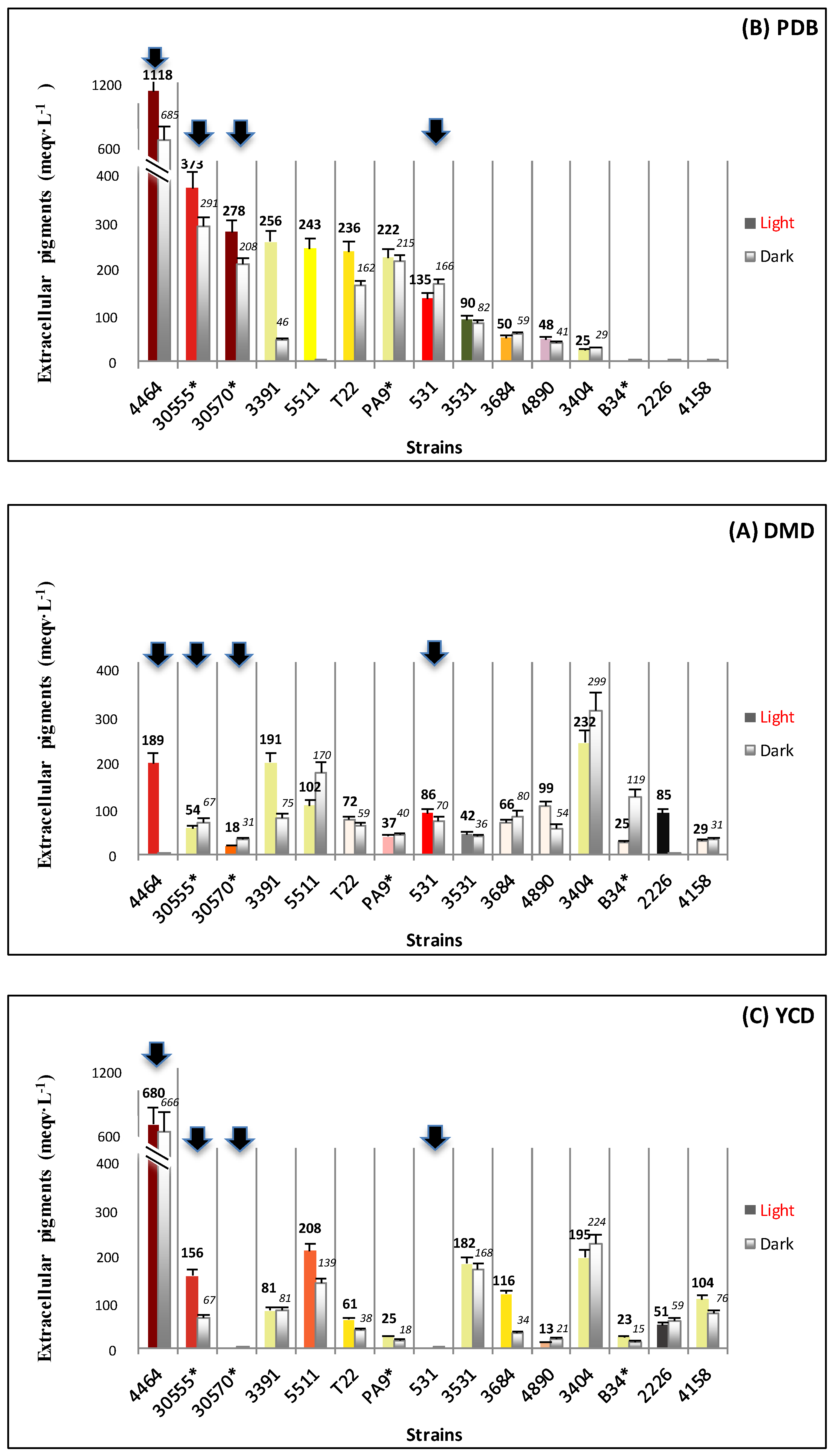
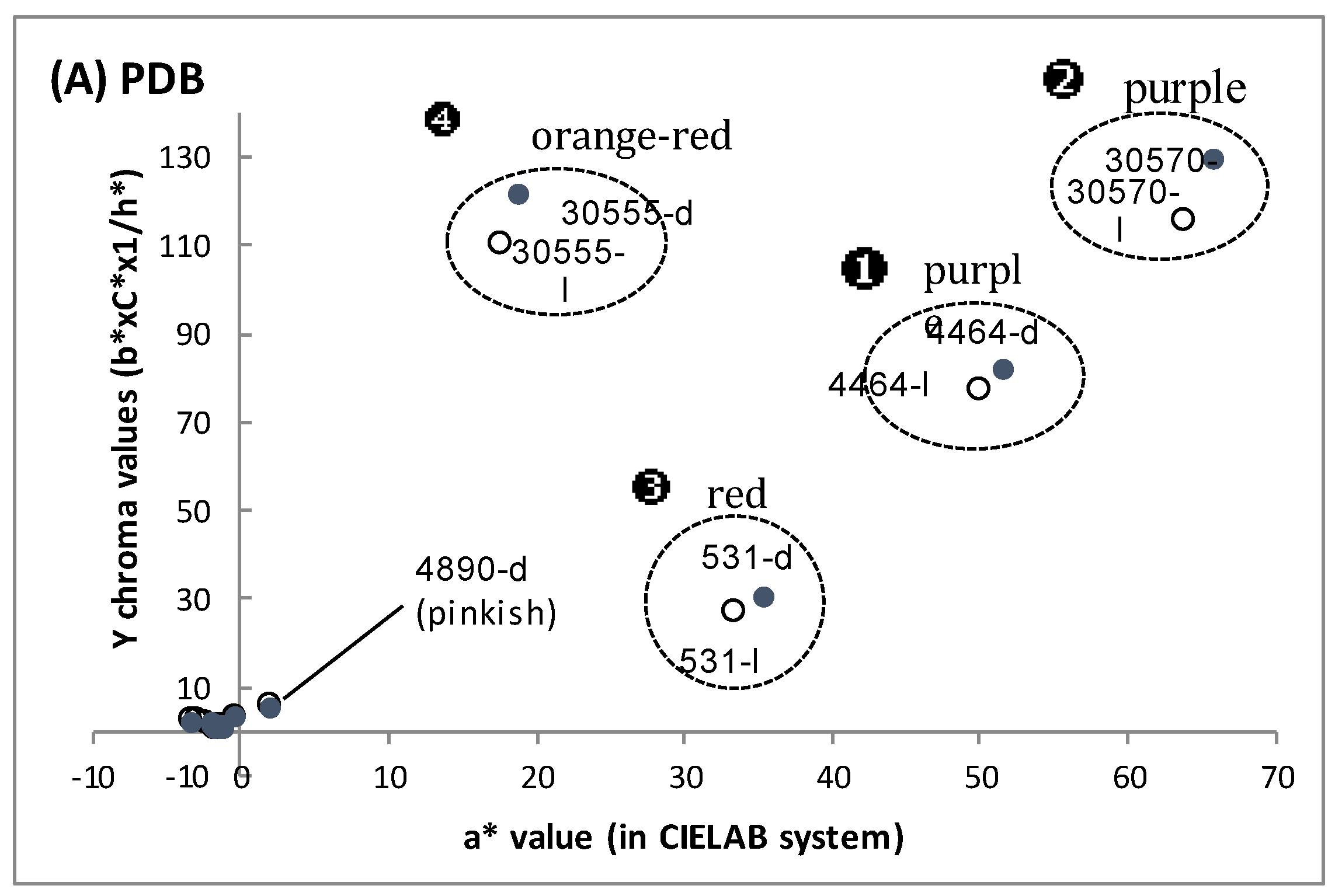
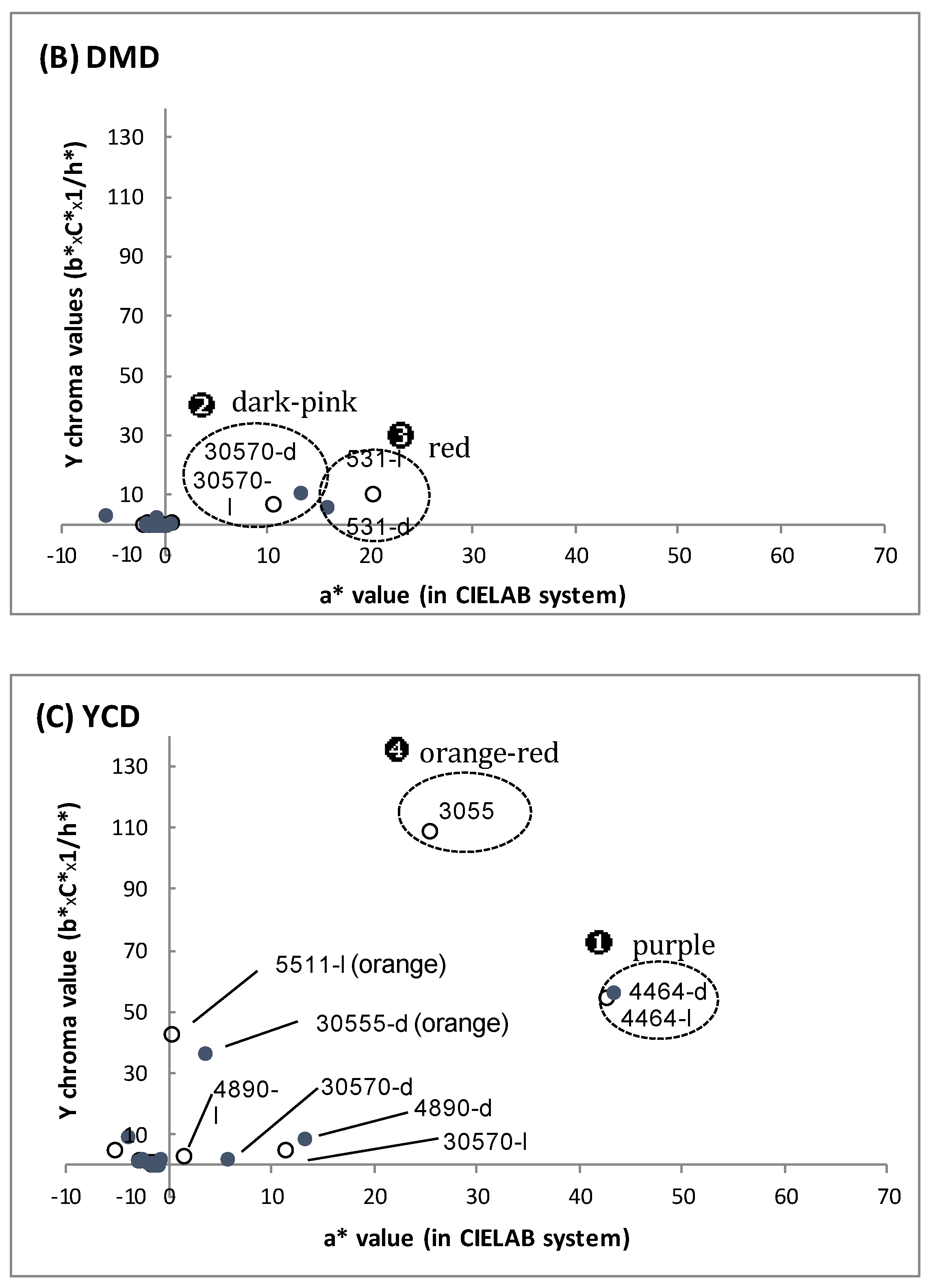
| Fungal Strain | Broth | Biomass | Extracellular Pigments | a-Value (CIELab) | Intracellular Pigments Extracted | |
|---|---|---|---|---|---|---|
| (g/L) | (meqv. Carmine/L) | (Red Color) | (meqv/g Biomass) or (meqv/L Culture) | |||
| 30570 | in DMD | 6.2–7.1 | 18–31 | 10.8–13.4 | 23.8–24.7 | 148–176 |
| in PDB | 5.0–5.5 | 208–278 | 63.8–65.8 | 49.4–69.8 | 247–384 | |
| in YCD | 1.6–2.0 | - | 5.3–5.6 | 44.3–48.5 | 71–97 | |
| 4464 | in DMD | 8.4–8.5 | 1–189 | 0.7 – 0.9 | 57.9–83.2 | 487–707 |
| in PDB | 1.0–1.3 | 685–1118 | 49.9–51.5 | 69.2–116.0 | 90–116 | |
| in YCD | 1.6–1.7 | 666–680 | 42.8–43.5 | 22.5–71.2 | 36–121 | |
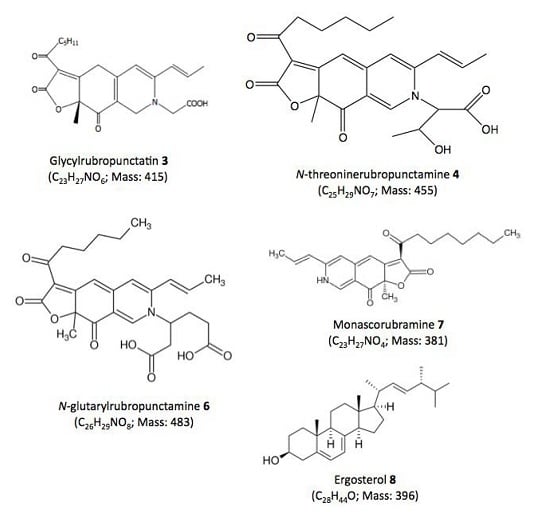
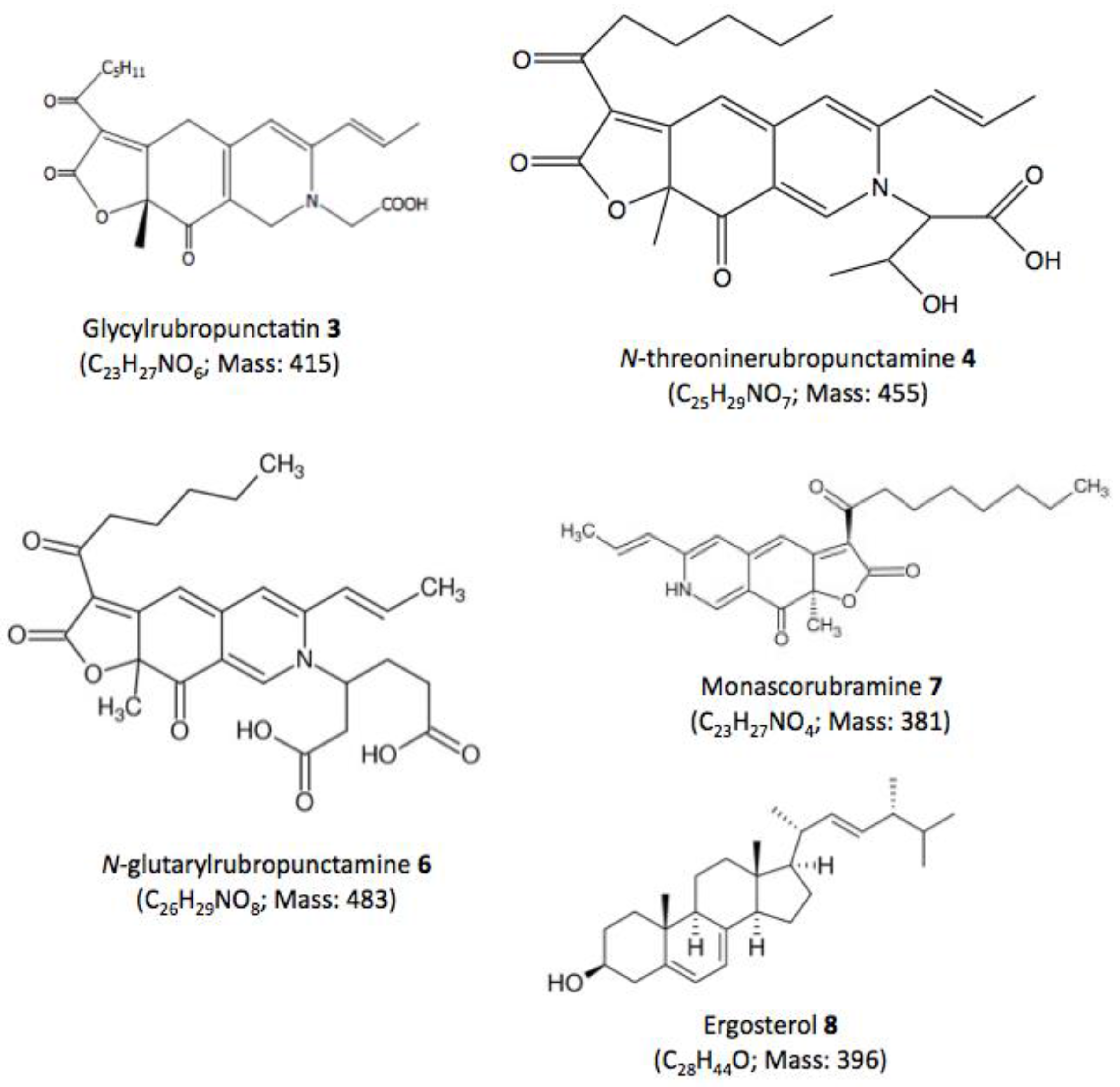
| Compound No. R.t. (mn) | λmax (nm) | Solvent | Tentative Identification | Mol. Peak (m/z) | |
|---|---|---|---|---|---|
| IE of Talaromyces spp. (305_70) | |||||
| 1 | 1.71 | 215, 244, 276, 418, 514, 524 | water | n.i. | n.d. |
| 2 | 26.07 | 246, 276, 425, 512 | MeOH | n.i. | 488 [M + H]+ |
| 3 | 29.60 | 245, 274, 421, 518 | MeOH | Glycylrubropunctatin | 416 [M + H]+ |
| 4 | 30.97 | 245, 274, 424, 521 | MeOH | N-threoninerubropunctamine | 456 [M + H]+ |
| 5 | 32.66 | 218, 250, 287, 424, 546 | MeOH | n.i. | 498 [M + H]+ |
| 6 | 38.04 | 246, 273, 427, 521 | MeOH | N-glutarylrubropunctamine | 484 [M + H]+ |
| 7 | 43.95 | 245, 272, 424, 519 | MeOH | Monascorubramine | 381 [M + H]+ |
| 8 | 69.78 | 272, 282, 293 | MeOH | Ergosterol | 393 [M + H]+ |
| IE of Penicillium purpurogenum rubisclerotium (4464) | |||||
| 8 | 69.78 | 272, 282, 293 | MeOH | Ergosterol | 393 [M + H]+ |
| 9 | 1.57 | 216, 292, 492 | MeOH | n.i. | n.d. |
| 10 | 22.05 | 262, 322, 430 | MeOH | n.i. | n.d. |
| 11 | 23.50 | 284, 389 | MeOH | n.i. | n.d. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and New Extraction Method of Polyketide Red Pigments Produced by Ascomycetous Fungi from Terrestrial and Marine Habitats. J. Fungi 2017, 3, 34. https://doi.org/10.3390/jof3030034
Lebeau J, Venkatachalam M, Fouillaud M, Petit T, Vinale F, Dufossé L, Caro Y. Production and New Extraction Method of Polyketide Red Pigments Produced by Ascomycetous Fungi from Terrestrial and Marine Habitats. Journal of Fungi. 2017; 3(3):34. https://doi.org/10.3390/jof3030034
Chicago/Turabian StyleLebeau, Juliana, Mekala Venkatachalam, Mireille Fouillaud, Thomas Petit, Francesco Vinale, Laurent Dufossé, and Yanis Caro. 2017. "Production and New Extraction Method of Polyketide Red Pigments Produced by Ascomycetous Fungi from Terrestrial and Marine Habitats" Journal of Fungi 3, no. 3: 34. https://doi.org/10.3390/jof3030034