Research Progress on the Regulation and Developmental Utilization of Bioactive Metabolites Synthesis in Floccularia luteovirens
Abstract
1. Introduction
2. Methodology
2.1. Search Strategies and Number of References
2.2. Screening Criteria and Process
2.3. Search the Database
2.4. Search Keywords
2.5. Time Range
3. Biological Characteristics and Ecological Adaptations of F. luteovirens
3.1. Geographical Distribution and Habitat
3.2. Adaptation Mechanisms
3.2.1. Environmental Adaptability of Macro Growth Cycle
3.2.2. Adaptation Mechanisms at the Molecular Level
4. Bioactive Metabolites of F. luteovirens
4.1. Diversity of Bioactive Metabolites
4.1.1. Metabolome Characteristics and Product Diversity of Mycelium
4.1.2. Key Signature Metabolites
4.2. Biological Function of Key Active Ingredients
4.2.1. Polysaccharides
4.2.2. Terpenoids
4.2.3. Other High-Value Compounds
4.3. Major Biosynthetic Pathways
4.3.1. Polysaccharide Biosynthetic Pathway
4.3.2. Triterpenoid Biosynthetic Pathways
5. Genetic and Environmental Regulation of Bioactive Metabolites Synthesis in F. luteovirens
5.1. Molecular Basis and Genetic Regulation of Synthesis
5.2. Regulatory Role and Mechanisms of Environmental Factors
5.2.1. Regulation by Abiotic Stresses
5.2.2. Cross-Talk of Stress Signals
6. Biotechnological Utilization: From Mycelial Production to Functional Products
6.1. Strategies for Enhanced Metabolite Production
6.1.1. Fermentation Process Optimization
6.1.2. Artificial Culture and Condition Optimization
6.2. Downstream Processing for Product Development
6.2.1. Extraction Process Innovation
6.2.2. Influence of Post-Treatment (Drying) Methods
6.3. Cultivation Mode Comparison and Scale-Up Potential
7. Conclusions and Outlook
7.1. Conclusions
7.2. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hibbett, D.; Nagy, L.G.; Nilsson, R.H. Fungal Diversity, Evolution, and Classification. Curr. Biol. 2025, 35, R463–R469. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S.C.; Perez-Moreno, J.; Rahman, M.M.; Rashid, M.H.; Phengsintham, P.; Rizal, L.; et al. Reviewing the World’s Edible Mushroom Species: A New Evidence-Based Classification System. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1982–2014. [Google Scholar] [CrossRef]
- Sangeeta; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom Processing: Innovative Techniques and Technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [CrossRef]
- Ni, Y.; Liao, Q.; Gou, S.; Shi, T.; Li, W.; Feng, R.; Zhao, Z.; Zhao, X. Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens. J. Fungi 2024, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dia, Z.M. Study on the nutritive composition in Armillaria luteo-virens and sustainable utilization of Qing Hai province. J. Shaanxi Norm. Univ. (Nat. Sci. Ed.) 2008, 36, 93–98. (In Chinese) [Google Scholar] [CrossRef]
- Taji, S.; Yamada, T.; Wada, S.; Tokuda, H.; Sakuma, K.; Tanaka, R. Lanostane-Type Triterpenoids from the Sclerotia of Inonotus obliquus Possessing Anti-Tumor Promoting Activity. Eur. J. Med. Chem. 2008, 43, 2373–2379. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically Active Polysaccharide from Edible Mushrooms: A Review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ren, J.L.; Zhang, H. Research Progress of Terpenoids and Bioactivities in Edible Mushroom. Sci. Technol. Food Ind. 2019, 40, 305–310. (In Chinese) [Google Scholar] [CrossRef]
- Mao, X.; Zhao, M.; Li, T.; Liu, Y.; Kong, X.H.; Ye, Y. Research progress in liquid fermentation technology and application of bioactive components in edible fungi. J. Food Saf. Qual. 2025, 16, 195–202. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhao, L.Z.; Li, N.; Lei, J.Q.; Zhang, F.M. The correlation of geographic distribution and ecological environment of endemic species Floccularia luteovirens on Qinghai-Tibet Plateau. Acta Ecol. Sin. 2016, 36, 2851–2857. (In Chinese) [Google Scholar]
- Zhao, L.Z. An overview of the study of Armillaria luteo-virens on the Tibetan Plateau. Edible Fungi 2015, 37, 1–3. (In Chinese) [Google Scholar]
- Chen, Q.L.; Diao, Z.M.; Han, Y. The Economic Value and Sustainable Utilization of Armillaria luteo-virens. World Notes Antibiot. 2011, 32, 161–164, 173. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Hu, B.; Deng, M.; Li, W.; Guo, J.; Song, Y.; Zheng, Q.; Song, X.; Ma, F.; et al. An Integrative Multi-Omics Analysis of Histone Modifications and DNA Methylation Reveals the Epigenomic Landscape in Apple under Drought Stress. Plant Biotechnol. J. 2025, 23, 4440–4460. [Google Scholar] [CrossRef]
- Xu, C.; Gui, Z.; Huang, Y.; Yang, H.; Luo, J.; Zeng, X. Integrated Transcriptomics and Metabolomics Analyses Provide Insights into Qingke in Response to Cold Stress. J. Agric. Food Chem. 2023, 71, 18345–18358. [Google Scholar] [CrossRef]
- Yang, F.-S.; Liu, M.; Guo, X.; Xu, C.; Jiang, J.; Mu, W.; Fang, D.; Xu, Y.-C.; Zhang, F.-M.; Wang, Y.-H.; et al. Signatures of Adaptation and Purifying Selection in Highland Populations of Dasiphora fruticosa. Mol. Biol. Evol. 2024, 41, msae099. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Liu, J.; Wang, L.; Liu, P.; Yin, Z.; Guo, S.; Ma, J.; Lu, Z.; Wang, T.; et al. Proteomic Discovery of H2O2 Response in Roots and Functional Characterization of PutGLP Gene from Alkaligrass. Planta 2018, 248, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xie, Z.; Jiang, H.; Xu, H.; Liu, B.; Meng, Q.; Peng, Q.; Tang, Y.; Duan, Y. The Molecular Mechanism of Yellow Mushroom (Floccularia luteovirens) Response to Strong Ultraviolet Radiation on the Qinghai-Tibet Plateau. Front. Microbiol. 2022, 13, 918491. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, Q.; Li, W.; Cao, L.; Feng, R.; Zhao, Z.; Zhao, X. Selection and Validation of Reference Genes for Normalization of Gene Expression in Floccularia luteovirens. Fungal Biol. 2024, 128, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Gao, Q.; Zhang, F.; Fu, P.; Wang, J.; Yan, H.; Chen, S. Genetic Variation and Phylogenetic Relationships of the Ectomycorrhizal Floccularia luteovirens on the Qinghai-Tibet Plateau. J. Microbiol. 2017, 55, 600–606. [Google Scholar] [CrossRef]
- Gan, X.; Bao, X.; Liu, B.; Li, Y.; Cao, D.; Zhang, H.; Zong, Y. Chemical Constituents and Molecular Mechanism of the Yellow Phenotype of Yellow Mushroom (Floccularia luteovirens). J. Fungi 2022, 8, 314. [Google Scholar] [CrossRef]
- Xing, R.; Yan, H.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Microbial Communities Inhabiting the Fairy Ring of Floccularia luteovirens and Isolation of Potential Mycorrhiza Helper Bacteria. J. Basic. Microbiol. 2018, 58, 554–563. [Google Scholar] [CrossRef]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef]
- Xie, Z.L.; Xu, H.Y.; Guo, J.; Meng, Q.; Dai, D.R.; Mao, Y.J. Study on Nutritional Types of Floccularia luteovirens and Identification of Genes Related to Environmental Adaptation; Qinghai University: Qinghai, China, 2021. (In Chinese) [Google Scholar]
- Xie, Z.L.; Guo, J.; Meng, Q. Analysis of genes related to the environmental adaptation of Floccularia luteovirens. In Proceedings of the 2018 Annual Academic Conference of the Mycological Society of China, Tai’an, China, 11 August 2018; p. 62. (In Chinese). [Google Scholar]
- Bai, S.J.; Bao, J.Y. Qualitative analysis on active ingredients of Armillaria luteo-virens. North. Hortic. 2012, 3, 161–163. (In Chinese) [Google Scholar]
- Fan, T.; Ren, R.; Tang, S.; Zhou, Y.; Cai, M.; Zhao, W.; He, Y.; Xu, J. Transcriptomics Combined with Metabolomics Unveiled the Key Genes and Metabolites of Mycelium Growth in Morchella importuna. Front. Microbiol. 2023, 14, 1079353. [Google Scholar] [CrossRef]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of Untargeted Metabolomics Approach and Molecular Networking Analysis to Identify Unique Natural Components in Wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Wu, I.-W.; Liao, Y.-C.; Tsai, T.-H.; Lin, C.-H.; Shen, Z.-Q.; Chan, Y.-H.; Tu, C.-W.; Chou, Y.-J.; Lo, C.-J.; Yeh, C.-H.; et al. Machine-Learning Assisted Discovery Unveils Novel Interplay between Gut Microbiota and Host Metabolic Disturbance in Diabetic Kidney Disease. Gut Microbes 2025, 17, 2473506. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, H.; Zhang, X.; Chen, Q. The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai–Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation. J. Fungi 2021, 7, 887. [Google Scholar] [CrossRef]
- Liu, Y. The Molecular Staructure, Conformation and Immunomodulatory Activity of Glucans from Floccularia luteovirens. Ph.D. Thesis, Tianjin University of Science and Technology, Tianjin, China, 2025. (In Chinese). [Google Scholar]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical Characterization, Antioxidant Properties and Anticancer Activity of Exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Xu, H.M.; Zhang, X.; Zhu, Q.Y.; Chen, Y.J.; Chen, Q.H.; Liu, Z.J. Optimization of Fermentation Conditions and Biological Activities of Exopolysaccharides from Floccularia luteovirens. J. Nucl. Agric. Sci. 2023, 37, 1598–1608. (In Chinese) [Google Scholar]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of Floccularia luteovirens Regulate Intestinal Immune Response, and Oxidative Stress Activity through MAPK/Nrf2/Keap1 Signaling Pathway in Immunosuppressive Mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Mueed, A.; Ma, Y.; Ibrahim, M.; Su, L.; Wang, Q. Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice. Foods 2024, 13, 3881. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Wang, S.; Li, C.; Chen, C.; Wan, X.; Li, D.; Li, Y. Polysaccharides of Floccularia luteovirens Alleviate Oxidative Damage and Inflammatory Parameters of Diabetic Nephropathy in Db/Db Mice. Front. Biosci.-Landmark 2023, 28, 82. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhang, X.; Zhu, Q.Y.; Wu, M.Y.; Zhang, L.Z.; Chen, Q.H.; Liu, Z.J. Effect of Extracellular Polysaccharides of Floccularia luteovirens on the Quality of Pacific White Shrimp during Refrigeration. Food Res. Dev. 2024, 45, 44–51+66. (In Chinese) [Google Scholar]
- Yang, S.; Xu, H.M.; Yin, X.L.; Ma, M.Z.; Miao, W.H.; Chen, Q.H.; Liu, Z.J.; Zhao, Y.D. Effect of polysaccharides of Floccularia luteovirens composite freshness coating on the storage quality of Tuna. Food Ferment. Ind. 2025. (In Chinese) [Google Scholar] [CrossRef]
- Schmidt-Dannert, C. Biosynthesis of Terpenoid Natural Products in Fungi. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–61. ISBN 978-3-319-20107-8. [Google Scholar]
- Zhang, X.; Zhu, Q.Y.; Xu, H.M.; Wu, M.Y.; Chen, X.E.; Chen, Q.H.; Liu, Z.J. Optimization of extraction process and activity of protoilludane sesquiterpene aryl esters from Floccularia luteovirens. J. Zhejiang Univ. (Agric. Life Sci.) 2023, 49, 813–824. (In Chinese) [Google Scholar]
- Dang, J.; Chen, C.; Ma, J.; Dawa, Y.; Wang, Q.; Tao, Y.; Wang, Q.; Ji, T. Preparative Isolation of Highly Polar Free Radical Inhibitor from Floccularia luteovirens Using Hydrophilic Interaction Chromatography Directed by On-Line HPLC-DPPH Assay. J. Chromatogr. B 2020, 1142, 122043. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, J.; Hou, L.; Gao, Y.; Sun, J.; Zhang, N.; Fan, B.; Wang, F. The Small Molecule Fractions of Floccularia luteovirens Induce Apoptosis of NSCLC Cells through Activating Caspase-3 Activity. Int. J. Mol. Sci. 2021, 22, 10609. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.M.; Chen, C.B.; Li, Y. Anti-inflammatory and analgetic effects of the aqueous extract of Floccularia luteovirens on NTG-induced migraine in rats. Mycosystema 2020, 39, 917–922. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.E. GC-MS analysis of supercritical carbon dioxide extraction products from Floccularia luteovirens. Mycosystema 2015, 34, 321–327. (In Chinese) [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-Inflammatory Potential of Mushroom Extracts and Isolated Metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Yu, G.; Ge, X.; Wang, Y.; Mo, X.; Yu, H.; Tan, L.; Yang, S. Discovery of Novel Terpenoids from the Basidiomycete Pleurotus ostreatus through Genome Mining and Coculture Optimization. J. Agric. Food Chem. 2023, 71, 11110–11123. [Google Scholar] [CrossRef]
- Ziemert, N.; Alanjary, M.; Weber, T. The Evolution of Genome Mining in Microbes—A Review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef]
- Luo, P.; Huang, J.-H.; Lv, J.-M.; Wang, G.-Q.; Hu, D.; Gao, H. Biosynthesis of Fungal Terpenoids. Nat. Prod. Rep. 2024, 41, 748–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, Y.H.; Mao, Y.J.; Meng, L.J.; Liu, H.L. Analysis on Isolation, Purification, Component and Structure of Armillaria luteo-virens. J. Chang. Univ. Sci. Technol. (Nat. Sci. Ed.) 2007, 02, 102–105. (In Chinese) [Google Scholar] [CrossRef]
- Brosnan, J.T. Interorgan Amino Acid Transport and Its Regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [CrossRef]
- Adil, B.; Xiang, Q.; He, M.; Wu, Y.; Asghar, M.A.; Arshad, M.; Qin, P.; Gu, Y.; Yu, X.; Zhao, K.; et al. Effect of Sodium and Calcium on Polysaccharide Production and the Activities of Enzymes Involved in the Polysaccharide Synthesis of Lentinus Edodes. AMB Expr. 2020, 10, 47. [Google Scholar] [CrossRef]
- Zan, X.-Y.; Wu, X.-H.; Cui, F.-J.; Zhu, H.-A.; Sun, W.-J.; Jiang, L.-H.; Tao, T.-L.; Zhao, X. UDP-Glucose Pyrophosphorylase Gene Affects Mycelia Growth and Polysaccharide Synthesis of Grifola frondosa. Int. J. Biol. Macromol. 2020, 161, 1161–1170. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the Fungal Terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, H.; Zhong, J. Biosynthesis of a Ganoderic Acid in Saccharomyces cerevisiae by Expressing a Cytochrome P450 Gene from Ganoderma lucidum. Biotech. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Macías-Rubalcava, M.L.; Trujillo-Roldán, M.A. Overview of Fungal Terpene Synthases and Their Regulation. World J. Microbiol. Biotechnol. 2023, 39, 194. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Godio, R.P.; Fouces, R.; Martín, J.F. A Squalene Epoxidase Is Involved in Biosynthesis of Both the Antitumor Compound Clavaric Acid and Sterols in the Basidiomycete H. sublateritium. Chem. Biol. 2007, 14, 1334–1346. [Google Scholar] [CrossRef]
- Cui, M.; Yang, H.; He, G. Submerged Fermentation Production and Characterization of Intracellular Triterpenoids from Ganoderma lucidum Using HPLC-ESI-MS. J. Zhejiang Univ. Sci. B 2015, 16, 998–1010. [Google Scholar] [CrossRef]
- Hu, G.; Zhai, M.; Niu, R.; Xu, X.; Liu, Q.; Jia, J. Optimization of Culture Condition for Ganoderic Acid Production in Ganoderma lucidum Liquid Static Culture and Design of a Suitable Bioreactor. Molecules 2018, 23, 2563. [Google Scholar] [CrossRef]
- Li, H.-J.; Zhang, D.-H.; Han, L.-L.; Yu, X.; Zhao, P.; Li, T.; Zhong, J.-J.; Xu, J.-W. Further Improvement in Ganoderic Acid Production in Static Liquid Culture of Ganoderma lucidum by Integrating Nitrogen Limitation and Calcium Ion Addition. Bioprocess. Biosyst. Eng. 2016, 39, 75–80. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, Q.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in Content of Triterpenoids and Polysaccharides in Ganoderma Lingzhi at Different Growth Stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.-J. A Novel Three-Stage Light Irradiation Strategy in the Submerged Fermentation of Medicinal Mushroom Ganoderma lucidum for the Efficient Production of Ganoderic Acid and Ganoderma Polysaccharides. Biotechnol. Prog. 2008, 24, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.X.; Zhao, M.W. Methyl Jasmonate Induces Ganoderic Acid Biosynthesis in the Basidiomycetous Fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef]
- Jiang, A.-L.; Liu, Y.-N.; Liu, R.; Ren, A.; Ma, H.-Y.; Shu, L.-B.; Shi, L.; Zhu, J.; Zhao, M.-W. Integrated Proteomics and Metabolomics Analysis Provides Insights into Ganoderic Acid Biosynthesis in Response to Methyl Jasmonate in Ganoderma lucidum. Int. J. Mol. Sci. 2019, 20, 6116. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-L.; Huang, W.-F.; Ren, Y.; Onac, E.; Zhou, G.-F.; Peng, S.; Wang, X.-J.; Li, H.-H. LED Lights Increase Bioactive Substances at Low Energy Costs in Culturing Fruiting Bodies of Cordyceps militaris. Sci. Hortic. 2014, 175, 139–143. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural Product Drug Discovery in the Genomic Era: Realities, Conjectures, Misconceptions, and Opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Dhakal, D.; Pham, V.T.T.; Nguyen, H.T.; Sohng, J.-K. Recent Advances in Strategies for Activation and Discovery/Characterization of Cryptic Biosynthetic Gene Clusters in Streptomyces. Microorganisms 2020, 8, 616. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Ishikawa, J.; Hara, H.; Suzuki, H.; Ikenoya, M.; Ikeda, H.; Yamashita, A.; Hattori, M.; Horinouchi, S. Genome Sequence of the Streptomycin-Producing Microorganism Streptomyces griseus IFO 13350. J. Bacteriol. 2008, 190, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, M.; Samborskyy, M.; Lester, J.B.; Mironenko, T.; Scott, N.; Dickens, S.; Haydock, S.F.; Leadlay, P.F. Complete Genome Sequence of the Erythromycin-Producing Bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 2007, 25, 447–453. [Google Scholar] [CrossRef]
- Kalkreuter, E.; Pan, G.; Cepeda, A.J.; Shen, B. Targeting Bacterial Genomes for Natural Product Discovery. Trends Pharmacol. Sci. 2020, 41, 13–26. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, J.; Meng, Q.; Zhang, H.; Zhou, G.; Li, M.; Wu, P.; Zhao, Y.; Chen, C.; Qin, Q. A New High-Quality Draft Genome Assembly of the Chinese Cordyceps Ophiocordyceps sinensis. Genome Biol. Evol. 2020, 12, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, Q.; Baiyintala; Wuhanqimuge. The Whole-Genome Sequence Analysis of Morchella Sextelata. Sci. Rep. 2019, 9, 15376. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Cao, D.; Zhang, Z.; Cheng, S.; Wei, L.; Li, S.; Liu, B. Draft Genome Assembly of Floccularia luteovirens, an Edible and Symbiotic Mushroom on Qinghai-Tibet Plateau. G3 2020, 10, 1167–1173. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Ren, A.; Li, M.-J.; Shi, L.; Mu, D.-S.; Jiang, A.-L.; Han, Q.; Zhao, M.-W. Profiling and Quantifying Differential Gene Transcription Provide Insights into Ganoderic Acid Biosynthesis in Ganoderma lucidum in Response to Methyl Jasmonate. PLoS ONE 2013, 8, e65027. [Google Scholar] [CrossRef]
- Cao, P.-F.; Wu, C.-G.; Dang, Z.-H.; Shi, L.; Jiang, A.-L.; Ren, A.; Zhao, M.-W. Effects of Exogenous Salicylic Acid on Ganoderic Acid Biosynthesis and the Expression of Key Genes in the Ganoderic Acid Biosynthesis Pathway in the Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Li, X.-B.; Miao, Z.-G.; Shi, L.; Jaing, A.-L.; Zhao, M.-W. Transcript and Metabolite Alterations Increase Ganoderic Acid Content in Ganoderma lucidum Using Acetic Acid as an Inducer. Biotechnol. Lett. 2014, 36, 2529–2536. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Xia, X.-X.; Zhong, J.-J. Induced Effect of Na+ on Ganoderic Acid Biosynthesis in Static Liquid Culture of Ganoderma lucidum via Calcineurin Signal Transduction. Biotechnol. Bioeng. 2013, 110, 1913–1923. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.-J.; Cao, P.-F.; Chen, T.-X.; Zhang, G.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. Heat Stress Modulates Mycelium Growth, Heat Shock Protein Expression, Ganoderic Acid Biosynthesis, and Hyphal Branching of Ganoderma lucidum via Cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-L.; Zhang, G.; Ren, A.; Dang, Z.-H.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. The pH-Responsive Transcription Factor PacC Regulates Mycelial Growth, Fruiting Body Development, and Ganoderic Acid Biosynthesis in Ganoderma lucidum. Mycologia 2016, 108, 1104–1113. [Google Scholar] [CrossRef]
- Hu, Y.; Ahmed, S.; Li, J.; Luo, B.; Gao, Z.; Zhang, Q.; Li, X.; Hu, X. Improved Ganoderic Acids Production in Ganoderma lucidum by Wood Decaying Components. Sci. Rep. 2017, 7, 46623. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, J.-J.; Geng, A. Improvement of Ganoderic Acid Production by Fermentation of Ganoderma lucidum with Cellulase as an Elicitor. Process Biochem. 2014, 49, 1580–1586. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, P.; Liu, J.; Luo, Y.; Sangzhu, T.; Li, H.; Zhao, Y.; Tang, C.; Yang, M. Metabolomics-Based Discrimination of Geographical Variability in Quality and Antioxidant Activity of Golden Mushroom (Floccularia luteovirens) from the Qinghai-Tibet Plateau. Food Biosci. 2025, 68, 106536. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, T.-J.; Lu, X.-X.; Ma, B.-L.; Ren, A.; Shi, L.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Membrane Fluidity Is Involved in the Regulation of Heat Stress Induced Secondary Metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef]
- Mei, X.L.; Chen, R.Y.; Li, B.M.; Chen, X.D.; Zhao, Z.; Lan, J. Effect of light quality on growth and triterpenic acid content of Ganoderma lucidum mycelium. Chin. Tradit. Herb. Drugs 2013, 44, 3546–3550. (In Chinese) [Google Scholar]
- Liu, R.; Shi, L.; Zhu, T.; Yang, T.; Ren, A.; Zhu, J.; Zhao, M.-W. Cross Talk between Nitric Oxide and Calcium-Calmodulin Regulates Ganoderic Acid Biosynthesis in Ganoderma lucidum under Heat Stress. Appl. Environ. Microbiol. 2018, 84, e00043-18. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Lu, X.-X.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Conversion of Phosphatidylinositol (PI) to PI4-Phosphate (PI4P) and Then to PI(4,5)P2 Is Essential for the Cytosolic Ca2+ Concentration under Heat Stress in Ganoderma lucidum. Environ. Microbiol. 2018, 20, 2456–2468. [Google Scholar] [CrossRef]
- Gao, T.; Shi, L.; Zhang, T.; Ren, A.; Jiang, A.; Yu, H.; Zhao, M. Cross Talk between Calcium and Reactive Oxygen Species Regulates Hyphal Branching and Ganoderic Acid Biosynthesis in Ganoderma lucidum under Copper Stress. Appl. Environ. Microbiol. 2018, 84, e00438-18. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.-Y.; Chen, Q.-H.; Xie, H.-C.; Jiao, Y.-C. Anthocyanin Extract from Lycium ruthenicum Enhanced Production of Biomass and Polysaccharides during Submerged Fermentation of Agaricus bitorquis (Quél.) Sacc. Chaidam. Bioprocess. Biosyst. Eng. 2021, 44, 2303–2313. [Google Scholar] [CrossRef]
- Wang, H.; You, X.Q.; Yu, M. Studies on the fermentation culture of extracellular polysaccharide production by Armillaria luteo-virens. North. Hortic. 2010, 22, 174–176. (In Chinese) [Google Scholar]
- Diao, Z.M. Preliminary studies on the nutritional and physiological properties of the mycelium of Armillaria luteo-virens. J. Microbiol. 1997, 01, 14–17. (In Chinese) [Google Scholar]
- Cai, X.; Zhang, Y.; Wang, X.L. Study on Medium Optimization of Yellow-green Armillaria luteo virens and Condition of Artificial Culture. Anhui Agric. Sci. Bull. 2013, 19, 33–34+50. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H.Z.; Wang, H.X.; Liu, Q.H.; Feng, K. Preliminary Study on Mycelium Growing Conditions of Armillaria luteovirens. Edible Fungi China 2007, 04, 16–19. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, J.S.; Jiao, Y.C.; Sheng, H.Y.; Xiong, H.Y.; Yang, C.J. Effects of Triacontanol, Culture Medium pH, and Incubation Temperature on the Mycelial Growth of Armillaria luteovirens. Edible Fungi 2011, 33, 8–9. (In Chinese) [Google Scholar]
- Liu, Z.J. Study on Physiological Characteristics, Importantbioactive Compounds, the Multi-Omics Elucidation Andgenetic Characterization of Floccularia luteovirens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2022. (In Chinese). [Google Scholar]
- Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. Mutational Analysis of MpPhy Reveals Magnetoreception and Photosensitivity Involvement in Secondary Metabolites Biosynthesis in Monascus Purpureus. J. Photochem. Photobiol. B 2021, 217, 112164. [Google Scholar] [CrossRef]
- Zhu, L.; Su, Y.; Ma, Z.; Guo, L.; Yang, S.; Yu, H. Comparative Proteomic Analysis Reveals Differential Protein Expression of Hypsizygus marmoreus in Response to Different Light Qualities. Int. J. Biol. Macromol. 2022, 223, 1320–1334. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.B.; Zhang, B.; Tong, X.D.; Wang, S.M.; Li, Y. Antioxidant activities and extraction technique optimization of crude polysaccharides from the fruiting body of Floccularia luteovirens. Mycosystema 2019, 38, 1681–1688. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, M.; Wang, T.; Tang, C.; He, M.; Pu, X.; Zhao, T.; Li, Y. Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis. J. Fungi 2025, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Yang, W.; Shi, Y.; Sun, Y.; Mariga, A.M.; Zhao, L.; Fang, Y.; Ma, N.; An, X.; Hu, Q. Comparison of Freeze-Drying with Three Different Combinations of Drying Methods and Their Influence on Colour, Texture, Microstructure and Nutrient Retention of Button Mushroom (Agaricus bisporus) Slices. Food Bioprocess. Technol. 2014, 7, 702–710. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi-a Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Yu, M. Modelling of mycelial dynamics in the batch fermentation of Armillaria luteo-virens. Sci. Technol. Food Ind. 2010, 31, 135–137. (In Chinese) [Google Scholar] [CrossRef]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
| Research Dimension | Key Findings | References |
|---|---|---|
| Genome and Transcriptome | Genomic annotation of 205 environmental adaptation genes; transcriptome analysis identified 225 Differentially Expressed Genes (DEGs) involved in environmental signal transduction, DNA damage repair and pigment metabolic pathways; | [17] |
| Functional Genomics | To validate the expression stability of 13 candidate endogenous genes in a wide range of abiotic stresses (salt, drought, oxidation, heat, extreme pH, cadmium stress); | [18] |
| Population Genomics | Discovery of 15 genotypes based on analysis of rDNA markers (ITS/IGS-1/LSU) to provide a genetic basis for environmental adaptation; | [19] |
| Transcriptome and Metabolome | The transporter gene FlMCH5, which exhibits highly specific overexpression, is a key transport regulatory gene responsible for generating the yellow phenotype; | [20] |
| Microbial Ecology | Comparison of microbial communities in different regions (IN/ON/OUT) of the fungal ring; Microbial diversity was lower in the ON zone; mycorrhizal promoting bacteria (MHB) such as Bradyrhizobium and Paenibacillus were more abundant in the ON zone; soil nutrient and physical changes shaped the ON zone community | [21] |
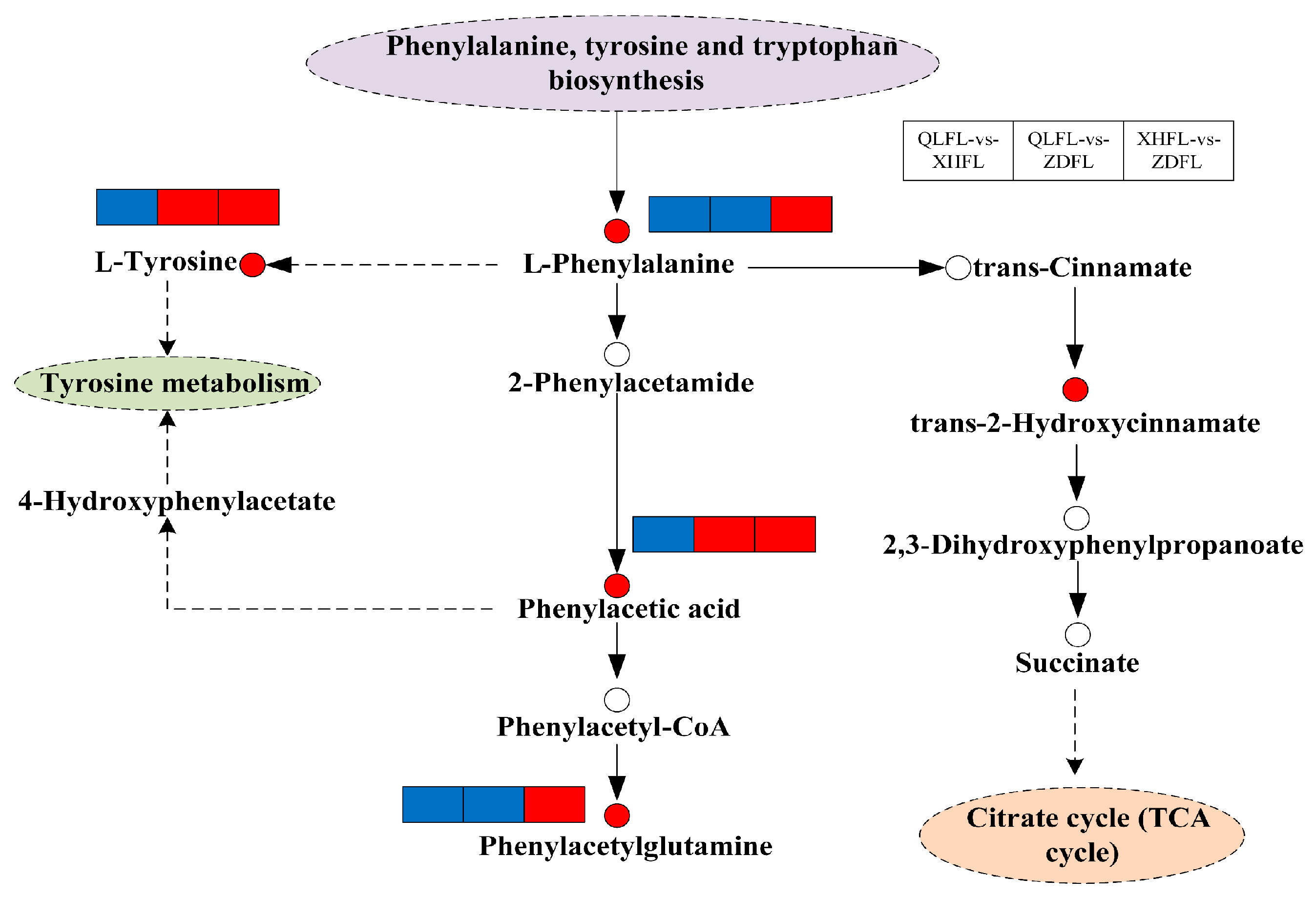
| Metabolomics | Identification of 5782 metabolites; vanillic acid hypothesized to be a key antioxidant marker; phenylalanine biosynthesis and metabolism as a major differential pathway; | [22] |
| Transcriptomics | Forty-five core genes for environmental adaptation were identified and classified into signal sensing (28.89%), metabolic reprogramming (26.67%) and stress response (44.44%) categories; | [23,24] |
| Metabolite Category | Specific Ingredients | Main Biological Functions | Research Modeling/Methodology | References |
|---|---|---|---|---|
| Polysaccharide | Extracellular Polysaccharide (FLEP) | Antioxidant; Antitumor; Neuroprotective; Antibacterial and Preservation | In vitro assays: chemical analysis; antitumor (PC9, NCI-H460); neuroprotective (PC12); antibacterial & preservation (shrimp model) | [31,32,36] |
| Fruiting body crude Polysaccharides (FLP1, FLPs) | Anti-fatigue, Anti-diabetic Nephropathy, Moisture-absorbing and Moisturizing | Mouse anti-fatigue model, db/db diabetic mouse model, in vitro physicochemical analysis | [33,34,37] | |
| Terpenoid | Melleolides (Capacity) | Genetic basis of terpenoids with antimicrobial and insecticidal activity | Genome mining | [29,38] |
| Protoilludane Sesquiterpene Aryl Esters | Antioxidant, Antibacterial | In vitro chemical analysis | [39] | |
| Other high-value compounds | L-(+)-ergothioneine—A rare amino acid | Strong antioxidant, known as the “longevity vitamin”. | HILIC chromatography combined with HPLC-DPPH screening, structure identification | [40] |
| Low molecular weight components (<6 kDa) | Anti-tumor: rich in amino acids, nucleosides, terpenes, alkaloids, etc. | Tumor cell lines, hollow fiber membrane separation | [41] | |
| aqueous extract (FLW) | Anti-type II diabetes, anti-migraine | Type II Diabetes Mellitus Rat Model, Nitroglycerin Induced Migraine Rat Model | [35,42] | |
| Unsaturated fatty acid | Contains linoleic acid, etc., nutrition and health care. | GC-MS analysis | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Tang, L.; Gou, S.; Li, W.; Xu, C.; Zhao, X. Research Progress on the Regulation and Developmental Utilization of Bioactive Metabolites Synthesis in Floccularia luteovirens. J. Fungi 2025, 11, 854. https://doi.org/10.3390/jof11120854
Shi T, Tang L, Gou S, Li W, Xu C, Zhao X. Research Progress on the Regulation and Developmental Utilization of Bioactive Metabolites Synthesis in Floccularia luteovirens. Journal of Fungi. 2025; 11(12):854. https://doi.org/10.3390/jof11120854
Chicago/Turabian StyleShi, Tongjia, Lihua Tang, Siyuan Gou, Wensheng Li, Chunxiao Xu, and Xu Zhao. 2025. "Research Progress on the Regulation and Developmental Utilization of Bioactive Metabolites Synthesis in Floccularia luteovirens" Journal of Fungi 11, no. 12: 854. https://doi.org/10.3390/jof11120854
APA StyleShi, T., Tang, L., Gou, S., Li, W., Xu, C., & Zhao, X. (2025). Research Progress on the Regulation and Developmental Utilization of Bioactive Metabolites Synthesis in Floccularia luteovirens. Journal of Fungi, 11(12), 854. https://doi.org/10.3390/jof11120854






